Strategies for cardio-protection are essential in coronary artery bypass graft surgery. The authors explored the relationship between cardioplegia volume, left ventricular mass index and ischemia time by means of the infused cardioplegia index and its relationship with post-operative low cardiac output syndrome.
DesignAll patients undergoing coronary artery bypass graft surgery between January 2013 and December 2015 were included. Low cardiac output syndrome was defined according to criteria of the SEMICYUC's consensus document. The perioperative factors associated with low cardiac output syndrome were estimated, and using a ROC curve, the optimum cut-off point for the infused cardioplegia index to predict the absence of low cardiac output syndrome was calculated.
ResultsOf 360 patients included, 116 (32%) developed low cardiac output syndrome. The independent risk predictors were: New York Heart Association Functional Classification (OR 1.8 [95% CI=1.18–2.55]), left ventricle ejection fraction (OR 0.95 (95% CI=0.93–0.98]), ICI (OR 0.99 [95% CI=0.991–0.996]) and retrograde cardioplegia (OR 1.2 [95% CI=1.03–1.50]). The infused cardioplegia index showed an area under the ROC curve of 0.77 (0.70–0.83; p<.001) for the absence of postoperative low cardiac output syndrome using the optimum cut-off point of 23.6ml·min−1 (100g/m2 of LV).
ConclusionsThe infused cardioplegia index presents an inverse relationship with the development of post-operative low cardiac output syndrome. This index could form part of new strategies aimed at optimizing cardio-protection. The total volume of intermittent cardioplegia, especially that of maintenance, should probably be individualized, adjusting for ischemia time and left ventricle mass index.
La cardioprotección es esencial en la revascularización coronaria quirúrgica. En este estudio exploramos la relación existente entre el tiempo que una masa miocárdica permanece en situación de isquemia y la dosis de cardioplejía utilizada para su preservación, reflejada a través del índice de cardioplejía infundida, con el desarrollo de bajo gasto cardiaco postoperatorio.
DiseñoSe incluyeron todos los pacientes sometidos a revascularización coronaria quirúrgica entre enero de 2013 y diciembre de 2015. El síndrome de bajo gasto cardiaco postoperatorio se definió siguiendo los criterios del documento de consenso de la SEMYCIUC. Se analizaron los factores perioperatorios asociados al síndrome de bajo gasto cardiaco y, mediante la curva ROC, se determinó el punto de corte del índice de cardioplejía infundida para predecir la ausencia del mismo.
ResultadosDe los 360 pacientes incluidos, 116 (32%) presentaron bajo gasto postoperatorio. Los factores de riesgo independientes fueron: clasificación funcional de la New York Heart Association (OR 1,8 [IC 95%=1,18–2,55]), la fracción de eyección del ventrículo izquierdo (OR 0,95 [IC 95%=0,93–0,98]), el empleo de cardioplejía retrógrada (OR 1,2 [IC 95%=1,03–1,50]) y el índice de cardioplejía infundida (OR 0,99 [IC 95%=0,991–0,996]), que mostró un área bajo la curva ROC de 0,77 (0,70–0,83; p<0,001) para la ausencia de síndrome de bajo gasto cardiaco postoperatorio, usando como punto de corte óptimo 23,6ml·min−1 (100g/m2 de VI).
ConclusionesEl índice de cardioplejía infundido es inversamente proporcional a los requerimientos postoperatorios de inotropos, pudiendo constituir una estrategia para optimizar la cardioprotección. El volumen total de cardioplejía intermitente debería calcularse, de forma individualizada, en base al índice de masa del ventrículo izquierdo y el tiempo de isquemia.
Left ventricular systolic dysfunction is an important prognostic factor in heart surgery patients.1 Myocardial stunning is a frequent cause of postoperative systolic dysfunction characterized by a decrease in contractility secondary to ischemia/reperfusion damage without causing cell death,2 and may contribute to the development of postoperative low cardiac output syndrome (LCOS). The longer the ischemia time, the greater the resulting molecular damage and secondary cellular alterations.3 It has been estimated that 10–15% of all patients subjected to coronary revascularization surgery develop left ventricular dysfunction.4,5
During heart surgery it is essential to adopt cardioprotection measures in order to minimize ischemia/reperfusion damage. In this regard, cardioplegia represents the main intraoperative myocardial protection strategy. Different methods have been developed to optimize cardioplegia, ranging from variations in its content or temperature to the use of different administration flows and routes. However, to the best of our knowledge, few studies have examined cardioplegia adjustment according to other factors associated with myocardial susceptibility to ischemia/reperfusion damage, such as the degree of ischemic involvement or increased left ventricular mass. The latter factor is associated with increased myocardial work, with the consequent increase in myocardial oxygen consumption, and should be taken into account in designing effective cardioprotection strategies.
The present study uses the infused cardioplegia index (ICI) to explore the relationship between cardioplegia volume, ischemia time and left ventricular mass, and the appearance of myocardial damage in patients subjected to myocardial revascularization surgery.
Patients and methodsThe study was approved by the local Ethics Committee.
Study design and populationThe study was carried out in the 24-bed medical-surgical Intensive Care Unit (ICU) of Canarias University Hospital (Canary Islands, Spain). An ambispective cohort design was used, including all patients subjected to coronary revascularization surgery between January 2013 and December 2015.
We defined postoperative LCOS based on the consensus definition of the SEMICYUC,6 with the inclusion of those patients who after the optimization of preload presented a measured cardiac index (CI) of <2.2l/min/m2; a clinical condition consistent with LCOS in non-monitored individuals with oliguria (diuresis <0.5ml/kg/h), central venous saturation <60% (with normal arterial saturation) and/or lactate >3mmol/l; a need for inotropic drugs and/or balloon counterpulsation in the immediate postoperative period to guarantee adequate hemodynamic conditions; and criteria of cardiogenic shock (CI <2l/min/m2), systolic blood pressure <90mmHg and oliguria.
Data compilationProspective data compilation was carried out, including demographic information, comorbidities, functional class, study of the main affected coronary arteries, SYNTAX score, left ventricular ejection fraction (LVEF), tricuspid annular plane systolic excursion (TAPSE) determined by echocardiography, and preoperative critical condition defined by the need for mechanical ventilation, inotropic support and ventricle assist, and/or acute renal failure with diuresis <10ml/h. Surgical risk was calculated based on the EuroSCORE.7 Surgical variables were also recorded: ischemia time, extracorporeal circulation (ECC) time, type and volume of cardioplegia (the only information collected on a retrospective basis), and complete or incomplete revascularization (according to the principal vessels involved). Lastly, postoperative data were compiled: hemodynamic parameters, vasopressor (amine) dose, right ventricular dysfunction (defined as hypocontractility evidenced by the surgeon upon weaning from the pump), duration of mechanical ventilation, need for renal replacement techniques, ICU and hospital stay, and mortality after 30 days.
Blood samples were analyzed in the immediate postoperative period, after 24h of admission, and every 24h as required. The following biochemical myocardial damage markers were measured: troponin I, creatine kinase MB, and N-terminal brain natriuretic peptide (NT-proBNP). Lactate in turn was recorded as systemic hypoperfusion marker. The preoperative echocardiographic study was carried out following the guidelines of the American Society of Echocardiography8 in the echocardiography laboratory. Left ventricular mass was calculated based on the modified formula of Devereux et al.,9 indexed according to body surface area, and LVEF was estimated from the modified Simpson equation.
Study variablesThe primary independent variable was ICI. This index relates total cardioplegia infused during the ischemia time to the left ventricular mass index (LVMI) (Σ Cardioplegia/ischemia time/LVMI). The primary dependent variable was the absence of criteria of postoperative LCOS.
Anesthetic and surgical protocolsAll patients were subjected to preoxygenation, and induction was carried out with propofol (1.5–1.8mg/kg b.w.), remifentanil (0.1–0.3μg/kg/min) and rocuronium (1mg/kg). Anesthesia was maintained with sevoflurane (1.5–2vol%) and remifentanil in continuous perfusion. The extracorporeal circulation system consisted of a membrane oxygenator (Optima XP, Cobe, Denver, CO, USA or Quantum Lifestream International, Inc. Woodlands, TX, USA), a Tygon extracorporeal circuit (Dideco, Mirandola, Italy) and Medtronic Biopump centrifuge pump (Minneapolis, MN, USA). Under hypothermia (30–32°C), the pump flow was adjusted to guarantee mean arterial pressure values of >60mmHg and CI 2.2l/min/m.2 All patients initially received anterograde cardioplegia, while maintenance was carried out with anterograde or retrograde cardioplegia according to surgeon criterion. For cardiac arrest we administered 1200–1500ml of cold crystalloid cardioplegia (500ml Ringer solution, 40mEq KCl, 25mEq HCO3Na, 35mg lidocaine) as intermittent infusion in 4:1 proportion with blood cardioplegia. Additional doses of 500ml of saline solution with 20mEq of KCl were administered every 20–25min or when considered necessary.
Statistical analysisContinuous variables were reported as the mean, standard deviation (SD), median and percentiles 25–75. The Student t-test or Mann–Whitney U test was used for comparison purposes, depending on the distribution of the variables (as determined with the Kolmogorov-Smirnov test). Homoscedasticity of the variances was evaluated with the Leven test. Categorical variables were reported as frequencies and percentages, with use of the chi-squared test or Fisher exact test for comparing differences between groups. The variables found to be significantly associated to LCOS with p<0.10 in the univariate analysis (age, dyslipidemia, calcium antagonists, LVEF, EuroSCORE, New York Heart Association (NYHA) functional class, preoperative critical condition, type of surgery, degree of revascularization, ICI and retrograde cardioplegia) were entered in the logistic regression analysis in order to identify independent predictors of LCOS. The ECC time, indexed LVMI and total cardioplegia volume were excluded from the regression analysis, since they were included in the calculation of ICI with the purpose of avoiding colinearity. We explored the association between ICI and LCOS stratifying according to the two risk covariables of the final model: NYHA (class I-II versus III-IV) and LVEF (using the cut-off point associated to the appearance of LCOS: 56%). The associations among ICI, myocardial damage biomarkers and inotropic drug doses were estimated based on the Spearman rho statistic. We grouped ICI into tertiles, and its association to the dobutamine requirements was examined with the Kruskal–Wallis test. The reliability of ICI in predicting the absence of LCOS was verified by analyzing the area under the receiver operating characteristic (ROC) curve. Cases with severe right ventricular dysfunction were excluded. For establishing the optimum cut-off point we calculated general precision, sensitivity, specificity, positive/negative predictive values and the positive/negative likelihood ratio. The optimum cut-off point for the absence of LCOS was selected by means of Youden's index. Lastly, a univariate analysis of the clinical impact of LCOS was carried out. Statistical significance was considered for p<0.5 in two-tailed testing. The IBM SPSS version 20.0 statistical package (IBM SPSS, Inc., Armonk, NY, USA) was used throughout.
ResultsThe study comprised a total of 362 patients with a mean age of 68±9 years (280 males; 77.3%). The mean EuroSCORE was 9.2±12.5. Medical-surgical variables: 348 (96.1%) were first surgeries, 225 (62.2%) elective surgeries, 127 (35.1%) emergency surgeries, and 10 (2.8%) were emergent surgeries, with a mean ischemia time of 68±39min and an ECC time of 114±78min. Nine patients (2.5%) were in preoperative critical condition. Two patients (0.5%) of 12 (3%) died in the operating room and were therefore not included in the analysis.
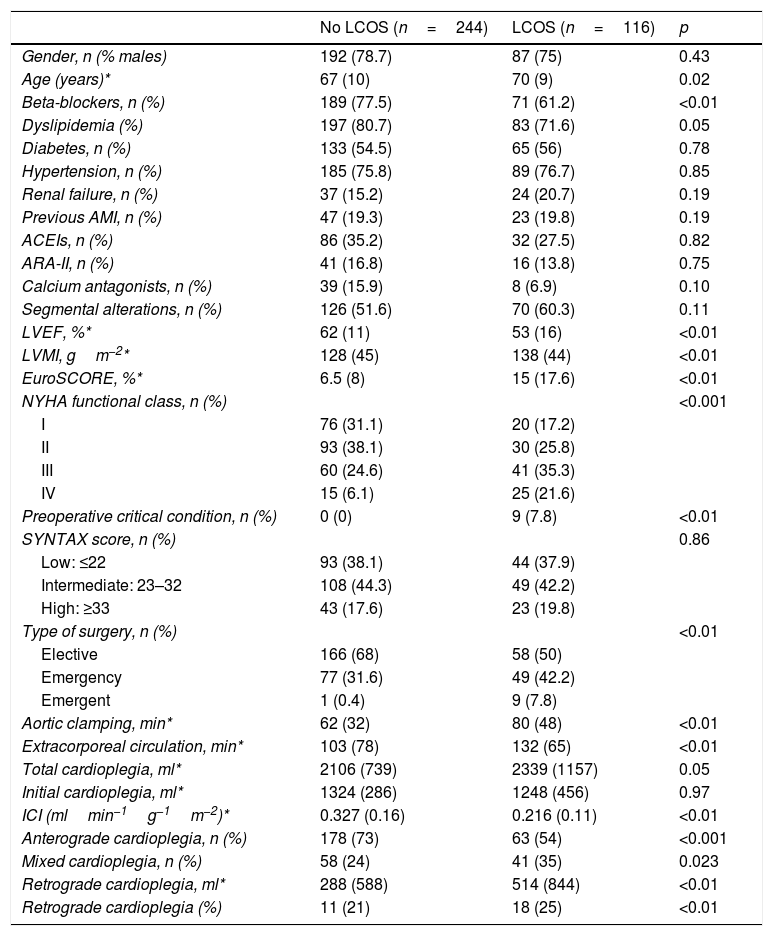
A total of 116 patients (32%) met criteria of postoperative LCOS. Table 1 describes the demographic and preoperative variables in relation to the presence of LCOS. The variables found to be significantly associated to the development of LCOS were: lesser preoperative beta-blocker use, old age, greater LVMI, higher EuroSCORE, poorer NYHA functional class, preoperative critical condition, non-elective surgery, longer ischemia or ECC times, lesser ICI, and greater proportion of retrograde cardioplegia with respect to total infused cardioplegia (all with p<0.01).
Preoperative characteristics of the patients with and without LCOS.
| No LCOS (n=244) | LCOS (n=116) | p | |
|---|---|---|---|
| Gender, n (% males) | 192 (78.7) | 87 (75) | 0.43 |
| Age (years)* | 67 (10) | 70 (9) | 0.02 |
| Beta-blockers, n (%) | 189 (77.5) | 71 (61.2) | <0.01 |
| Dyslipidemia (%) | 197 (80.7) | 83 (71.6) | 0.05 |
| Diabetes, n (%) | 133 (54.5) | 65 (56) | 0.78 |
| Hypertension, n (%) | 185 (75.8) | 89 (76.7) | 0.85 |
| Renal failure, n (%) | 37 (15.2) | 24 (20.7) | 0.19 |
| Previous AMI, n (%) | 47 (19.3) | 23 (19.8) | 0.19 |
| ACEIs, n (%) | 86 (35.2) | 32 (27.5) | 0.82 |
| ARA-II, n (%) | 41 (16.8) | 16 (13.8) | 0.75 |
| Calcium antagonists, n (%) | 39 (15.9) | 8 (6.9) | 0.10 |
| Segmental alterations, n (%) | 126 (51.6) | 70 (60.3) | 0.11 |
| LVEF, %* | 62 (11) | 53 (16) | <0.01 |
| LVMI, gm−2* | 128 (45) | 138 (44) | <0.01 |
| EuroSCORE, %* | 6.5 (8) | 15 (17.6) | <0.01 |
| NYHA functional class, n (%) | <0.001 | ||
| I | 76 (31.1) | 20 (17.2) | |
| II | 93 (38.1) | 30 (25.8) | |
| III | 60 (24.6) | 41 (35.3) | |
| IV | 15 (6.1) | 25 (21.6) | |
| Preoperative critical condition, n (%) | 0 (0) | 9 (7.8) | <0.01 |
| SYNTAX score, n (%) | 0.86 | ||
| Low: ≤22 | 93 (38.1) | 44 (37.9) | |
| Intermediate: 23–32 | 108 (44.3) | 49 (42.2) | |
| High: ≥33 | 43 (17.6) | 23 (19.8) | |
| Type of surgery, n (%) | <0.01 | ||
| Elective | 166 (68) | 58 (50) | |
| Emergency | 77 (31.6) | 49 (42.2) | |
| Emergent | 1 (0.4) | 9 (7.8) | |
| Aortic clamping, min* | 62 (32) | 80 (48) | <0.01 |
| Extracorporeal circulation, min* | 103 (78) | 132 (65) | <0.01 |
| Total cardioplegia, ml* | 2106 (739) | 2339 (1157) | 0.05 |
| Initial cardioplegia, ml* | 1324 (286) | 1248 (456) | 0.97 |
| ICI (mlmin−1g−1m−2)* | 0.327 (0.16) | 0.216 (0.11) | <0.01 |
| Anterograde cardioplegia, n (%) | 178 (73) | 63 (54) | <0.001 |
| Mixed cardioplegia, n (%) | 58 (24) | 41 (35) | 0.023 |
| Retrograde cardioplegia, ml* | 288 (588) | 514 (844) | <0.01 |
| Retrograde cardioplegia (%) | 11 (21) | 18 (25) | <0.01 |
ARA-II: type II angiotensin receptor antagonists; LVEF: left ventricular ejection fraction; AMI: acute myocardial infarction; ICI: infused cardioplegia index; ACEIs: angiotensin converting enzyme inhibitors; LVMI: left ventricular mass index; NYHA: New York Heart Association; LCOS: low cardiac output syndrome.
a Mixed cardioplegia: anterograde and retrograde.
b Values expressed as mean and percentiles 25 and 75.
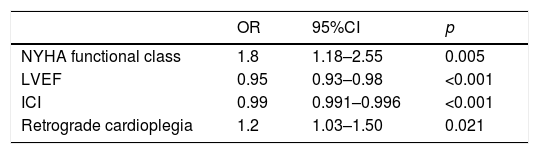
In the final logistic regression model, NYHA functional class and retrograde cardioplegia were identified as independent risk factors, while LVEF and ICI were identified as protective factors (Table 2). No first-order interactions were observed.
Regression model.
| OR | 95%CI | p | |
|---|---|---|---|
| NYHA functional class | 1.8 | 1.18–2.55 | 0.005 |
| LVEF | 0.95 | 0.93–0.98 | <0.001 |
| ICI | 0.99 | 0.991–0.996 | <0.001 |
| Retrograde cardioplegia | 1.2 | 1.03–1.50 | 0.021 |
LVEF: left ventricular ejection fraction; CI: confidence interval; ICI: infused cardioplegia index; NYHA: New York Heart Association; OR: odds ratio.
After stratification according to NYHA functional class, in patients with NYHA class I-II, a lower ICI tertile of 20.7 (18.4–28.8) versus 32 (24–42.5)ml/min−1 (100g/m2 of LV)−1 was associated to the appearance of LCOS (p<0.001). Likewise, in patients with NYHA class III-IV, a lower ICI tertile of 20.4 (14.6–25.8) versus 27.6 (21.9–33.3)ml/min−1 (100g/m2 of LV)−1 was associated to LCOS (p<0.001). On the other hand, in patients with LVEF >56%, a lower ICI tertile of 21.6 (18.3–26.4) versus 31.5 (22.9–41.9)ml/min−1 (100g/m2 of LV)−1 was associated to LCOS (p<0.001). In those patients with LVEF <56%, a lower ICI tertile of 19.5 (13.9–28.1) versus 29.4 (25.2–39.5)ml/min−1 (100g/m2 of LV)−1 was associated to postoperative LCOS (p<0.001).
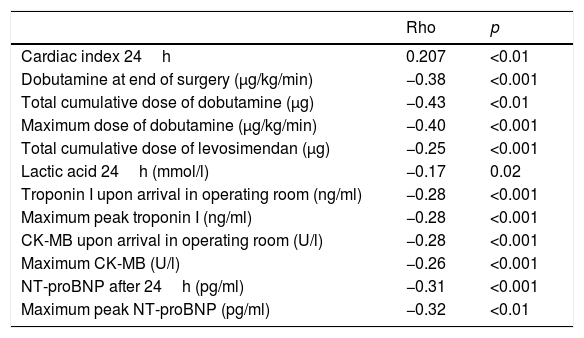
The ICI was directly correlated to CI after 24h (p<0.01) and inversely correlated to the dobutamine dose at the end of surgery (p<0.001), the total cumulative dose (p<0.001) and maximum dose of dobutamine (p<0.001), the total cumulative dose of levosimendan (p<0.001), and the lactate levels after 24h (p=0.02). The ICI was also inversely correlated to the biochemical myocardial damage markers recorded upon admission to the ICU and their postoperative maximum peaks: troponin I (p<0.001, respectively) and creatine kinase MB (p<0.001, respectively); and to NT-proBNP after 24h (p<0.001) and its postoperative peak (p<0.01) (Table 3).
Correlations between ICI and hemodynamic parameters and biochemical myocardial damage markers.
| Rho | p | |
|---|---|---|
| Cardiac index 24h | 0.207 | <0.01 |
| Dobutamine at end of surgery (μg/kg/min) | −0.38 | <0.001 |
| Total cumulative dose of dobutamine (μg) | −0.43 | <0.01 |
| Maximum dose of dobutamine (μg/kg/min) | −0.40 | <0.001 |
| Total cumulative dose of levosimendan (μg) | −0.25 | <0.001 |
| Lactic acid 24h (mmol/l) | −0.17 | 0.02 |
| Troponin I upon arrival in operating room (ng/ml) | −0.28 | <0.001 |
| Maximum peak troponin I (ng/ml) | −0.28 | <0.001 |
| CK-MB upon arrival in operating room (U/l) | −0.28 | <0.001 |
| Maximum CK-MB (U/l) | −0.26 | <0.001 |
| NT-proBNP after 24h (pg/ml) | −0.31 | <0.001 |
| Maximum peak NT-proBNP (pg/ml) | −0.32 | <0.01 |
ICI: infused cardioplegia index; CK-MB: creatine kinase-MB; NT-proBNP: N-terminal brain natriuretic peptide.
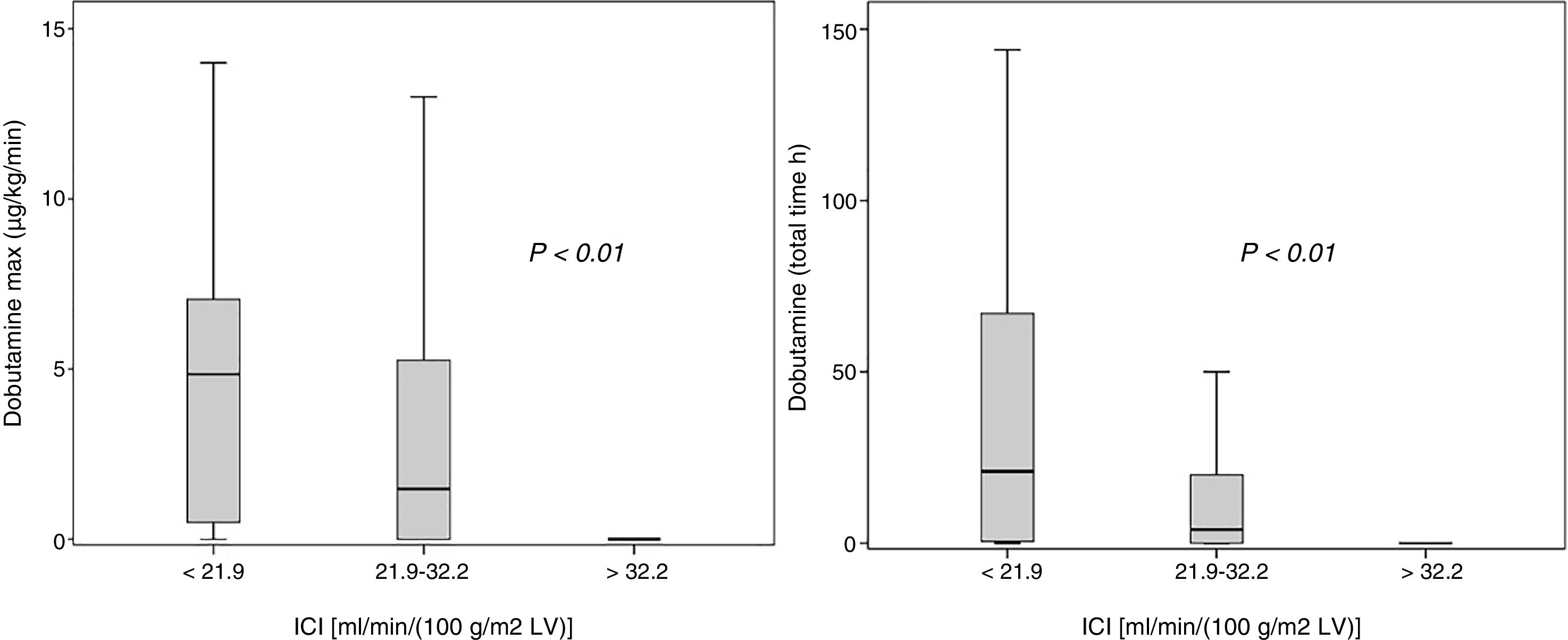
The upper ICI tertile [ml min−1 (100g/m2 of LV)−1] was associated to lower doses of dobutamine (maximum dose and administration time): maximum dose of dobutamine (μg/kg/min), ICI<21.9: 4.85 (0.5–7.1); ICI 21.9–32.2: 1.44 (0–5.3) and ICI >32: 0 (0–0); dobutamine time (hours): ICI<21.9: 21 (0.5–67); ICI 21.9–32.2: 4 (0–20) and ICI >32: 0 (0–0) (p<0.001, respectively) (Fig. 1).
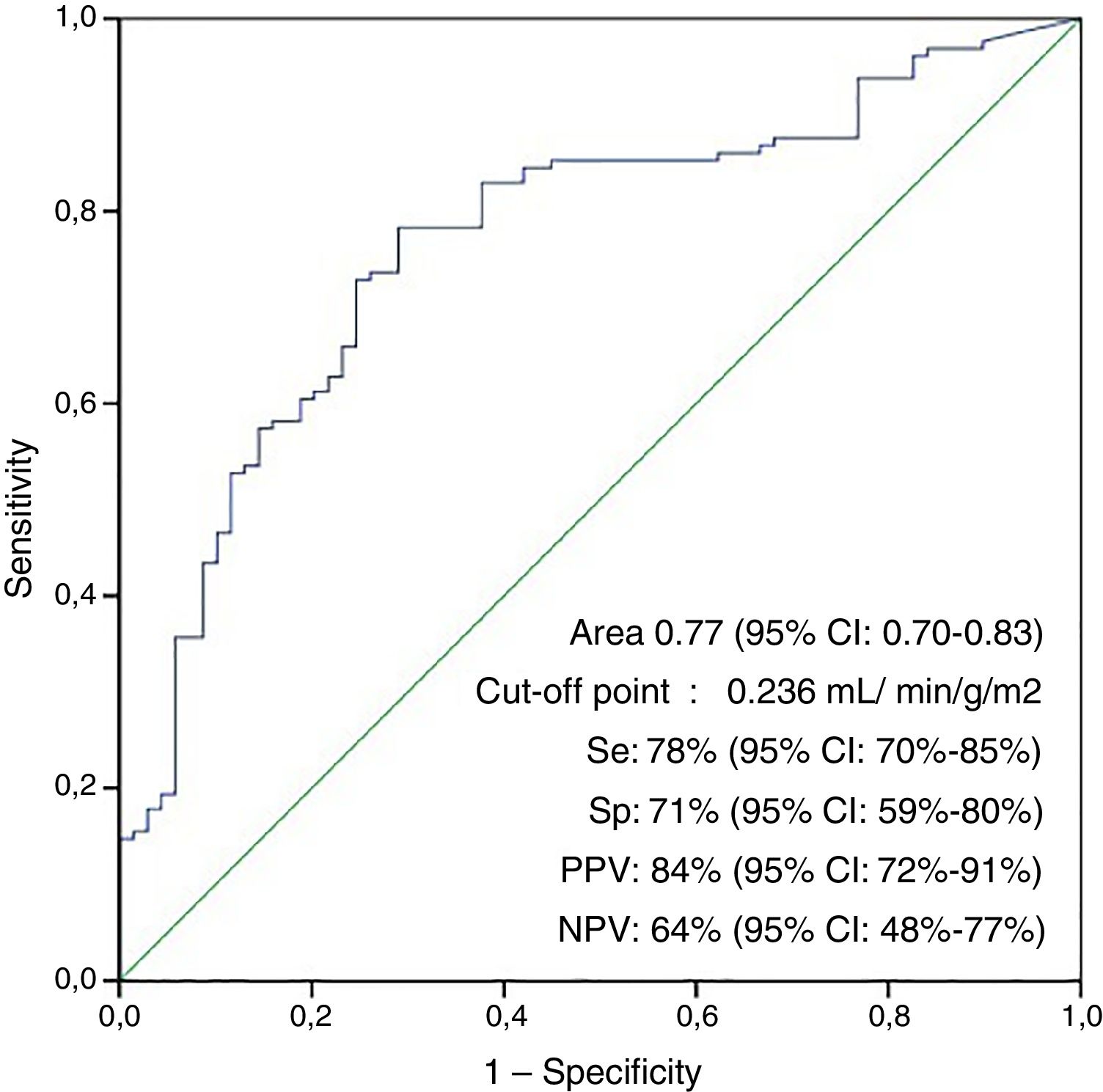
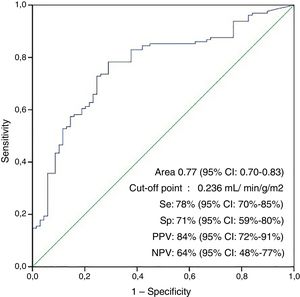
The area under the ROC curve for the non-appearance of LCOS according to the cardioplegia dose per unit of mass and ischemia time (ICI) was 0.77 (95% confidence interval [95%CI]: 0.70–0.83; p<0.001), with an optimum cut-off point of 23.6ml/min−1 (100g/m2 of LV)−1, a sensitivity of 78%, a specificity of 71%, a positive predictive value of 84%, a negative predictive value of 64%, a positive likelihood ratio of 2.7 and a negative likelihood ratio of 3.27 (Fig. 2).
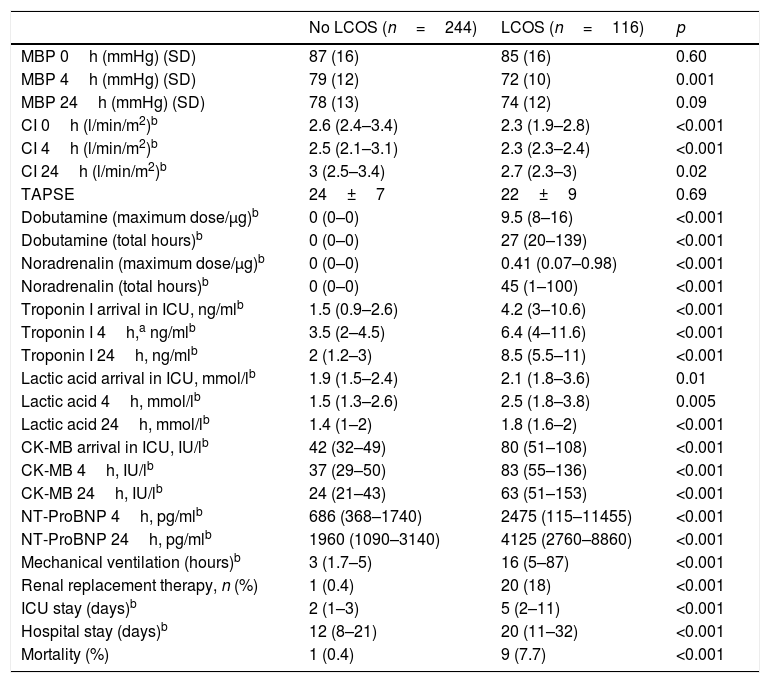
The patients with postoperative LCOS persisted with this clinical syndrome for a median of 27h (20–139), requiring a median maximum dose of dobutamine of 9.5μg/kg/min (8–16). The biochemical markers of myocardial damage and hypoperfusion were significantly higher among these individuals in both the immediate postoperative period and after 24h. Likewise, they had higher levels of NT-proBNP after 24h. Nine patients developed right ventricular dysfunction, and two of them died. They also required longer mechanical ventilation, suffered a higher incidence of acute renal failure with the need for renal replacement techniques, showed longer ICU and in-hospital stays, and presented greater mortality – with statistically significant differences in all cases (p<0.001) (Table 4).
Postoperative variables in patients with and without LCOS.
| No LCOS (n=244) | LCOS (n=116) | p | |
|---|---|---|---|
| MBP 0h (mmHg) (SD) | 87 (16) | 85 (16) | 0.60 |
| MBP 4h (mmHg) (SD) | 79 (12) | 72 (10) | 0.001 |
| MBP 24h (mmHg) (SD) | 78 (13) | 74 (12) | 0.09 |
| CI 0h (l/min/m2)b | 2.6 (2.4–3.4) | 2.3 (1.9–2.8) | <0.001 |
| CI 4h (l/min/m2)b | 2.5 (2.1–3.1) | 2.3 (2.3–2.4) | <0.001 |
| CI 24h (l/min/m2)b | 3 (2.5–3.4) | 2.7 (2.3–3) | 0.02 |
| TAPSE | 24±7 | 22±9 | 0.69 |
| Dobutamine (maximum dose/μg)b | 0 (0–0) | 9.5 (8–16) | <0.001 |
| Dobutamine (total hours)b | 0 (0–0) | 27 (20–139) | <0.001 |
| Noradrenalin (maximum dose/μg)b | 0 (0–0) | 0.41 (0.07–0.98) | <0.001 |
| Noradrenalin (total hours)b | 0 (0–0) | 45 (1–100) | <0.001 |
| Troponin I arrival in ICU, ng/mlb | 1.5 (0.9–2.6) | 4.2 (3–10.6) | <0.001 |
| Troponin I 4h,a ng/mlb | 3.5 (2–4.5) | 6.4 (4–11.6) | <0.001 |
| Troponin I 24h, ng/mlb | 2 (1.2–3) | 8.5 (5.5–11) | <0.001 |
| Lactic acid arrival in ICU, mmol/lb | 1.9 (1.5–2.4) | 2.1 (1.8–3.6) | 0.01 |
| Lactic acid 4h, mmol/lb | 1.5 (1.3–2.6) | 2.5 (1.8–3.8) | 0.005 |
| Lactic acid 24h, mmol/lb | 1.4 (1–2) | 1.8 (1.6–2) | <0.001 |
| CK-MB arrival in ICU, IU/lb | 42 (32–49) | 80 (51–108) | <0.001 |
| CK-MB 4h, IU/lb | 37 (29–50) | 83 (55–136) | <0.001 |
| CK-MB 24h, IU/lb | 24 (21–43) | 63 (51–153) | <0.001 |
| NT-ProBNP 4h, pg/mlb | 686 (368–1740) | 2475 (115–11455) | <0.001 |
| NT-ProBNP 24h, pg/mlb | 1960 (1090–3140) | 4125 (2760–8860) | <0.001 |
| Mechanical ventilation (hours)b | 3 (1.7–5) | 16 (5–87) | <0.001 |
| Renal replacement therapy, n (%) | 1 (0.4) | 20 (18) | <0.001 |
| ICU stay (days)b | 2 (1–3) | 5 (2–11) | <0.001 |
| Hospital stay (days)b | 12 (8–21) | 20 (11–32) | <0.001 |
| Mortality (%) | 1 (0.4) | 9 (7.7) | <0.001 |
CK-MB: creatine kinase-MB; SD: standard deviation; CI: cardiac index; NT-ProBNP: N-terminal brain natriuretic peptide; MBP: mean blood pressure; LCOS: low cardiac output syndrome; TAPSE: tricuspid annular plane systolic excursion; ICU: Intensive Care Unit.
The aim of the present study was to identify a parameter allowing adjustment of the cardioplegia dose according to LVMI, thereby providing a tool (the ICI) that could be useful for optimizing myocardial preservation during surgery, reducing post-ischemia myocardial damage and the development of postoperative LCOS.
Myocardial stunning is defined as post-ischemia ventricular dysfunction persisting after reperfusion in the absence of irreversible myocardial damage.10 Clinically, it is characterized by a need for postoperative inotropic drugs despite the obtainment of satisfactory surgical outcomes. In high risk patients with a low cardiac reserve, myocardial stunning is associated with more days of mechanical ventilation, an increased risk of renal failure, longer ICU and in-hospital stays, and greater mortality, as was evidenced in those patients that developed LCOS.
Postoperative LCOS is one of the most frequent complications following coronary revascularization surgery. The incidence recorded in our study (32%) is higher than that reported in other publications (16.1–13.5%).5,11,12 A number of factors could explain these differences. On one hand, the number of emergency and emergent surgeries in our population was higher than in other studies (Algarni et al.1 documented 8–10% of emergency surgeries, while Ding et al.5 recorded 8.3% of emergent surgeries in the group that presented LCOS). On the other hand, in our study surgeries without ECC were not performed. Lastly, the definition of LCOS used by other authors is more restrictive, with the exclusion of patients who although needing inotropic drugs did not exceed a certain dose or treatment time threshold.5,11,12 These inclusion criteria could limit sensitivity in detecting less severe forms of myocardial dysfunction in the immediate postoperative period. However, as contemplated by the hemodynamic monitoring guidelines of the SEMICYUC,13 “the routine and systematic monitoring of cardiac output in hemodynamically unstable patients is not recommended”. On the other hand, the clinical criteria suffice to adequately classify patients with postoperative LCOS, as has been recently evidenced by the ESBAGA study, where the clinically classified patients showed a postoperative course with a clear increase in morbidity-mortality very similar to that seen in the group diagnosed on the basis of hemodynamic monitoring parameters.14
Although consensus is lacking on the causes underlying the postoperative elevation of biochemical myocardial damage markers, three important clinical trials have identified suboptimal myocardial protection as the main factor responsible for ischemia/reperfusion damage.15 In our study, those patients with a lower ICI showed higher concentrations of these markers (troponins, creatine kinase MB and NT-proBNP) and of lactate (as a surrogate indicator of hypoperfusion), requiring more inotropic support in the immediate postoperative period. Moreover, the following were identified as risk factors for the development of postoperative LCOS: older patient age, greater LVMI, lower postoperative LVEF, poorer NYHA functional class, higher EuroSCORE, emergency surgery, prolonged ECC time, and cardioplegia volume and administration route – all this being consistent with the findings of previous studies.5,11,12,16
As has been mentioned, a number of factors have been shown to contribute to the etiopathogenesis of post-ischemia myocardial dysfunction in heart surgery patients. Based on these findings, an increase in LVMI implies greater morbidity-mortality among patients undergoing heart surgery17; this is because increased LVMI implies a higher perioperative myocardial ischemia risk secondary to an imbalance between myocardial oxygen demand and supply. Left ventricular hypertrophy is associated to coronary vascular structural alterations, with increases resistances, flow alterations and an increased left ventricular end-diastolic pressure – with the consequent decrease in subendocardial perfusion. Likewise, it is associated to an increase in oxygen demand and greater wall stress. During surgery, patients with severe ventricular hypertrophy are at an increased risk of suboptimal myocardial preservation. The combination of these factors entails a poorer prognosis.18 On the other hand, LVMI has been related to an increase in postoperative arrhythmias19 and to longer stay,17 and it has even been identified as an independent predictor of patient mortality after 30 days, exhibiting a practically linear correlation.18
Given the importance of myocardial preservation, a number of cardioprotection strategies during surgery have been developed, with the identification of cardioplegia as a key element. Among the different forms of cardioplegia, the gold standard in the last 30 years has been a depolarizing hyperpotassemic solution that induces rapid and easily reversible cardiac arrest – though it is not without inconveniences. The increase in potassium concentration may be associated with different degrees of vasoconstriction and vasospasm, giving rise to an irregular distribution of cardioplegia20 and thus to a decrease in myocardial protection.
Furthermore, extracellular potassium concentrations are characterized by a narrow therapeutic margin, where minor variations in such concentrations can give rise to persistent ionic currents resulting in an increase in intracellular calcium and sodium.21,22 In an attempt to restore the ionic balance, transmembrane pumps are activated, with a consequent increase in energy consumption that in turn contributes to myocardial stunning.23 This requires keeping the extracellular potassium concentrations stable through an adequate supply adjusted to a myocardial mass in the presence of coronary arterial disease.
Despite the many studies that explore the different cardioplegia types and administration modes, to the best of our knowledge, no strategies have been developed to allow cardioplegia dose adjustment to LVMI. In a study carried out in the mid-1980s, Matsuda et al.24 evaluated the initial optimum cardioplegia doses in patients with severe ventricular hypertrophy subjected to aortic valve replacement surgery. However, we found no posterior studies validating the conclusions drawn or suggesting cardioprotection strategies based on the results of the mentioned authors. In our study we used ICI to relate LVMI and the amount of cardioplegia. The main conclusion was that there is a relationship among the ischemia time to which a given myocardial mass is exposed, the cardioplegia dose used for preservation, and the development of postoperative LCOS. Specifically, we found ICI >23.6ml/min−1 (100g/m2 of LV)−1 to be associated with a lesser incidence of LCOS, and to be inversely correlated to the postoperative inotropic drug requirements. This is probably because these doses afford greater stability of the ionic concentrations.
Lastly, our results indicate that intermittent retrograde cardioplegia is associated to lesser cardioprotection. In patients with coronary disease, the retrograde administration of cardioplegia is better able to cover the territory of the anterior descending coronary artery than the anterograde route,25 since the ventricular territory can receive retrograde flow through the Thebesian collaterals.26 However, the exclusive administration of retrograde cardioplegia may result in insufficient cardioprotection due to scant perfusion of the right ventricle, posterior wall and interventricular septum, unless it is accompanied by anterograde administration through the coronary ostium.27 Nevertheless, a number of studies have shown that high-flow retrograde cardioplegia can improve the right ventricle and interventricular septum preservation outcomes.25 The findings of our study show that the greater the proportion of cardioplegia administered via the retrograde route, the greater the incidence of postoperative LCOS. This could be because the volume of cardioplegia via the retrograde route or the combination with intermittent cardioplegia proved insufficient.
The present study has limitations. In effect, it is a single-center observational study exclusively involving intermittent hyperpotassemic cardioplegia, with no comparison against other methods of administration and with no predefined method for administering it (route, volume and subsequent doses) – the decision in this respect being left to surgeon criterion. However, each milliliter of cardioplegia administered per gram of myocardium and minute of ischemia lessened the probability of LCOS by a little under 1%.
In sum, two factors have been shown to be crucial in the development of postoperative myocardial stunning: the aortic clamping time and an adequate individualized myocardial preservation strategy. Our study shows that adjusting the cardioplegia dose according to the ischemia time and LVMI may represent a useful tool for improving the degree of myocardial protection during coronary revascularization surgery, thereby minimizing the deleterious effects of suboptimal cardioprotection.
A possible application of the findings of this study in the management of hyperpotassemic cardioplegia could be adjustment of the initial cardioplegia dose according to LVMI. In patients with mild hypertrophy (LVMI<130g/m2), we would administer 1.2l, while in those with moderate or severe hypertrophy we would administer at least 1.5l. With respect to the maintenance dose, it would be important to observe administration intervals of between 20 and 25min, depending on the surgical procedure involved. This maintenance dose could be calculated by multiplying 25ml by the minutes of ischemia time (20 or 25 maximum), multiplied by a myocardial mass correction factor (LVMI/100).
Further studies would be needed to confirm the usefulness of this strategy in clinical practice. It also would be interesting to evaluate ICI with another cardioplegia solution.
Contribution of the authorsJJJR, CLJ and JLIS designed the project and conducted the statistical analysis. JLA specifically performed the echocardiographic studies, while GYB carried out the hemodynamic studies and calculated the SYNTAX score. RAP and RMS performed the surgery and together with the rest of the authors implemented the research protocol, discussed the data and contributed to final drafting of the manuscript.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Jiménez Rivera JJ, Llanos Jorge C, Iribarren Sarrías JL, Brouard Martín M, Lacalzada Almeida J, Pérez Vela JL, et al. Índice de cardioplejía infundida. Med Intensiva. 2019;43:337–345.