Mechanically ventilated patients had higher exhaled breath condensate (EBC) acidity, representing a marker of acute lung injury. EBC pH became more acidic during clinical deterioration. Independent of collection/gas standardization methods, the EBC pH remained constant throughout the study, and hence it did not seem to be a useful biomarker for predicting successful weaning, ventilator associated pneumonia or death.
Los pacientes con asistencia respiratoria mecánica pueden desarrollar mayor acidificación del Condensado del aire exhalado (CAE). Este dato fue señalado como biomarcador de injuria pulmonar aguda y el pH del CAE disminuía en relación al deterioro clínico del paciente. Nuestra conclusión fue el pH del CAE se mantuvo constante a lo largo de este estudio; y por tanto no pudo anticipar la desconexión exitosa del respirador, o la neumonía asociada al respirador o aun la muerte. Al no medir otro marcador en el CAE y no tener grupo control obligan a nuevos estudios.
Collection of exhaled breath condensate (EBC) is a promising method for obtaining samples from the lungs.1 Its non-invasive nature makes EBC collection attractive to clinicians. The pH is one of the most studied variable of EBC.2,3 However, its measurement yields greatly variable results depending on the gas standardization procedure. From intubated subjects without lung disease, the mean pH of deaerated samples is likewise 7.7 with no difference from matched oral collections.4 In another study, the pH of EBC samples from intubated subjects undergoing cardiothoracic surgery was reported to be between 5 and 7.5 Mechanically ventilated patients had higher EBC acidity and it was suggested to represent a marker of acute lung injury caused by or accompanied by pulmonary inflammation.6 Walsh and colleagues found that the EBC pH became more acidic during clinical deterioration and normalized with recovery.7
A low EBC pH could be a key clue to be cautious in mechanically ventilated patients; but because in most studies assessments of EBC pH was obtained with coolant chamber and deareated with argon, the procedure was not sufficiently simple to be extensively used. Spontaneous EBC in the expiratory trap was readily available. To our knowledge there were no studies comparing spontaneous EBC pH in the expiratory trap of a ventilator with EBC pH collected with a coolant chamber and standardized with argon in acute critically ill patients.
The aims of this trial were: (1) to compare spontaneous EBC pH with or without argon deaeration with EBC pH obtained by coolant chamber collection of EBC pH with or without argon deaeration. (2) To assess the prognostic value of EBC pH for ventilator-associated pneumonia (VAP) and mortality.
Materials and methodsPatientsThis prospective clinical trial was conducted in a 9-bed intensive care unit located in a teaching hospital. Critically ill adults (>18 yrs of age) who required endotracheal intubation and mechanical ventilation (MV) for at least 48h due to non-pulmonary direct cause and for whom their close acquaintances gave the informed consent were eligible for enrolment. Reasons for exclusion were: pregnancy, denied surrogate decision-makers to give informed consent. Patients who required MV due to pneumonia, severe respiratory infection, massive hemoptysis, acute severe asthma, bronchiectasis, COPD exacerbation, or acute lung injury were excluded. Finally, patients who expected to be ventilated for less than 2 days were also excluded. The Hospital institutional review board approved the study. No commercial entities providing equipment or devices had a role in any aspect of this study.
MethodsEach patient was enrolled after the close acquaintances gave the informed consent. Then, a sample of at least 2ml spontaneously EBC was obtained from the trap of the expiratory arm of ventilation circuit. From this original sample one ml was taken in a syringe and rapidly sent to the lab where pH was measured with a blood gas analyzer, ABL5, Radiometer Copenhagen after routine calibration. The rest of the EBC was collected in Eppendorf tube and pH was measured after deaeration of the condensate with argon (350ml/min) for 10min as a gas standardization procedure as previously described by Hunt and colleagues.2 The expiratory trap was not emptied during the last 8h before collecting and the heated humidifier of the ventilator remained constant throughout the study. For obtaining non-spontaneous condensation, the expiratory arm of the ventilator circuit was passed through the condensing chamber and inside it, the expiratory arm was surrounded by coolant at −3°C. The ad-hoc assembled, condensing chamber was a Styrofoam box with two holes, at each side and across them we passed the expiratory arm of the ventilator. It was not commercially available. At least 2ml of exhaled airway vapor condensate was obtained during 10min for all the patients. Then, similarly to the spontaneous EBC, the sample was divided into two equal parts; at least one ml for directly pH measurement and the rest of the sample for gas standardization with argon before stable pH measurement. The collection procedure, as it was describe above, was repeated every 72h in each patient until the development of ventilator associated pneumonia (VAP) as defined by the American Thoracic Society and the Infectious Disease Society of America,8 or successful weaning or death. Ventilatory settings remained constant during both collections. The ICU staff did not use EBC information for making clinical or therapeutic decisions.
Statistical analysisComparisons between samples obtained from different methods were performed by Tukey's Multiple Comparison Test. Normal distribution was confirmed by D’Agostino and Pearson omnibus normality test. Parametric tests were used for EBC because data were normally distributed.
Paired t-tests were applied for comparison of EBC pH at baseline and after VAP diagnosis, just before successful weaning or before death. Unpaired t-tests were applied for comparison of EBC pH between dead and survivor patients.
Bland-Altman plot were used to assess the relationship between the four collection methods.9 Data were expressed as mean±SD, and significance defined as p<0.05. The Institutional Review Board and the ICU chief received every 4 months a follow-up information from the authors in order to decide the continuity of the study.
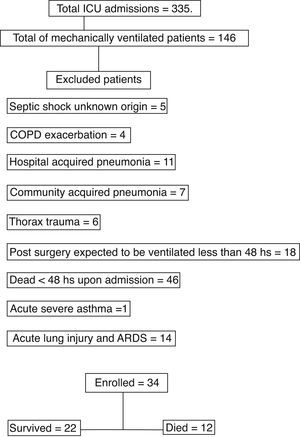
ResultsFrom August 2007 until April 2008, 355 patients were admitted of whom 146 patients were mechanically ventilated; and only 34 complied with the inclusion criteria (Fig. 1). The baseline characteristics of the included patients were shown in Table 1. We collected and registered 79 samples by each one of the four procedures and summary results were shown in Table 2. The first sample was taken at a mean time of 1.7±1.51 days of MV. The mean duration of MV until the last sample was 3.97±2.93 days. Twenty-two patients were successfully extubated and discharge while 12 patients died in the ICU. We obtained only one sample of EBC pH (with the four procedures) in 15 patients due to rapid successful weaning in 12 patients and 3 deaths within 48h. In the remaining 19 patients the mean time elapsed between the first and the last measurement of EBC pH was 5.45±2.50 days of MV and the mean change in EBC pH was −0.007±0.05. In patients who died, the baseline EBC pH by coolant chamber and argon was 6.70±0.43 and the last EBC pH was 6.76±0.38; p=0.77. This last measurement was done 4.88 days before deaths (range 1-10). The survival group had a mean baseline EBC pH 6.66±0.44 and before successful weaning a mean=6.66±0.36; p=0.52. There was no correlation between duration of MV and change in EBC pH (r=−0.12) We did not find any correlation between initial C Reactive Protein (CRP) and baseline EBC pH. Also, APACHE score, PaO2/FiO2, Tidal Volume, PEEP, arterial pH, PaCO2 or HCO3− did not show any correlation with EBC pH at its collection time.
Baseline demographics, clinical characteristics and co-morbidities of 34 mechanically ventilated patients.
| Mean | SD | |
| Age, years | 54.85 | 19.86 |
| Male sex, n (%) | 26 (76.5%) | |
| APACHE II score | 23.58 | 14.70 |
| PaO2/FiO2ratio | 240.00 | 98.29 |
| PaCO2mm Hg | 36.35 | 9.15 |
| Arterial pH | 7.37 | 0.08 |
| Respiratory rate (rpm) | 14.0 | 2.1 |
| Tidal volume (ml/kg PBW) | 7.98 | 0.92 |
| PEEP cm H2O | 3.47 | 2.72 |
| CRP mg/dl | 10.22 | 10.02 |
| Primary cause for MV | ||
| Stroke | 18 | |
| Successful CPR | 3 | |
| Post surgery | 5 | |
| Hemorrhagic shock | 2 | |
| Myasthenia gravis | 1 | |
| Meningitis | 1 | |
| Traumatism | 4 | |
| Comorbidities | ||
| Diabetes, n (%) | 7 | |
| Malignancy | 1 | |
| Smoker/ex-smoker | 18 | |
Abbreviations: APACHE, acute physiology and chronic health evaluation; CRP, “C” reactive protein; CPR, cardiopulmonary resuscitation; PaO2/FIO2, ratio of the partial pressure of arterial oxygen and the fraction of the inspired oxygen; PEEP, positive end-expiratory pressure; stroke, hemorrhagic or ischemic neurologic events; PBW, predicted body weight.
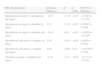
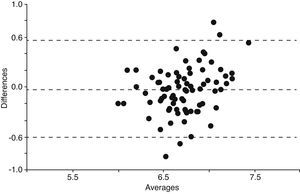
When we compared all the 79 samples of EBC pH measurement done by each one of the four procedures, the means were significantly different with the exception of the comparison between Spontaneous collected and then deaerated with argon EBC pH versus EBC pH from coolant chamber deaerated with argon (mean difference 0.041; q=2.026; 95% CI of difference −0.034 to 0.12; p=NS). These results were shown in Table 3. In order to confirm if EBC pH obtained by coolant chamber and deaerated with argon could be extrapolated or replaced by spontaneous collection of EBC pH deaerated with argon, we applied the Bland and Altman method and it demonstrated that both procedures had significant deviation from the mean (Fig. 2).
Tukey's multiple comparison test.
| EBC pH procedures | pH mean difference | Q | p-Value | 95% CI of difference |
| Spontaneous no argon vs spontaneous and argon | −0.23 | 11.42 | <0.05 | −0.3084 to −0.1579 |
| Spontaneous no argon vs chamber no argon | 0.10 | 5.124 | <0.05 | 0.02943 to 0.1799 |
| Spontaneous no argon vs chamber and argon | −0.19 | 9.39 | <0.05 | −0.2670 to −0.1166 |
| Spontaneous and argon vs chamber no argon | 0.34 | 16.54 | <0.05 | 0.2626 to 0.4130 |
| Spontaneous and argon vs chamber and argon | 0.04 | 2.026 | 0.13 | −0.03385 to 0.1166 |
| Chamber no argon vs chamber and argon | −0.30 | 14.51 | <0.05 | −0.3717 to −0.2212 |
Analysis according to Bland and Altman method to test reproducibility for EBC pH by spontaneous collection deaerated with argon vs EBC pH by coolant chamber deaerated with argon. Averages and differences of both EBC pH methods: spontaneous collected and coolant chamber from 79 samples. Horizontal lines are mean difference±2 standard deviation. These methods could not be extrapolated.9
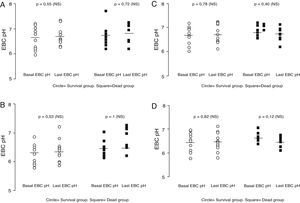
There were no differences between baseline and last mean EBC pH in patients who developed VAP, neither between baseline and final EBC pH in patients who died nor between baseline and weaning from MV (Fig. 3a–d). This lack of difference occurred with the four studied procedures. The mean change from baseline EBC pH in subjects who died was not statistically significant (mean difference=0.01±0.69 for coolant chamber with argon). Four patients died due to VAP and the mean change from baseline EBC pH was also not significant (mean=0.03±0.4). None of the four procedures showed a significant change in EBC pH when VAP occurred; neither before successful weaning nor before death.
Open circles=11 survived patients with more than one EBC pH measurement. Black square=9 patients who died and had at least 2 EBC pH measurements. Basal EBC pH was obtained at least 24h after initiating mechanical ventilation and last EBC pH before successful weaning in survival group (mean time elapsed=4.36±2.62 days). In patients who died the mean time elapsed between first and last sample was 4.88±3.48 days. Last EBC pH was obtained before developing VAP in 4 patients. Paired t test (NS)=not statistically significant within groups and unpaired t test between groups; p=not significant. All the samples showed in these four figures were obtained at the same time point. (a) Comparison between baseline and last EBC pH obtained by coolant chamber and measured after argon deaeration in alive versus dead patients. Mean coefficient of variation between basal and last EBC pH in alives=1.3% and for dead group=1.63%. Fine horizontal lines represented mean. (b) EBC pH obtained by coolant chamber and immediately measured without argon standardization. Paired t test (NS)=not statistically significant within groups and unpaired t test between groups; p=not significant (p not shown). Mean coefficient of variation between basal and last EBC pH in alives=1.12% and for dead group=−0.10%. Fine horizontal lines represented mean. (c) EBC pH spontaneously collected in the trap of ventilator expiratory arm and dearated with argon. Paired t test (NS)=not statistically significant within groups and unpaired t test between groups; p=not significant (p not shown). Mean coefficient of variation between basal and last EBC pH in alives=0.29% and for dead group=−1.16%. Fine horizontal lines represented mean. (d) EBC pH spontaneously collected in the trap of the ventilator expiratory arm without argon standardization. Paired t test (NS)=not statistically significant within groups and unpaired t test between groups; p=not significant (p not shown). Mean coefficient of variation between basal and last EBC pH in alives=−0.51% and for dead group=−2.02%. Fine horizontal lines represented mean.
The Institutional review board recommended to stop the trial for futility after 34 patients were enrolled when a planned interim analysis showed differences between the 4 methods and particularly EBC pH obtained by spontaneous collection seemed not to be able to replace EBC pH by coolant chamber and argon. But importantly, none of the 4 procedures showed a predictive value for VAP, duration of MV or mortality. Furthermore, with a sample size of 34 patients (due to stop decision of IRB), the power to detect a minimum significant difference was 90, alpha: 0.05 and beta: 0.1 for a minimum difference of 0.24 to be detected and a standard deviation of population of 0.3.
DiscussionAt least to our knowledge, simultaneous measurement of pH in the spontaneous EBC collected in the trap of expiratory arm of the ventilator, and EBC pH obtained by coolant chamber around the expiratory arm, until death, VAP or extubation, have not been published in initially non-pulmonary critically ill patients.
The major disappointing finding was that EBC pH measurements did not show increasing acidity throughout the study, neither in patients who died nor in patients with VAP, using our ad-hoc designed coolant chamber or spontaneous EBC pH. This finding was independent of the condensate and deaeration method and the probability of a type II error was not possible because with 34 patients the power of this sample size was 90 and β was 0.1. In fact, the coefficient of variation between first and last measurements were lower than prior publication,4 independent of the EBC procedure (Fig. 3a–d). That means, EBC pH remained constant throughout the study in each one of the 4 procedures The other disappointing finding (although expected) was the significant differences between spontaneous EBC pH and EBC from coolant chamber (Table 2). Our attempt to simplify the method by obviating coolant chamber and argon was frustrated by the significant lower pH in the Spontaneous EBC without argon deaeration (mean difference=−0.19; 95% CI=−0.267 to −0.116). Spontaneous EBC pH and collection by coolant chamber EBC pH, both with argon; was the only one comparison that resulted in no differences by Tukey's Multiple Comparison Test (Table 2). However, both procedures could not be extrapolated as it was shown by Bland and Altman method (Fig. 2). Then, we confirmed that despite the controversy regarding the role of gas standardization, this procedure added a difference to EBC pH measurements. Argon deaeration was suggested as a method of improving the reproducibility of pH readings1,2 as, in theory, inert gas removes all volatile components of EBC allowing the measurement of nonvolatile acidity. Gas standardization or deaeration of EBC prior to measurement of pH provided different information than measurement of pH immediately after collection. The significant increase in EBC pH that we found after applying the deaeration procedure with argon supported the fact that it was technically irreproachable (Table 3). Although, both methodologies may have advantages, in our study EBC pH did not appear to be a useful biomarker for mechanically ventilated patients independent of the applied procedure. Furthermore, the coefficients of variation between basal EBC pH and last EBC pH were even lower than 4.5% reported by Vaughan et al.4 despite such opposite circumstances as alive and dead groups in our study (Fig. 3a–d).
In the meantime the current trial was running, and Kullmann and colleagues10 published a new method using CO2 gas standardization that had an excellent reproducibility and could detect even very small changes in EBC pH. Our results argued against the concept that EBC pH measurement was a robust variable with a great future4,7 unless, standardized by CO2 method could revert these observations in critically-ill patients. Our results did not show instability or lack of reproducibility; by contrast, EBC pH remained constant despite different outcomes. Furthermore, recent studies also shed some doubts on the usefulness of EBC pH as a biomarker in asthma11 and COPD.12
Could systemic inflammation induce airway acidity in this group of ventilated patients? The increased mean CRP supported the presence of systemic inflammation, but by itself could not explain the low EBC pH along the study and we did not find a correlation between EBC pH and CRP. Gessner and colleagues published that EBC pH measured directly in 35 ventilated patients with acute lung injury was 5.85 and was measured after argon dearation was 5.98. These authors found a correlation between acidity and clinical scores of lung injury.6 But interestingly, they did not find any correlation with systemic markers of inflammation such as serum IL-6 and serum IL-8.6
Other studies in MV patients have detected significant correlations between the EBC NO2/Vt ratio and the Lung Injury Score and PaO2/FIO2 ratio, suggesting that increases in the EBC NO2/Vt ratio may be explained by greater mechanical alveolar stress.13
Nitrite from EBC has also been reported to correlate with both the tidal volume (adapted to ideal body weight) and to the extent of lung injury, and thus it may be indicative of mechanical stress in ventilated lungs.14 Even when we could not refuse the alveolar mechanical stress as source of acidity, hypoventilation strategy was a common practice in the ICU (mean tidal volume 7.98ml/kg [predicted body weight] and respiratory rate of 14/min; Table 1) and the mean PEEP was also low (3.47cm H2O; Table 1). The low PaO2/FiO2 at baseline (240) despite non-pulmonary disease could be due to the proximity to intubation procedure in our otherwise critically-ill patients. Then, recovered to a mean PaO2/FiO2 of 334 (data not shown).
There was a lack of explanation in the literature of the wide variability of EBC pH even in healthy subjects with pH values as low as 7.2415 and as high as 8.26.16 In a recent publication with similar inclusion criteria to our study, EBC samples were collected from ten critically-ill patients weaning from longer MV without lung disease and the mean EBC deaerated pH was 7.49 and 8.07 in normal controls.13 Effros and colleagues found that EBC pH averaged 7.24 in normal subjects, and 6.67 in stable COPD subjects with 40% predicted FEV1.15 Furthermore, only one study reported intra-day and intra-week coefficients of variation of EBC pH measurements in healthy subjects to be 3.5% and 4.5% respectively.4 Then, it could be suspected that a yet undisclosed factor might induce the substantially wide range of pH values between different studies. This issue rendered it very difficult to find a cutoff point or the minimal clinically significant change in EBC pH values.
Our study had many limitations. Other exhaled biomarkers as comparators have not been measured. The length of follow-up could not be enough to find a difference in EBC pH. Autopsies were not done in any patient. We did not have a normal control group; however, in ten stable asthmatic subjects under ICS treatment (FEV1 89±23% predicted), we had measured a mean EBC pH of 7.39 (range 7.14-7.7)17 with the same procedure as Hunt and colleagues.2 Additionally, it was published that patients on ICS have higher EBC pH values that do not differ from healthy controls.3
We concluded that, independent of collection methods; the EBC pH remained constant throughout the study and hence, it did not seem to be a useful biomarker for predicting successful weaning, VAP or death in these mechanically ventilated patients. However, volatile and non-volatile acids, different buffer systems, technical issues such as the device for collecting EBC samples and gas standardization; all of them pointed out the necessity of more studies on clinical application of EBC pH in mechanically ventilated patients.
Author's contributionLJN as the firs author has been involved in the conception, delineation of hypothesis, design of the trial, acquisition, analysis and interpretation of data and he has written the draft article.
RQ and DHB have been involved in delineation of hypothesis, trial design, acquisition of data, interpretation of information and in the revision of the article prior to submission.
MD, MR, RN and GG have been involved in trial design, acquisition of data, and revision prior to submission.
We are indebted to the intensive care unit nurses without whom this work would not have been possible and to Dr Juan Rossi head of central hospital lab as well as all the lab personnel.
Conflict of interestThe authors declare no conflict of interest.