Airway isolation by endotracheal intubation or tracheostomy impedes or even interrupts speech and swallowing. Pharyngeal and laryngeal impairment frequently occurs after extubation or de-cannulation, common consequences being dysphonia, dysphagia and the aspiration of oral secretions, food, or fluids. Aspiration often leads to pneumonia and eventually death. Although the literature reports a high frequency of dysphagia following intubation and tracheostomy, the data vary considerably, and the true incidence of oropharyngeal dysphagia following artificial airway isolation remains to be established.
We conducted a systematic review of the available evidence, in order to assess oropharyngeal dysphagia physiology, diagnosis and treatment.
El aislamiento de la vía aérea mediante intubación translaríngea o traqueotomía, dificulta, cuando no interrumpe las funciones faríngeas y laríngeas de fonación y deglución; tras retirar el tubo translaríngeo o cánula de traqueotomía, dichas funciones no se recuperan de forma inmediata, observándose con frecuencia disfonía, disfagia y aspiraciones traqueobronquiales. La aspiración de secreciones orofaríngeas, de alimentos, así como de contenido gástrico, pueden dar lugar a infecciones respiratorias nosocomiales, en pacientes frágiles o convalecientes de afecciones severas con un aumento significativo en su morbimortalidad.
La incidencia de incompetencia faríngea y laríngea, en pacientes que requieren uso de una vía aérea artificial no está bien determinada. Los estudios realizados hasta el momento sugieren una alta proporción de dichas alteraciones, tanto en pacientes recientemente extubados, como en pacientes traqueotomizados. El conocimiento de los mecanismos fisiopatológicos que condicionan la disfagia en dichos pacientes, junto con las alternativas diagnósticas y terapéuticas centrarán la actual revisión.
Dysphagia is defined as patient perceived difficulty in swallowing solid and liquid foods. It can manifest in variable forms—from a delay in food bolus formation or movement to pathological displacement with passage of the bolus into the airway.
The consequences of dysphagia can be serious, and may involve important mortality when dehydration, denutrition and/or tracheobronchial aspiration are produced.1–3
The penetration and aspiration of oropharyngeal secretions, food and gastric contents in the upper bronchial tree give rise to an inflammatory response that stimulates mucosal secretion. This in turn gives rise to bronchorrhea and to a series of respiratory alterations ranging from conditions characterized by a good prognosis, such as atelectasis or chemical pneumonitis (Mendelson's syndrome), to severe respiratory infections or acute respiratory distress syndrome (ARDS) in fragile patients or individuals who are recovering from serious conditions.1–5
The risk of bronchoaspiration in patients with oropharyngeal dysphagia is 11 times greater than in patients without swallowing difficulties.5,6
The incidence of pharyngeal and laryngeal incompetence in patients with disorders of neuromuscular origin is very high, with variations in its frequency of manifestation, severity and treatment options. Series of patients with disorders as common as cerebrovascular stroke have been reported in which 94% of the subjects were seen to suffer some degree of dysphagia—in an important number of cases with a good response to conventional rehabilitation therapy. The persistence of dysphagia in these patients was associated to a poorer prognosis, a prolongation of hospital stay, and to increased admission to homes for the elderly, worsening of quality of life, and dependency for activities of daily living.7–9
In patients with neuromuscular degenerative processes mostly in the form of amyotrophic lateral sclerosis or muscle dystrophy, the dysphagia associated to these processes is not reversible; as a result, such individuals require an alternative solution in the form of a gastrostomy, for example.
The incidence of pharyngeal and laryngeal incompetence (typically in the intra-glottic/subglottic region) (G/SG-I) in patients requiring an artificial airway has not been clearly established. Some studies suggest an incidence of over 40% in recently extubated patients, and of between 50 and 84% in patients requiring tracheotomy.4,9–13 A diagnosis of dysphagia secondary to artificial airway isolation (in studies conducted in tracheotomized patients) has been associated to a prolongation of admission to the Intensive Care Unit (ICU), and to a longer time to weaning from mechanical ventilation and decannulation.14 The use of simple screening methods or protocolization of the study of such disorders has been shown to be useful, allowing the identification of patients at high risk, with a view to adopting appropriate management measures.15–18
On the other hand, in the ICU, where a large proportion of patients require an artificial airway, pneumonia accounts for up to 25% of all nosocomial infectious complications. In turn, 90% of these pneumonias are associated to mechanical ventilation (MV), and this proportion moreover increases with the duration of MV. The mortality attributable to nosocomial respiratory overinfection in such Units has been estimated to reach 33–50%, depending on the series.19
Independently of its origin, oropharyngeal dysphagia increases patient morbidity and mortality, the mean duration of stay, and the economical, intangible and hospital opportunity costs.5,14
Classification of dysphagiaDepending on the anatomical level at which dysphagia originates, a distinction is made of the followingOropharyngeal dysphagiaOropharyngeal dysphagia is defined as food bolus penetration difficulty from the oropharynx to the cervical esophagus due to dysfunction in the oropharyngeal phase of swallowing, secondary to laryngeal problems or alterations in upper esophageal sphincter function. Tumors and neurological or muscular disorders are the most common causes of this type of dysphagia (Table 1), and the appearance of dysphagia immediately follows the act of swallowing.1
Causes of oropharyngeal dysphagia.
| - Structural causes: |
| ∘ Oropharyngeal strictures (stenosis): neoplastic/foreign body/inflammatory |
| ∘ Esophageal stenosis: esophageal membranes/Zenker's diverticulum/cicatricial |
| ∘ Mediastinal and neck masses |
| - Disorders of the central nervous system: |
| ∘ Cerebrovascular events (stroke) |
| ∘ Parkinson/Wilson/Alzheimer/Huntington chorea |
| ∘ Head injuries |
| ∘ Brainstem tumors |
| ∘ Amyotrophic lateral sclerosis/multiple sclerosis |
| ∘ Cerebral paralysis |
| ∘ Bulbar poliomyelitis–postpoliomyelitis syndrome |
| ∘ Guillain-Barré syndrome |
| ∘ Tardive dyskinesia |
| ∘ Familial dysautonomy (Riley-Day syndrome) |
| - Motor end plate diseases: |
| ∘ Myasthenia gravis, Eaton-Lambert syndrome, botulism |
| ∘ Toxic: aminoglycosides, phenytoin, penicillamine, procainamide, propanolol, organophosphorus compounds |
| - Peripheral neuropathy: |
| ∘ Diabetes mellitus–chronic alcoholism |
| ∘ Paralysis of the laryngeal recurrent nerve (neoplasms, surgery, etc.) |
| ∘ Others (diphtheria, tetanus, rabies, lead poisoning, syphilis, etc.) |
| - Myopathy: |
| ∘ Connective tissue diseases (overlap syndrome) |
| ∘ Metabolic myopathy (hyper-/hypothyroidism, amyloidosis, sarcoidosis, Cushing, Wilson) |
| ∘ Paraneoplastic syndrome |
| ∘ Drugs (amiodarone, HMG-CoA reductase inhibitors, corticosteroids, etc.) |
| ∘ Inflammatory myopathy (polymyositis, dermatomyositis, inclusion body myositis) |
| ∘ Myotonic and oculopharyngeal dystrophy |
| - Primary cricopharyngeal dysfunction |
| - Xerostomia or dry syndrome |
| - Secondary to artificial airway (translaryngeal intubation, tracheotomy or tracheostomy) |
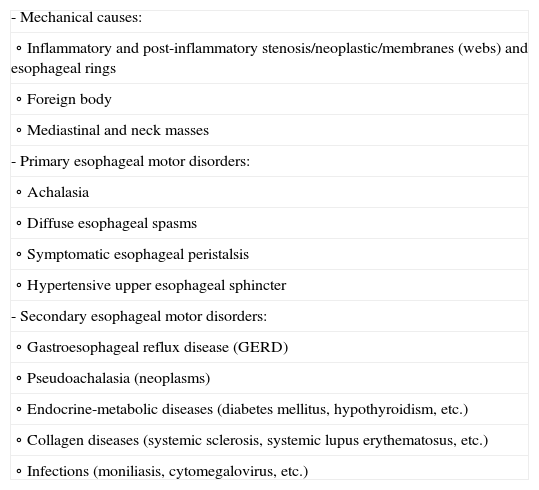
Esophageal dysphagia is defined as food bolus penetration difficulty from the upper esophageal sphincter to the stomach, due to alterations of the esophagus or lower esophageal sphincter. Mechanical or motility alterations are the most frequent underlying causes (Table 2). In contrast to patients with oropharyngeal dysphagia, the symptoms generally manifest several seconds after swallowing, and are typically referred to the retrosternal region.1
Causes of esophageal dysphagia.
| - Mechanical causes: |
| ∘ Inflammatory and post-inflammatory stenosis/neoplastic/membranes (webs) and esophageal rings |
| ∘ Foreign body |
| ∘ Mediastinal and neck masses |
| - Primary esophageal motor disorders: |
| ∘ Achalasia |
| ∘ Diffuse esophageal spasms |
| ∘ Symptomatic esophageal peristalsis |
| ∘ Hypertensive upper esophageal sphincter |
| - Secondary esophageal motor disorders: |
| ∘ Gastroesophageal reflux disease (GERD) |
| ∘ Pseudoachalasia (neoplasms) |
| ∘ Endocrine-metabolic diseases (diabetes mellitus, hypothyroidism, etc.) |
| ∘ Collagen diseases (systemic sclerosis, systemic lupus erythematosus, etc.) |
| ∘ Infections (moniliasis, cytomegalovirus, etc.) |
This term is used in reference to dysphagia of unknown origin.
Classification of dysphagia according to the degree of functional impairmentThis classification is typically used in patients with neurological, muscular or neuromuscular disorders.2
Mild degree dysphagiaMild degree dysphagia is predominantly oral dysphagia, typically characterized by a delay in swallowing, with loss of oral content, and difficulty in forming the food bolus. No dysphonia or cough is observed after swallowing, and there is a low risk of airway penetration or aspiration.
Moderate degree dysphagiaModerate degree dysphagia is characterized by a predominance of oral and pharyngeal dysfunction, with a loss of oral content due to lip incontinence and food leakage through the nasal passages. In addition, food bolus transport is slowed as a consequence of altered lip and tongue contractility, with the possible association of dysphonia. In these cases there is a risk of laryngeal penetration and/or bronchial aspiration.
Severe degree dysphagiaIn addition to alteration of the oral and pharyngeal phases of swallowing, severe degree dysphagia is characterized by laryngeal impairment with alteration of the airway protective reflexes. Food remains are often retained in the pharyngeal recesses, and patients may experience alteration or abolition of laryngeal and hyoid elevation and anteversion during swallowing. Coughing is not always observed. These individuals are at a high risk of airway penetration and/or aspiration.
Oropharyngeal dysphagia secondary to artificial airwayThe studies conducted to date in relation to laryngeal incompetence (G/SG-I) secondary to artificial airway isolation comprise a limited number of heterogeneous patients. In such studies it is important to note that hours after intubation the patients may develop laryngeal alterations that can persist for prolonged periods of time.4,5,10–13 A recent series has reported that in the first 24h after extubation, 44% of the patients suffered aspirations not accompanied by the cough reflex.20 The existing data in tracheotomized patients describe laryngeal dysfunction in 50–83% of the cases.12,15–18
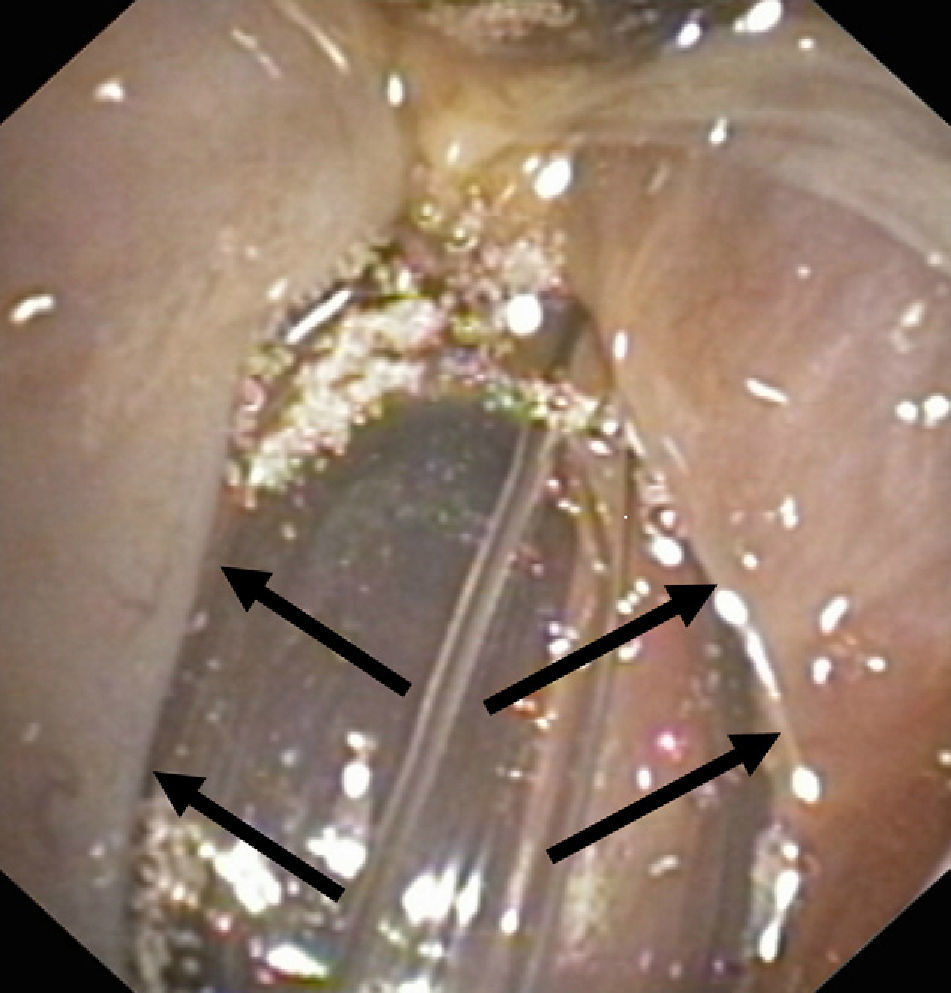
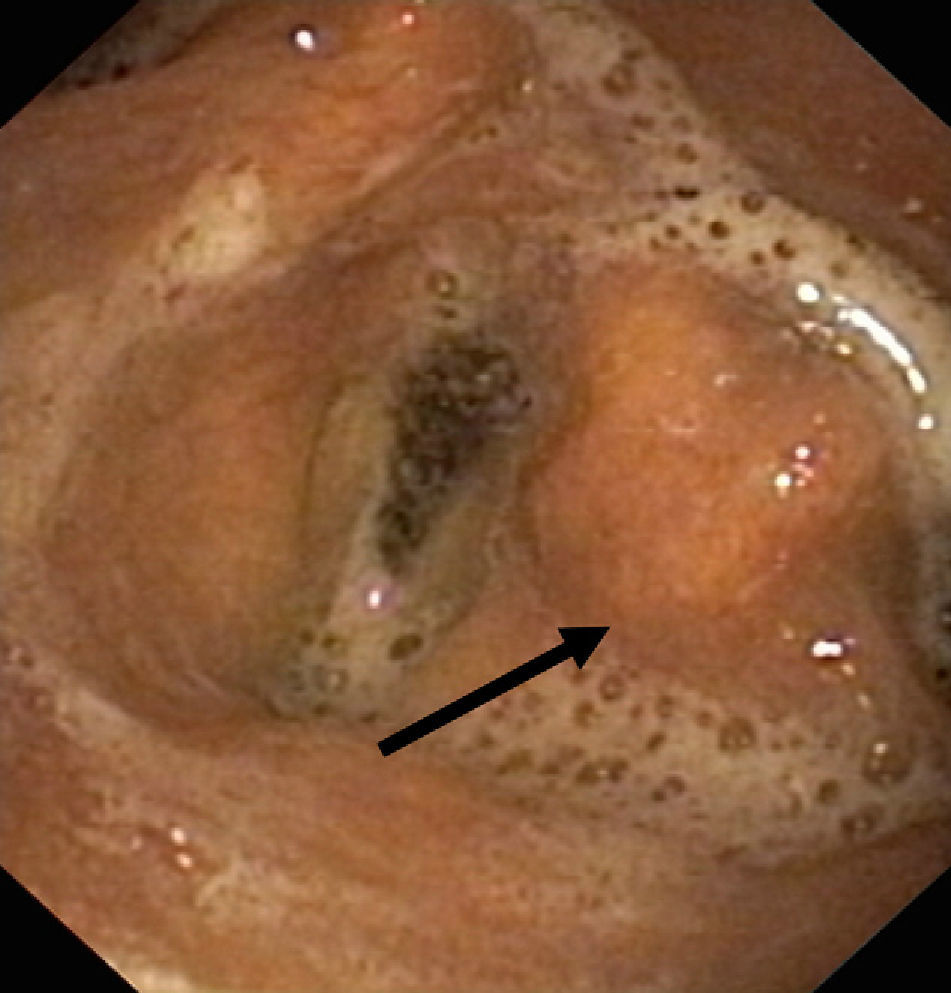
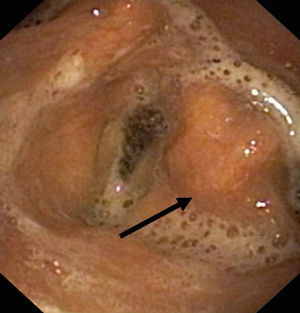
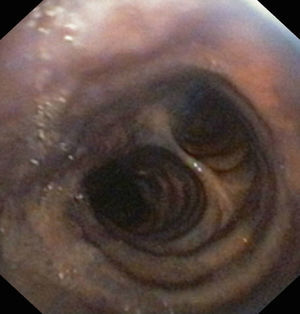
Lesion mechanismsTranslaryngeal intubation can affect the laryngeal structures as a result of direct impact during intubation, in the course of prolonged intubation, in restless patents, as a consequence of abrasion of the laryngeal mucosa, or secondary to the mere presence of the tube orotracheal. Common findings are vocal cord edema, as well as swelling of the supraglottic space (Fig. 1), with a less frequent observation of granulomas at this level. Other infrequent findings are arytenoid subluxation or luxation (Fig. 2), or vocal cord paralysis secondary to direct damage or involvement of the recurrent nerves. All these lesions adversely affect swallowing.10,11
The orotracheal (translaryngeal) tube keeps the glottis open for prolonged periods of time, abolishing the natural movements of the larynx and of pharyngeal muscles. This in turn leads to muscle atrophy, weakness of the pharyngolaryngeal muscles, and stiffness of the tongue, pharynx, hypopharynx and larynx.10 The intrinsic movements of the larynx, such as reflex glottic closure during swallowing, are affected. The edema produced as a result of the indwelling foreign body (translaryngeal tube) and the absence of correct stimulation of the laryngeal and hypopharyngeal mechanoreceptors cause a decrease in sensitivity to the presence of secretions, altering the complex swallowing mechanism, which in turn can facilitate laryngeal penetration or tracheal aspiration.17 In tracheotomized patients, the presence of the tracheotomy cannula can lead to aspirations, since it is a coadjuvant factor in G/SG-I.8,9 Correct insufflation of the pressure cuff in the tracheotomy cannula or translaryngeal tube does no fully prevent bronchial aspiration.18,21 Secretions and food tend to accumulate in the glottic and subglottic space, where they are difficult to eliminate in the presence of a tracheotomy cannula or translaryngeal tube (due to the reasons given above). It is from this space where penetration or bronchial aspiration ultimately takes place, unless the tube or cannula is equipped with an aspiration system located immediately above the pressure cuff (subglottic aspiration cannulas or tubes)—thereby minimizing penetrations/aspirations. This complication of tracheotomy, described in the medical literature,18,21 should be monitored, since it is the cause of an important increase in comorbidity that proves difficult to resolve. Increasing the pressure cuff effect in an attempt to make this compartment airtight can give rise to tracheal mucosal ischemia, associated to the posterior development of granulomas or even mucosal necrosis—with consequent scarring and strictures.
The placement of a tracheotomy cannula, in the presence of prolonged pressure cuff insufflation, directly alters the glottic closure reflex, which is normally triggered when the supraglottic mucosa comes into contact with solids or liquids.12
Tracheotomy in patients subjected to prolonged mechanical ventilation modifies normal function and the relationship between breathing and swallowing, causing them to become independent of each other (the normal situation being total dependency between the two phenomena)—thereby altering the normal airway protective reflexes.21 In addition, the motor response is reduced and the laryngeal adduction reflex is shortened in time. On the other hand, it has been postulated that in the presence of a tracheotomy cannula the normal laryngeal elevation and anteversion movement of the larynx is reduced and proves less effective in opening the upper esophageal sphincter and lowering the epiglottis.10 Different tracheotomy techniques have even been proposed to avoid such limitations.
The most deleterious effects of tracheotomy cannulas with pressure cuffs are muscle atrophy and atrophy of the nerve endings in the glottic and subglottic region. The interruption of air passage through the glottis, and the loss of pressure in the subglottic region, directly affect the cough reflex, laryngeal adduction and glottis closing capacity. The glottic and subglottic muscles are affected not only by polyneuropathy in the critical patient9,17,18 but also largely by a lack of use. In fact, restoring air passage through the glottis, with the use of fenestrated cannulas and particularly Passy-Muir type speaking valves, facilitates rehabilitation and posterior recovery from such alterations, as well as correction of the swallowing and speech mechanisms.9,10,22,23
Exploration and diagnosis of oropharyngeal dysphagiaIn order to explore, diagnose and classify the different types of oropharyngeal dysphagia, swallowing is evaluated clinically and with the use of instruments. In turn, the multidiscipline-based development of diagnostic protocols allows a rapid and firm diagnosis and makes it possible to adopt appropriate and effective therapeutic measures:
Physical examination- 1.
Inspection of the oral cavity, including the evaluation of dental and/or gingival disorders, alterations of the oral mucosa (dentition problems, herpes, mucositis, aphthae, etc.), reduced salivation (Sjögren syndrome, antihistamines, anticholinergic drugs, etc.), and the presence of masses.
- 2.
Inspection and palpation of rostral-caudad displacement and anteversion of the laryngeal structures, together with elevation of the floor of the mouth during swallowing.
- 3.
Oral motor and sensory function, including specific neurological exploration according to the background disorder involved:
- -
Cranial nerves V, VII, IX, X and XII. An evaluation is made of the movements of the lips, face, tongue, mandible and palate, with an assessment of muscle mass and tone, strength, symmetry, speed and range of action.
- -
Cough reflex. Loss of this reflex is a sign of diminished airway clearance. The presence of dysphonia, wet voice or “vocal cord bubbling” is common in patients with an abolished cough reflex. The nausea reflex is of no help in the exploration of swallowing.2
- -
- 4.
In tracheostomized patients, the evaluation of swallowing requires deflation of the pressure cuff and occlusion of the external orifice of the cannula, with the aim of restoring transglottic airflow and facilitating speech and cough:
- -
On deflating the pressure cuff, the presence of secretions above the cuff is suggestive of laryngeal incompetence (G/SG-I); the presence or absence of a cough reflex is significant, being preserved in patients with active airway defense mechanisms.
- -
- 5.
Small pieces of ice are administered as solids, along with 3–4ml of water and semisolids (purée or soufflé), with the observation of chewing efficacy. The movements of the laryngeal structures and floor of the mandibular region are monitored and palpated. Neck auscultation before, during and after swallowing is useful for determining the presence or absence of secretions or liquids in the larynx or pharynx.
Videofluoroscopy involves radioscopic assessment of the swallowing of barium contrast-based formulations of different consistencies. It is presently the gold standard for the study of oropharyngeal dysphagia.24 The technique allows visualization of the complete swallowing sequence from different angles, including elevation of the hyoid bone and larynx, pharyngeal contraction and relaxation of the upper esophageal sphincter. It also can be used to analyze formation of the food bolus, the function of different muscle groups and anatomical structures, and to precisely measure oropharyngeal transit times and diagnose laryngeal penetration (the foreign material is retained within the laryngeal vestibular zone, extending no further than the true vocal cords) or bronchial aspiration (the foreign material extends beyond the true vocal cords).24,25
Radioisotope transit testingThis technique is indicated for studying transit from the oral cavity to the stomach, using Tc99m-sulfur colloid (the administered dose varying from 150 to 300μCi), a radiodrug that is not absorbed and does not adhere to the gastrointestinal mucosa. Imaging is carried out in the context of a dynamic study immediately after swallowing, using a low-energy collimator and centering the image from the oral cavity to the stomach, in an anteroposterior projection. The technique is widely used to study esophageal dysphagia, and is sensitive and specific in diagnosing the esophageal motility pattern. In application to oropharyngeal dysphagia, it is less sensitive and specific from the anatomical–functional perspective than videofluoroscopy, though it offers the possibility of acquiring high-count static images that are able to correctly diagnose bronchial aspirations. The acquisition of images of this kind with a sufficient time margin after normal swallowing of the radioisotope also allows the exclusion of bronchial aspirations due to gastroesophageal reflux.26–28
Evans methylene blue testThis test is used to diagnose G/SG-I in tracheotomized patients, and involves the instillation of a few drops of methylene blue on the tongue, in tracheotomized patients under spontaneous breathing or who are able to maintain continuous positive airway pressure (CPAP) ventilation and can undergo pressure cuff deflation in the semi-sitting position (between 45° and 90°). Over the subsequent hours, the appearance of blue-stained secretions in the tracheal aspirations is evaluated as an indication of passage from the pharynx to the trachea.
To date, the Evans blue test has shown high sensitivity (82–100%) in application to abundant aspirations (>10% of the food bolus), with a high false-negative rate (up to 50%) in studies involving limited samples.29–31
In our Unit we have used the Evans blue test for years, incorporating modifications designed to improve its performance. In tracheotomized patients in which the test is indicated and the above described conditions apply, 2ml of methylene blue are placed on the middle-posterior third of the tongue, followed by the observation of cough (early or late) and the appearance or aspiration of blue-stained secretions in the tracheotomy cannula, as well as the appearance of stained saliva at the lip commissures. The use of 2ml corresponds to physiological swallowing, which is known to be volume dependent (study range involving a bolus of over 1ml and less than 20ml); the appearance of blue-stained saliva at the lip commissures is suggestive of the existence of alterations in the oral phase of swallowing.
The anatomical level at which normal airway defense reflexes are present can be established from the timing of cough with the appearance of methylene blue in the tracheotomy cannula: laryngeal during the first 30s, tracheal after between 30s and 2min, and bronchial when longer than 2min.32
Glucose in bronchial secretionsThe utilization of Medi-Test® reactive strips (commonly used in urine sediment studies) for glucose testing in the bronchial secretions of tracheotomized patients in which oral nutrition has been started constitutes a simple G/SG-I screening test. Positivity with this technique is strongly suggestive of the presence of glucose, and makes it necessary to resort to other tests for confirmation purposes.
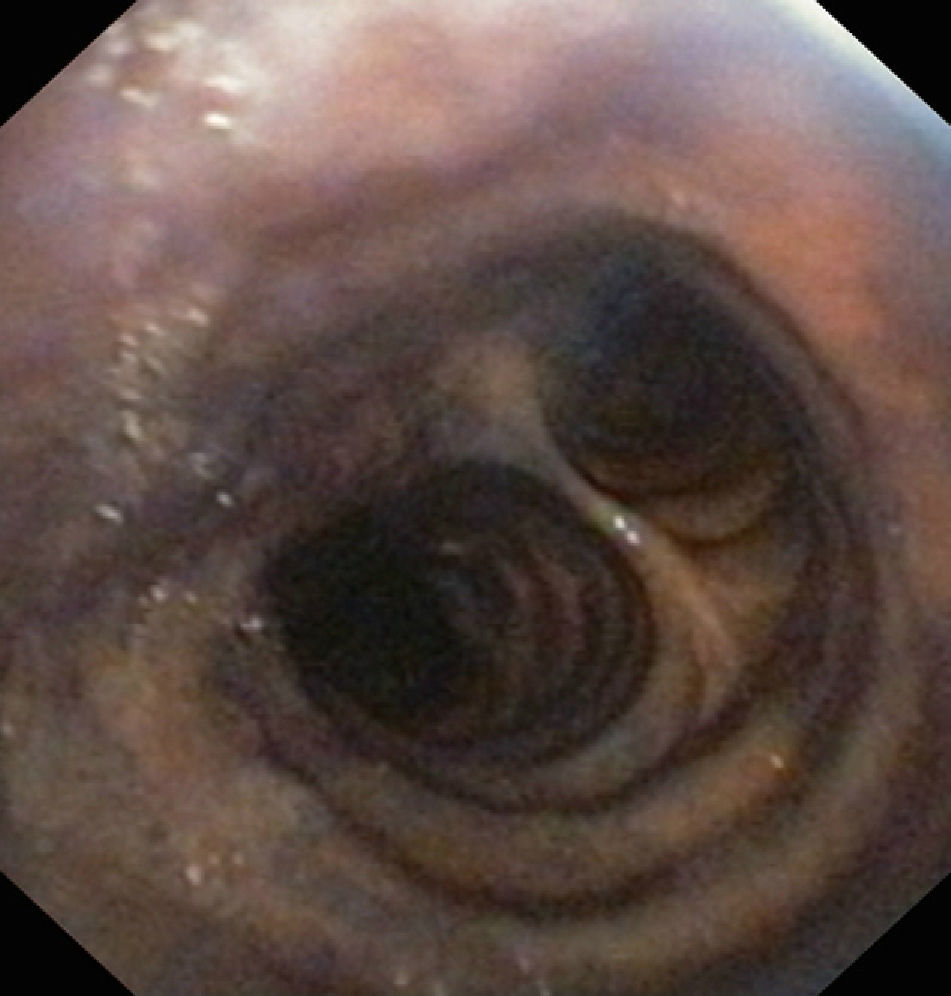
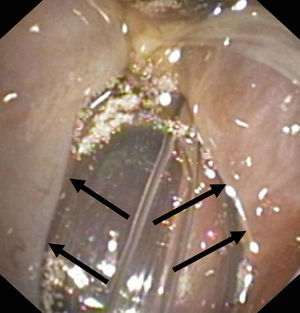
Instrumental testsFibroscopyTransnasal fibroscopy is able to visualize the laryngopharyngeal structures and their possible alterations. During the test a functional evaluation is made, including direct visualization of the formation of the food bolus (liquid or solid) in its pharyngeal phase, together with laryngeal tests to assess vocal cord adduction and cough reflexes.33–39
After swallowing, fibroscopy moreover allows the detection of retained food in the hypopharyngeal region (supraglottic recesses), and its visualization in the airway in the case of tracheal aspiration.36–38
Studies of this kind should be carried out by experienced specialists, and their sensitivity can be improved if the swallowed substances are stained with easily identifiable dyes. Despite such measures, however, the procedure involves significant interobserver variability39,40 (Fig. 3).
ManometryManometry allows direct evaluation of pharyngeal and esophageal contractility, including the study of both esophageal sphincters (upper and lower). It involves the use of a pressure probe or sensor that is inserted through the mouth or nose.
Manometry is widely used for studying esophageal dysphagia, and is an essential tool for diagnosing certain disorders.41,42 In the study of oropharyngeal dysphagia, manometry is used when dysfunction of the upper esophageal sphincter is suspected. It also provides diagnostic clues in patients with pathologically weakened pharyngeal contraction (bulbar dysfunction) or alterations in the coordination of cricopharyngeal contraction and upper esophageal sphincter relaxation. In the event of such findings, complementary studies are needed.42–44
A combination of techniques is sometimes used, comprising manometry and fluoroscopy (known as “manofluoroscopy”), to obtain objective data on the origin and mechanism of dysphagia, synchronizing fluoroscopic events with the manometric data.45
The performance and interpretation of pharyngeal manometry are complex, and should be carried out by experienced specialists.
ElectromyographySwallowing is a complex neurological and muscular phenomenon involving the participation of 26 muscle pairs and 5 cranial nerves. From the electromyographic perspective, the study of dysphagia and odynophagia is complex and affords useful information on the nerve stimulation and muscle response pattern of the patient, with differentiation of the different muscle groups.46,47
Recent studies have proposed electromyography with surface electrodes as a screening technique for oropharyngeal dysphagia, thereby avoiding the use of needles. The procedure is rapid and easily reproducible, and affords qualitative information that can influence posterior rehabilitation therapy and even the diagnostic orientation. This type of study moreover involves no radiation or discomfort for the patient, and is rapid and inexpensive; furthermore, in patients who require treatment, electromyographic monitorization can yield information on the patient course.48,49
Management approachThe indicated management approach depends on the cause of oropharyngeal dysphagia. In all cases the aim of management is to allow correct swallowing and the avoidance of bronchial aspiration.
In general, the management approach to dysphagia secondary to structural alterations such as tumors or diverticuli is surgical, while patients with dysphagia of esophageal origin are mostly treated with drugs or therapeutic endoscopic procedures.
This section focuses on the treatment of artificial airway dysphagia, distinguishing between extubated patients and tracheotomized patients.
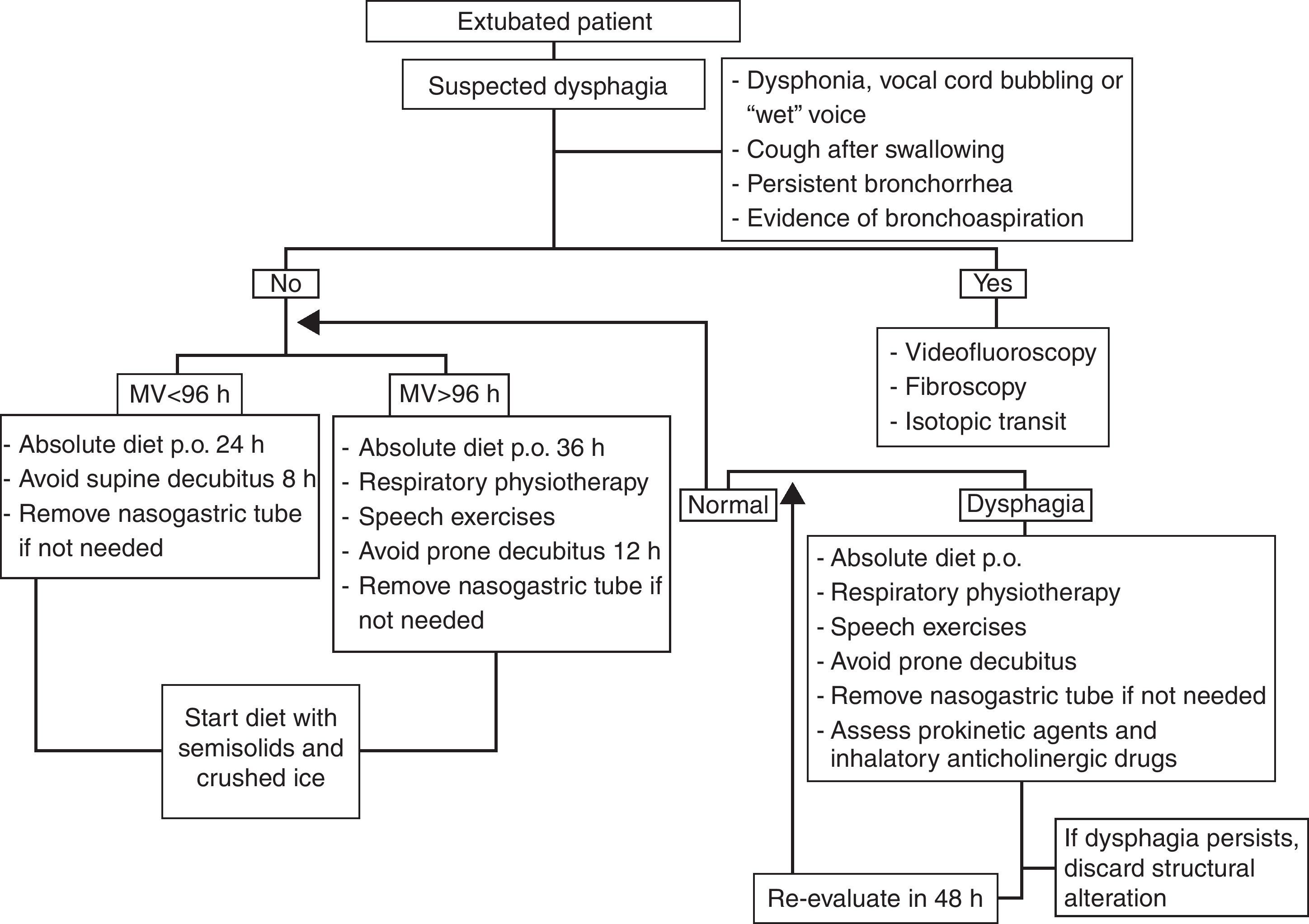
Extubated patientsNo studies in extubated patients have demonstrated the best time to reintroduce oral nutrition, despite evidence of a high frequency of bronchial aspirations not accompanied by cough reflex.4,9–11
Traditionally, in many Intensive Care Units (ICUs), these patients have been subjected to 12–24h of absolute diet in order to avoid possible complications associated to bronchoaspiration in the event reintubation proves necessary because of extubation failure.
The few studies made in this field point to the efficacy of transglottic airflow restoration and the increase in subglottic pressure following extubation in recovering effective glottic closure reflex and correct swallowing.4,8,9,22
Late reintroduction of oral feeding (probably after more than 24h) and exercises of the neck, laryngeal and pharyngeal muscles in the form of deep exhalations, forced coughing, the Valsalva maneuver, clearing of the throat, contained slow inspirations, the pronunciation of vocals, etc., are indicated in extubated patients—particularly in those with over 96h of translaryngeal intubation, where the probability of suffering some degree of artificial airway oropharyngeal dysphagia is greater.4,9–11
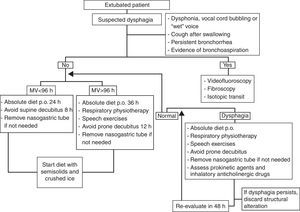
Once dysphagia has been diagnosed, frequent patient evaluation is required (at intervals of between 48 and 72h); if the dysphagia persists despite the above mentioned rehabilitation therapy, the possible existence of underlying structural damage should be evaluated, with adoption of the opportune management measures. Fibroscopy should be performed, with possible functional evaluation and laryngeal tests to assess vocal cord adduction and cough reflexes. On the other hand, it must be remembered that the presence of an orotracheal tube is not the only element that can affect swallowing, and that other factors also should be considered, such as the background disease leading to the need for mechanical ventilation, and the patient evolutive stage (Fig. 4).
Tracheotomized patientsTracheotomized patients are mostly individuals who have required prolonged mechanical ventilation due to extremely serious disease, associated complications, or previous or concomitant disease processes.
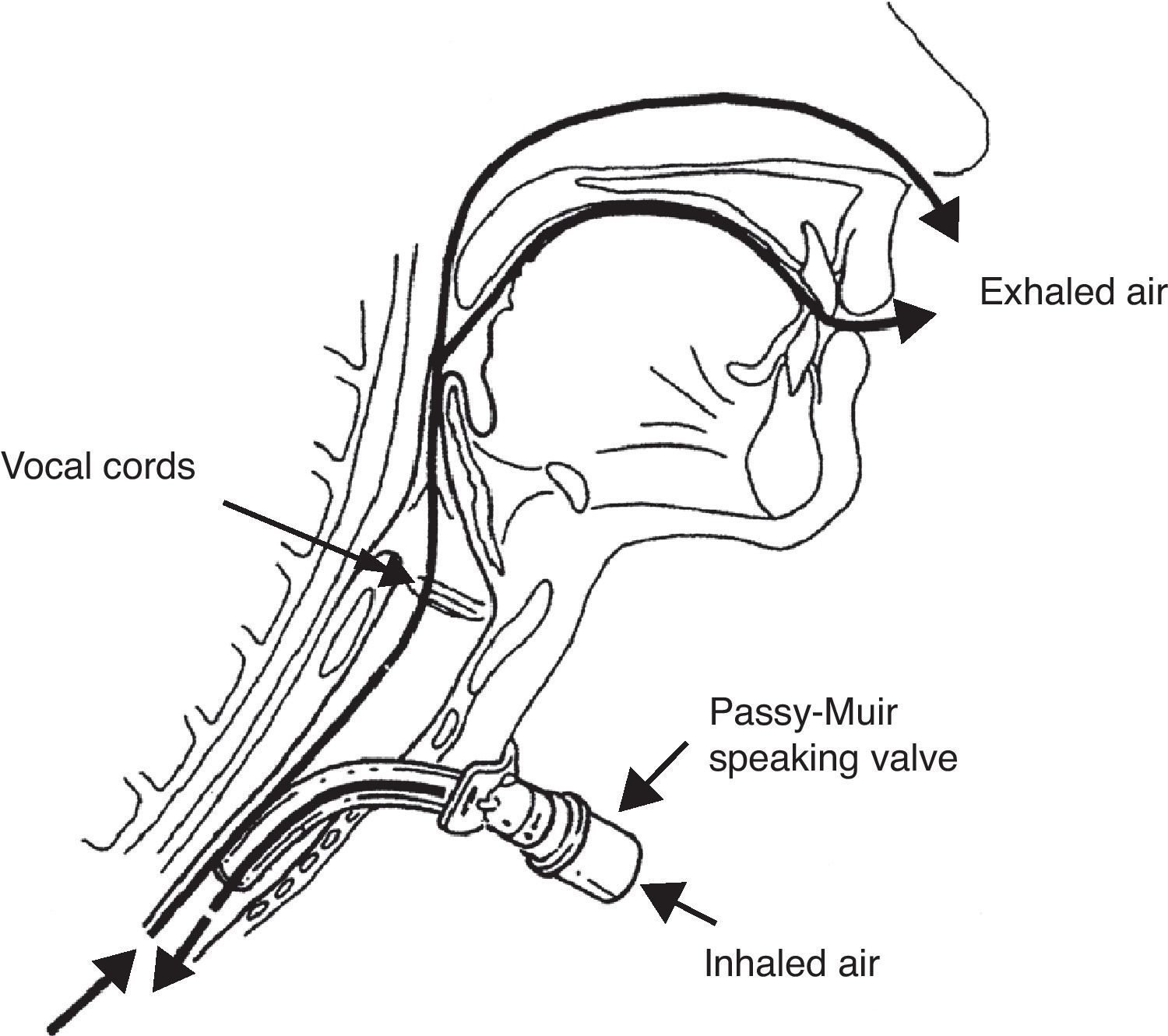
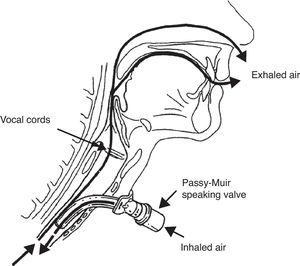
The management approach to G/SG-I is similar to that described for extubated patients. Rehabilitation aims to restore air passage through the glottis, with an increase in subglottic pressure and stimulation of the central and peripheral nerve endings. Use is made of fenestrated cannulas, Passy-Muir type speaking valves, and even intermittent sealing of the cannula with the deflated pressure cuff.50–53
The Passy-Muir type speaking valve is a unidirectional valve that occludes air outflow through the tracheotomy cannula during expiration, thereby forcing transglottic air passage (Fig. 5). The valve is contraindicated in patients with obstructive disease in the glottic–subglottic region produced by structural alterations (e.g., tumors), functional disorders (e.g., paralysis of both vocal cords) or edema, granulomas, etc., since in such cases it would induce immediate respiratory failure and asphyxia if left in place for a prolonged period of time. Likewise, it is essential to keep the tracheotomy cannula pressure cuff deflated during use of the speaking valve.
A simple test should be performed in spontaneously breathing patients, involving digital occlusion of the tracheotomy cannula with the pressure cuff deflated; if the patient is able to speak and breathe comfortably, then a speaking valve can be indicated. In order to ensure improved tolerance of the valve and avoid excessive muscle effort, it is advisable to replace the tracheotomy cannula with a cannula that is 1–2 ISO points smaller; this will lessen respiratory resistance (though at the expense of increasing inspiratory resistance) and also improve the management of secretions, which during use of the speaking valve must be expectorated through the natural airway.
An advantage of one of the Passy-Muir valve designs is that its dimensions adapt to the tracheotomy cannula as well as to the mechanical ventilator tube fittings; as a result, those patients who require ventilatory support and have no glottic structural or functional impairments can benefit from use of the valve during weaning from mechanical ventilation—accelerating the dysphagia rehabilitation process. Likewise, patients requiring chronic mechanical ventilation can greatly improve their quality of life thanks to the recovery of speaking capacity.54,55
In patients diagnosed with G/SG-I, this type of valve minimizes but does not avoid bronchoaspiration. As a result, it must not be used during sleeping periods, and in all cases should be employed concomitant to other measures such as raising the patient headrest 40° (or while the patient is in the sitting position) or using cannulas with subglottic aspiration systems, additional airway humidification, etc.51–53
Since the valve does not adapt to Trach-Vent® type airway passive humidification systems, it is not uncommon for mucosal plugs to develop. This problem can be overcome by monitoring the bronchial secretions, taking care not to exceed grade 3 secretion thanks to the use of aerosol therapy or water vapor active humidification measures—the latter being the most effective and adequate option.56,57
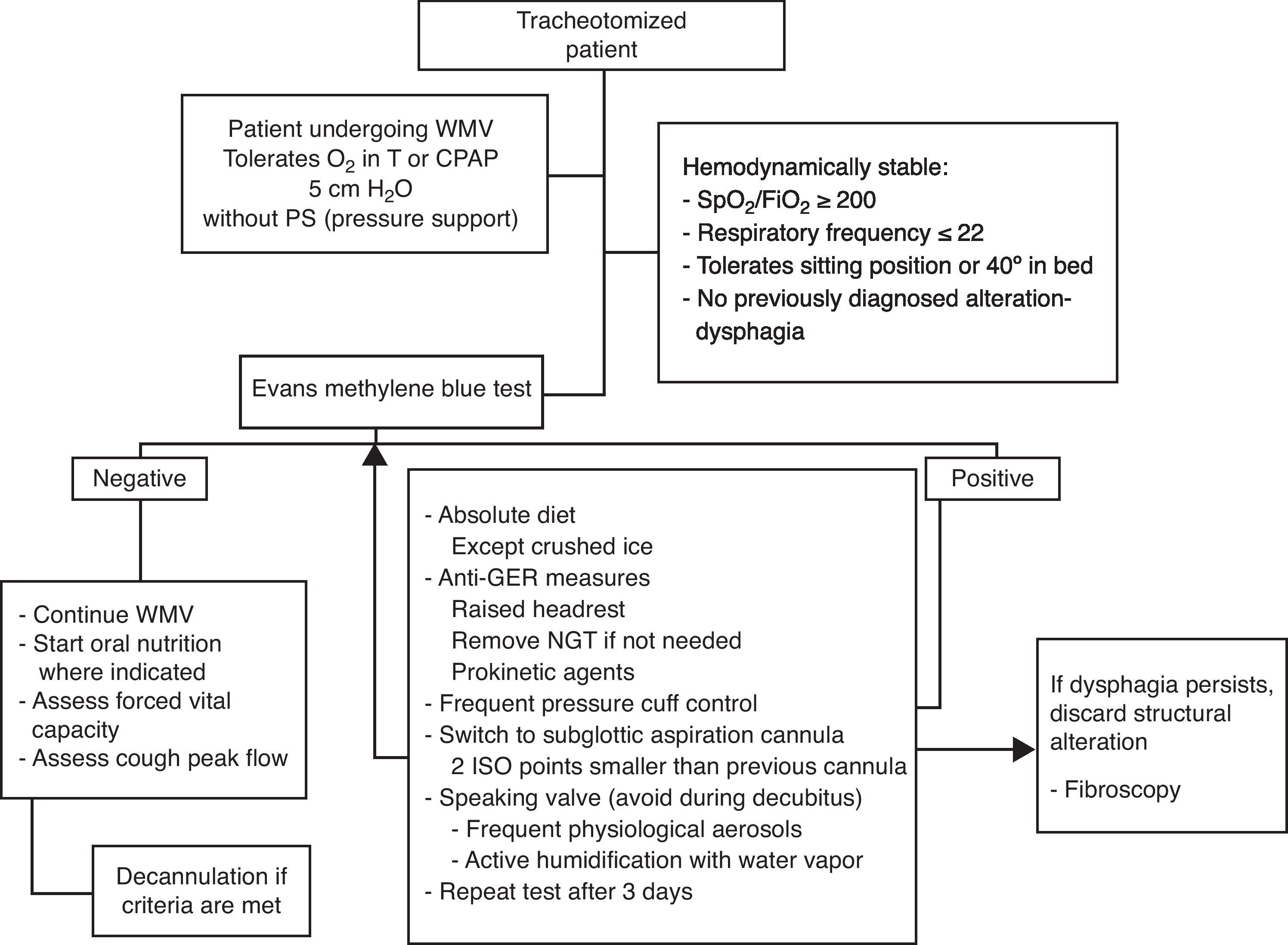
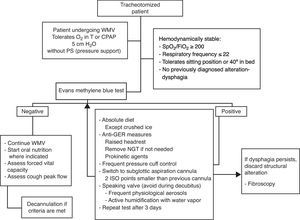
In the same way as in extubated patients, frequent re-evaluation of G/SG-I is required; in the case of persistent dysphagia, other complementary tests are needed to discard possible associated disorders (Fig. 6—diagnostic and therapeutic algorithm in tracheotomized patients).
Tracheotomized patients can moreover benefit from integral rehabilitation therapy with adequate breathing exercises, facilitating the recovery of effective vital capacity and cough, and specific swallowing, cough stimulation and laryngeal and muscle strengthening exercises. Likewise, and although supporting scientific evidence is lacking, electrostimulation can also be considered.58,59
Other therapeutic measures such as taste stimulation with small amounts of crushed ice, or central stimulation using inhaled essences (pepper derivatives), which prove effective in patients with disorders of neuromuscular origin, probably can also offer benefit.60
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Fernández-Carmona A, et al. Exploración y abordaje de disfagia secundaria a vía aérea artificial. Med Intensiva. 2012;36:423–33.