The pulmonary artery catheter has been a key tool for monitoring hemodynamic status in the intensive care unit for nearly 40 years. During this period of time, it has been the hemodynamic monitoring technique most commonly used for the diagnosis of many clinical situations, allowing clinicians to understand the underlying cardiovascular physiopathology, and helping to guide treatment interventions. However, in recent years, the usefulness of pulmonary artery catheterization has been questioned. Technological advances have introduced new and less invasive hemodynamic monitoring techniques.
This review provides a systematic update on the hemodynamic variables offered by cardiac output monitoring devices, taking into consideration their clinical usefulness and their inherent limitations, with a view to using the supplied information in an efficient way.
El catéter de la arteria pulmonar (CAP) ha constituido una herramienta fundamental para la monitorización hemodinámica en las unidades de cuidados intensivos durante los últimos 40 años. Durante este período de tiempo ha sido ampliamente usado en pacientes críticos para el diagnóstico y como guía del tratamiento, ayudando a los clínicos a entender la fisiopatología de muchos procesos hemodinámicos. Sin embargo, en los últimos años la utilidad del CAP ha sido sometida a un intenso debate. Paralelamente, los avances tecnológicos han permitido el desarrollo de nuevas técnicas, menos invasivas, para la monitorización cardiovascular. Esta puesta al día pretende dar a los clínicos una visión de los parámetros hemodinámicos que aportan los distintos métodos disponibles, considerando que es fundamental comprender tanto su potencial utilidad clínica como sus limitaciones para un uso eficaz de la información que proporcionan.
For the past 40 years, the pulmonary artery catheter (PAC) has been a fundamental tool in the hemodynamic monitorization of patients admitted to the Intensive Care Unit (ICU).1 During this period of time, it has been widely used in critical patients for diagnostic purposes and as a guide to treatment, helping clinicians to understand the physiopathology of a broad range of hemodynamic processes. However, in recent years the usefulness of the PAC has been the subject of intense debate, fundamentally due to the publication of studies in which its use was not found to be associated with benefits in terms of patient survival.2–7 In fact, several of these studies reported an increase in mortality associated with the use of the catheter.2,3 At the same time, technological advances have made it possible to use less invasive procedures for cardiovascular monitorization−reinforcing the idea that the systematic utilization of the PAC may have come to an end. Despite the controversy, however, there is no doubt that the PAC can be used to obtain unique, valuable and useful hemodynamic variables in critically ill patients.8,9
In recent years, new methods have come to replace the PAC in the determination of cardiac output (CO). These new technologies are highly diverse, ranging from very invasive to less invasive or even noninvasive, from intermittent to continuous, and involving different basic principles, methods and costs. Some of the methods offer dynamic fluid response indices, which are currently regarded as better predictors of the response to volume expansion, while others allow us to evaluate volumetric preload parameters or afford continuous central venous saturation measurements. All of these variables, together with CO, contribute to improve the hemodynamic monitorization of critical patients.10 However, to date, none of the mentioned techniques exhibit optimum or ideal characteristics, i.e., noninvasiveness, continuous measurement, reliability, reproducibility, convenience for both the patient and physician, accuracy and minimum side effects.11,12 Consequently, the utilization of each of them fundamentally depends on their availability and on the knowledge or aptitudes of the professional.
All of these techniques have been evaluated and validated by comparing their results with those of the gold standard, which continues to be intermittent thermodilution of the pulmonary artery.
The present update aims to offer clinicians a vision of the hemodynamic parameters afforded by the different methods which are currently available, considering that it is essential to understand both their potential clinical usefulness and their limitations in order to ensure effective use of the information obtained in each case.
Invasive methodsPulmonary artery or Swan–Ganz catheterThis catheter was introduced by J.C. Swan and W. Ganz in 1970. It is advanced through a large caliber vein to the right side of the heart and into the pulmonary artery, where its distal tip is positioned in a branch of the artery. The PAC offers information referred to three categories of different variables: measurements of blood flow (CO), intrathoracic intravascular pressures, and oximetric parameters.
Measurements of blood flowThe measurement of CO using this catheter is based on transcardiac thermodilution. After injecting a volume of liquid at a temperature below the temperature of the blood, the thermistor detects the temperature changes over time in the form of a curve. The area under this curve (AUC) is the minute volume. The details referred to the measurement of CO, and the technical limitations involved (tricuspid valve insufficiency, etc.), have been extensively addressed in previous “Updates in hemodynamic monitorization”.13
Measurement of intrathoracic intravascular pressuresThe PAC, when correctly positioned, allows us to record pressures in three different locations: right atrium (central venous pressure, CVP), pulmonary artery (pulmonary artery pressure, PAP) and the pulmonary veins (also called pulmonary occlusion or wedge pressure, PWP). Originally, the PAC was developed for the measurement of PWP, which corresponds to the pulmonary venous pressure distal to the pulmonary capillary bed (hence the commonly used term of pulmonary capillary wedge pressure, or PCWP), affording an indirect estimate of left atrial pressure (LAP). In fact, even today PCWP affords the best patient bedside estimate of pulmonary venous pressure, contributing to assess both pulmonary resistances and left atrial preload. To this effect there is no practical alternative to PCWP. Recently, a series of pulmonary venous flow measurements have been proposed, using Doppler echocardiography, for the estimation of PCWP,14 though the variables obtained using Doppler ultrasound derived from transmitral flow (TMF) and pulmonary venous flow (PVF) are inexact, time consuming to obtain, cannot be recorded in all patients, and require important experience beyond the basic principles of echocardiography.15 Nevertheless, in recent years new parameters have been developed, based on tissue Doppler ultrasound, which afford increased accuracy. In any case, the usefulness of PCWP in the critical patient requires redefinition. It has been repeatedly and consistently shown that PCWP has low predictive value in the evaluation of volume response. As a result, it is not advisable for clinicians to use the absolute PCWP values at the patient bedside in predicting the response to fluid administration. In this regard, the variables obtained from the analysis of the arterial pressure curve during positive pressure ventilation, such as the variation in pulse pressure or the variation in systolic volume, predict volume response much more reliably.16 However, measurements of PCWP remain very useful in diagnosing the origin of pulmonary hypertension, and in distinguishing between primary (non-cardiogenic) and secondary (cardiogenic) lung edema.
Mixed venous saturation and other oximetric variablesOxygen saturation measured at distal pulmonary artery level or mixed venous oxygen saturation (SvO2) is probably the best isolated indicator of the adequacy of global oxygen transport (DO2), since it represents the amount of oxygen remaining in the systemic circulation after passing through the tissues. The use of central venous oxygen saturation (SvcO2) has been proposed as a simple method, replacing SvO2, for assessing the adequacy of global perfusion in different clinical scenarios. However, the fact that SvcO2 reflects SvO2 has been strongly debated, particularly in the critical patient. Moreover, based on the recorded CO and SvO2 values and the arterial oxygenation values, we can calculate global oxygen transport and consumption (DO2 and VO2, respectively), as well as the pulmonary oximetric shunt and the oximetric gradient in situations of acute interventricular septal rupture.
Advantages and inconveniencesThe PAC appears ideal for identifying and monitoring different forms of circulatory shock (hypovolemic, cardiogenic, obstructive and distributive), with the determination of key parameters in any of these scenarios: CO, PCWP and oximetry. The use of these three measurements, associated to direct measurements of mean blood pressure (MBP), allows us to establish the origin of shock and to monitor the treatment response.17 Based on these arguments, the PAC has been one of the cornerstones in the management of our patients in the ICU, being used as a systematic monitorization tool. However, the data obtained have been largely ignored or simply used to assess patient stability. This scantly specific and indiscriminate use is at least partly responsible for the lack of efficacy (in terms of patient survival) reported in different studies.18–20
Another contributing factor is the repeated confirmation of the scant knowledge clinicians have when it comes to interpreting the information obtained with the PAC, as for example in the analysis of PCWP wave morphology, and the lack of adequate comprehension of the physiological variables obtained when transferring the information to the clinical setting.21 Clearly, no hemodynamic monitorization system can improve the patient prognosis, unless the information it provides is associated to a choice of treatment that effectively serves to improve patient survival.
In contraposition to the above, several studies have shown the use of the PAC in objective-guided treatment to improve the patient prognosis. When the resuscitation strategies have been guided by hemodynamic variables obtained with the PAC, such as DO2, the cardiac index (CI) or SvO2, significant reductions have been recorded in hospital stay, with improved patient survival.22–24 It therefore seems that the PAC is a useful tool, capable of improving survival when associated to a treatment algorithm with specific physiological goals or objectives, applied in adequately selected patients. No benefits have been documented when using the technique in low surgical risk populations or in guiding resuscitation in late stage disease, once organic damage has been established.25 Likewise, the PAC has not yielded benefits when comparing volume expansion management strategies guided by PCWP versus CVP, as in the recent FACCT study,7 since neither of the variables used are reliable measurements of preload or volume response.
In addition to the debate referred to the patient prognosis, emphasis has been placed on the potential complications of the PAC, as a reinforcement of the arguments against its use. Evidently, and in the same way as with any invasive procedure, there are potential risks and complications. Many studies have shown that the local complications resulting from insertion of the PAC are no different from those derived from the insertion of any other central venous catheter.26 However, the PAC has been related to an increased risk of infections (incidence of bacteremia of 0.7–1.3%)30 and to thrombotic phenomena during prolonged use (>48h), as well as to an increased risk of arrhythmias during insertion (though with a minimum incidence of serious arrhythmias, and no impact upon the prognosis). It therefore can be deduced that the contribution of PAC use to the improvement or not of the patient prognosis will depend on how, when, where and in what type of patient the catheter is used.
Minimally invasive methodsIn recent years we have seen the introduction of different commercial systems, involving new and less invasive methods for quantifying CO. Most of them are based on the analysis of arterial pulse wave morphology according to a classical model allowing estimation of the stroke volume from variations in the morphology of the pulse wave (the Windkessel model described by Otto Frank in 1899).27 The existing systems differ in a number of aspects: in the way of transforming the information provided by the morphology of the arterial pressure into systolic volume and beat-to-beat CO; in the algorithms used in each case; in the calibration employed (since some require manual calibration while others require no external calibration); in the arterial cannulation site involved; in the parameters analyzed; and in the accuracy with which CO is determined in each case.
The methods and systems available on the market for analyzing pulse wave morphology are the following: PiCCO® (Pulsion), PulseCO® (LiDCO), Modelflow (TNO/BMI), MostCare® (Vygon) and FloTrac®/Vigileo® (Edwards Lifesciences, Irvine, CA, USA). Of these systems, the PiCCO is calibrated by transpulmonary thermodilution, the LiDCO by lithium dilution, and the Modelflow by means of three or four conventional thermodilution measurements. In contrast, the FloTrac®/Vigileo® system and MostCare® require no external calibration.
All of these methods are based on the morphology of the arterial pressure curve. It is therefore important to obtain a precise curve morphology. Buffering of the arterial curve and insufficient zeroing−common problems in clinical practice–must be avoided in order to obtain a signal valid for the calculation of CO. The presence of severe arrhythmias and the use of an intraaortic counterpulsation balloon reduce the precision of the CO measurements. Furthermore, the analysis of pulse pressure is of limited accuracy during periods of hemodynamic instability, as for example in the rapid changes in vascular resistance found in septic patients and in cases of liver dysfunction.
The PiCCO® systemThe PiCCO® (PiCCO System, Pulsion Medical Systems AG, Munich, Germany) is currently the only commercially available monitor using transpulmonary thermodilution (TPTD) to measure CO. It requires only an arterial line and a venous line, which in any case are necessary in most critical patients. The system offers information on blood flows and intravascular volumes.
Measurements of blood flowCardiac output is calculated from the analysis of the TPTD curve using the Stewart–Hamilton equation. In order to determine CO, an indicator (normally isotonic saline solution) is injected as a bolus dose at a temperature different from that of the blood through the lumen of the central venous catheter in which the external temperature sensor is located. Once within the bloodstream, the thermistor in the tip of the arterial catheter detects the temperature variations, generating the thermodilution curve. Three measurements are recommended for initial calibration of the system. In addition, calibrations must be made every 8h, and whenever needed according to the hemodynamic condition of the patient. Parallel to the thermodilution process, an analysis is made of the systolic portion of the arterial pulse wave morphology, to determine aortic compliance. By using the study of the pulse pressure wave for the analysis of stroke volume (SV), we can also calculate percentage pulse pressure variation (PPV) or stroke volume variation (SVV), used to guide fluid therapy and evaluate the patient response to such therapy.
Measurement of volumesAnother advantage of this technique is its capacity to calculate different intravascular compartment volumes (not pressure as in the case of the PAC), as well as pulmonary extravascular fluid. Cardiac preloading is estimated based on two parameters: (a) the measurement of global end-diastolic volume (GEDV), defined as the sum of the volume of blood in the four heart cavities; and (b) the intrathoracic blood volume index (ITBV), regarded as the volume of blood in the four heart cavities and in the pulmonary vascular bed. None of these parameters are altered by mechanical ventilation. The measurement of extravascular lung water (EVLW) quantifies lung edema and vascular permeability, with calculation of the pulmonary vascular permeability index (PVPI). Both PPV and SVV offer information on the volemia status of ventilated patients. These are very sensitive preload parameters, and indicate the point of the patient on the Frank–Starling curve, and whether there will be a response to fluid expansion or not.
Recent studies indicate that PiCCO measurements are more consistent and are not influenced by the respiratory cycle, compared with the PAC. However, in order to compensate the inter-individual variations that can occur in vascular system compliance and resistance, as a result of the different clinical situations, frequent manual calibrations are needed−particularly in situations of hemodynamic instability−in order to secure increased precision of the CO values.28 This technique has been validated in different clinical situations in critical patients, comparing it with PAC thermodilution, including patients subjected to coronary revascularization surgery.29,30 TPTD can yield inexact measurements in patients with intracardiac shunts, aortic stenosis, aortic aneurysms, and in those subjected to extracorporeal circulation.
The measurement of volumes with this system can lead to changes in treatment strategy, allowing more precise management of fluid resuscitation and the optimization of vasoactive drug use, as well as guiding depletion therapy with diuretics or dialysis.
While minimally invasive, the PiCCO system–in the same way as the PAC–can give rise to complications, which are all catheter-related, including infection, thrombosis, bleeding and vascular damage secondary to ischemia of the extremity, or pseudoaneurysms.
The LiDCO plus® systemIn a way similar to the PiCCO device, the Lithium Dilution Cardiac Output system (LiDCO plus®, London, UK) measures CO from a lithium chloride dilution wave using a peripheral lithium indicator sensor to generate a curve similar to the thermodilution curve, which in turn is used for the continuous beat-by-beat calibration of CO, based on the analysis of pulse strength. Calibration is carried out by injecting a bolus dose of the lithium chloride tracer (0.002–0.004M/kg) into a central or peripheral venous line. An electrode placed in a central or peripheral arterial line detects the blood lithium concentration and the time elapsed from administration of the tracer, calculating CO using the area under the concentration–time curve. The stroke volume is calculated from pulse strength after calibration with the lithium solution. From the lithium mean transit time (MTt) we obtain the intrathoracic blood volume (ITBV) as an indicator of preload. As in the PiCCO system, using the study of the pulse pressure wave for the analysis of SV also allows us to calculate percentage PPV or SVV in predicting the patient response to fluid therapy. With the manual introduction of certain variables, we obtain the systemic or peripheral vascular index or resistance (SVRI/SVR) and the oxygen transport index (IDO2). The latter may contribute to maximize oxygen supply to the tissues, with optimization of the hemodynamic conditions in patients at risk. In the same way as with the PiCCO, the dilution curve can be altered in patients with intracardiac shunts. The use of non-depolarizing muscle relaxants and lithium salt treatments also give rise to errors in the determination of CO.
The LiDCO technique offers acceptable accuracy when frequently recalibrated, and is less invasive that the PiCCO system, since it requires no central venous access (catheterization of the radial artery is sufficient). On the other hand, calibration is rapid and with few complications, and offers continuous information on a range of variables. The CO measurement obtained through lithium dilution has been validated in comparison with PAC thermodilution.31 The continuous measurement of CO obtained from the pulse wave has also been validated,32,33 in the same way as its stability–no recalibration being needed for up to 24h.34 Nevertheless, recalibration is recommended whenever there is a substantial change in the hemodynamic condition of the patient, particularly after modifications in the hemodynamic support measures.
In the case of the more recent LiDCO rapid®, lithium dilution has been replaced by a normogram derived from the in vivo data to estimate CO on a continuous basis. It uses the same pulse pressure algorithm as the LiDCO plus (PulseCO®). This system is simple and easy to use, and was designed to offer reliable parameters of use in application to objective-guided fluid therapy. A number of studies have made use of the LiDCO rapid as a guide for fluid administration and for assessing the blood pressure response to such treatment.
The FloTrac®/Vigileo® systemIn contrast to the above two devices, the FloTrac®/Vigileo® system (Edwards LifeSciences, Irvine, CA, USA), comprising the FloTrac® sensor and the Vigileo® monitor, analyzes arterial pulse wave morphology without the need for external calibration. The latter is replaced by correction factors that depend on the mean blood pressure (PAM) and on anthropometric measurements (patient age, gender, weight and height). The system is based on the principle that pulse pressure (the difference between systolic and diastolic pressure) is proportional to SV and inversely proportional to aortic compliance. In contrast to the indicator dilution methods used in manual calibration, the FloTrac®/Vigileo® system requires no central or peripheral venous access, or cannulation of a large caliber artery; only a radial arterial catheter is needed.
In addition to continuous CO monitorization, the system offers information on SV, SVV and SVR. With the implantation of a central venous catheter equipped with fiber optics, we can also monitor SvcO2.
Different studies have reported good reliability with the FloTrac/Vigileo® in different clinical scenarios compared with PAC thermodilution.35 However, the percentage error of the FloTrac/Vigileo® compared with the PAC in obese patients (body mass index (BMI)>30kg/m2) proved slightly greater than in patients of normal weight, due to the alteration of arterial compliance observed in these individuals. Likewise, the accuracy of the results is lower in patients with diminished SVR.36 The precision of the system has been increased as a result of successive software upgrades, and with incorporation of the latest algorithm version it shows acceptable correlation with intermittent thermodilution and continuous thermodilution in post-heart surgery patients.37
The determination of SVV with this system has revealed accuracy similar to that afforded by the PiCCO,38 with good performance during objective-guided fluid therapy, fewer complications, and a shorter hospital stay.39
A new hemodynamic monitorization device has recently been placed on the market: the VolumeView® system (Edwards LifeSciences, Irvine, CA. USA), which uses transpulmonary thermodilution for calculating CO. In the same way as the PiCCO system, CO is calculated by analyzing the TPTD curve, using the Stewart–Hamilton equation. In addition to continuous CO, the system allows us to determine SV, SVR and SVV. From the dilution curve it derives volumetric parameters such as EVLW, PVPI (for quantifying lung edema), GEDV and global ejection fraction (GEF). Although the experience gained is still limited, the results obtained to date are comparable to those of the PiCCO system.40
The MostCare® system (Vygon) (Vytech, Padova, Italy)The MostCare® system (Vygon)(Vytech, Padova, Italy) employs the pressure recording analytical method (PRAM), using a modified version of the Wesselings algorithm for analysis of the arterial pulse wave. The system requires only an arterial catheter (e.g., radial artery). The SV is proportional to the area under the diastolic portion of the arterial pressure wave divided by the aortic impedance characteristics, obtained from the morphological data of the pressure curve, without the need for calibration. The aortic impedance is determined by means of a formula that uses the principles of quantum mechanics and fluid dynamics. This formula is completely different from those of all the known methods. Stroke volume is calculated on a beat-by-beat basis, and CO is obtained by multiplying SV by heart rate. CO is reported as the mean of 12 beats.11 To date, the system has been validated in animal models in different clinical scenarios.41 Recent studies have revealed a significant correlation between the values obtained with the PRAM method and those obtained through thermodilution in hemodynamically unstable patients,42 as well as in septic patients, where the good correlation to TPTD was not affected by the changes in vascular tone induced by vasoactive drugs.43 The MostCare® system has an exclusive monitorization parameter, the cardiac cycle efficiency (CCE) or cardiac stress index, which corresponds to the work performed by the heart divided by an energy expenditure ratio. This parameter reflects the energy expenditure needed for the cardiovascular system to maintain a hemodynamic equilibrium. The MostCare® system requires validation through further studies.
The Modelflow-Nexfin® systemThe Modelflow-Nexfin® system (FMS, Amsterdam, The Netherlands) analyzes pulse pressure noninvasively using photoelectric plethysmography in combination with an inflatable finger cuff. Cardiac output is calculated through continuous monitorization of arterial pressure and analysis of pulse wave morphology, based on the study of the area of the systolic pressure wave and on the Windkessel triple elements model individualized for each patient (Modelflow method). The measurements obtained include continuous CO, SV, SVR and a left ventricle contractility index. Some studies, carried out in different clinical situations, suggest good correlation to thermodilution.44
The NICO® systemThe NICO® system (Novametrix Medical Systems, Wallingford, USA) is based on the Fick principle, using CO2 as indicator. With this method, CO is proportional to the change in production of CO2 divided by the end-tidal CO2 after a brief re-inhalation period. This system has a number of inconveniences that limit its utilization: small measurement errors give rise to important changes in the calculation of CO, due to the scant difference between PaCO2 and PvCO2–the results not being valid in patients with PCO2<30mmHg, and false changes in CO are obtained both in dead space and ventilation–perfusion alterations.
Few validation studies of this technique versus the PAC have been made, though a reasonably good correlation has been reported. In post-heart surgery patients, the measurement of CO through re-inhalation is underestimated with respect to the value obtained with the PAC. In conclusion, this system is presently no replacement for the PAC, but constitutes a feasible alternative in certain patients, such as those subjected to heart surgery.45,46
Noninvasive methodsTechniques such as transthoracic bioimpedance and esophageal Doppler ultrasound have been developed in recent years for the evaluation of CO, and have been well accepted in clinical practice, though with certain limitations.
The NICOM® thoracic electrical bioreactance systemBioimpedance is used to determine CO, SV and cardiac contractility from continuous measurements of the changes in thoracic impedance caused by fluctuation of the blood volume during the cardiac cycle. Bioreactance, a method used by the NICOM® system (Cheetah Medical Ltd., Maidenhead, Berkshire, UK), analyzes the changes in amplitude and frequency of the electrical impulses as they course through the chest. The advantage in this case with respect to bioimpedance is a significant reduction of factors such as electrical interferences, patient movements or positioning, or displacement of the electrodes, which can give rise to data error. A better signal-to-noise ratio is obtained compared with bioimpedance. Among the limitations of the technique, it should be mentioned that since the area under the pulse wave is proportional to the product of peak flow and ventricle ejection time, the precision of the CO determinations may be adversely affected under low flow conditions. The readings obtained offer an acceptable correlation to the results of CO measured with the PAC, in both humans and animals, and in different clinical scenarios.47–49
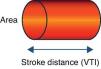
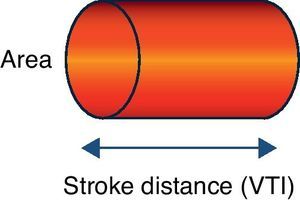
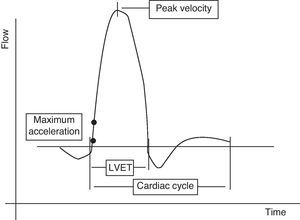
Doppler ultrasound (USCOM® system)The USCOM® system is a noninvasive technique that uses Doppler technology to obtain the measurements of stroke volume and its derived parameters. All medical devices based on Doppler ultrasound use a probe that emits ultrasound waves that are reflected from the constantly moving red blood cells (they either move closer to or further from the transducer), thereby obtaining a measure of flow. When the emitted wave comes into contact with the red cell, the wave that is reflected towards the transducer changes its original frequency according to the direction of blood flow. When the transducer is aligned with the blood flow, we record a maximum optimum velocity or frequency. In the case of the USCOM®, the probe is positioned at the suprasternal, supraclavicular or parasternal notch, seeking the maximum blood flows at the level of the aortic and pulmonary valve outflow tracts, respectively. The areas of the outflow tracts are estimated from an anthropometric algorithm. Based on these velocities and areas, we can obtain the measurements of stroke volume, cardiac output, cardiac index and vascular resistances (Figs. 1 and 2).
The main advantages of this method are those common to all ultrasound systems. In effect, the technique is totally noninvasive, and the compact size of the device makes it easier to use at the patient bedside. Learning to use the system is rapid, and no calibration is needed.
On the other hand, the USCOM® system is observer-dependent and does not afford information on a continuous basis. The acoustic window is also a limiting factor in the use of the device, despite the existence of a number of possible accesses (suprasternal, supraclavicular and parasternal) that help minimize this limitation. The use of the technique is not yet widespread, due to the lack of validation studies. Most of the existing studies have been carried out in surgical patients or following heart surgery, comparing the USCOM® device with the pulmonary artery catheter–the results varying greatly. Tom et al.50 compared 250 measurements obtained simultaneously in 89 patients, with the observation of a poor correlation between the two systems. The mean difference was 0.09L/min, but with a confidence interval of between 2.83 and −3.01L/min. Previously, Chand et al.,51 in the same type of patients (n=50), had recorded an excellent correlation, with a mean difference of 0.03L/min and with a confidence interval of between −0.19 and 0.13m/minm. A recent study, published by Horster et al.,52 has compared the cardiac output measurements obtained with the PiCCO and USCOM® systems in septic patients, and describes a good correlation between the USCOM® and the reference technique based on thermodilution (PiCCO). Seventy measurements in 70 patients showed a mean difference of −0.36L/min, with a confidence interval of ±0.99L/min.
Further research is needed to fully validate and implement this system at the patient bedside in the ICU.
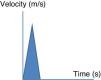
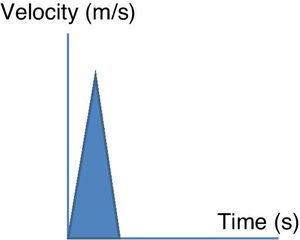
Esophageal Doppler ultrasoundEsophageal Doppler ultrasound began to be used in the 1990s in critical patients, with the purpose of allowing precise, rapid, continuous and especially minimally invasive hemodynamic monitorization, with the measurement of CO and other parameters of established clinical usefulness–thereby affording a sufficiently comprehensive view of the hemodynamic condition of the patient.53 Briefly, the device consists of a D-shaped Doppler probe that continuously emits ultrasound waves at a fixed frequency (generally 4–5MHz), and is positioned in the esophagus (via the nasal or oral route) with an inclination of 45° with respect to the explored blood vessel (in this case the descending aorta). The ultrasound waves are reflected from the circulating red blood cells and are again detected by the transducer. The signal received is analyzed, and the monitor displays the corresponding wave velocity–time tracings. The area under the velocity–time tracing is the systolic distance, i.e., the distance traveled by a blood column through the aorta with each contraction of the left ventricle. The product of the systolic distance and the cross-sectional area of the aorta at that point allow us to obtain the stroke volume.
The different esophageal Doppler monitors available on the market use a variety of principles. As an example, the CardioQ® (Deltex Medical, Chichester, West Sussex, UK) employs a normogram referred to patient age, weight and height to estimate the total stroke volume of the left ventricle from the flow measured in the descending aorta.
In addition to SV and CO, this technique affords particularly interesting information on the cardiovascular condition of the patient (preload, contractility and afterload), drawn from the analysis of the velocity–time curves (Fig. 3).
Although few studies have been made, the existing evidence in the medical literature suggests good reliability of the CO measurements obtained by esophageal Doppler compared with the classical thermodilution measurements. There is a large body of scientific evidence, supported by numerous randomized prospective studies, demonstrating the usefulness of esophageal Doppler in the preoperative optimization of volemia in the high risk surgical patient54–56–with clear improvement in the prognosis of these patients (shorter hospital stay and fewer postoperative complications). In the critical patient, esophageal Doppler has been postulated as an interesting monitorization tool, in view of the characteristics already commented above. Its use has been described in the monitorization of patients during lung recruitment maneuvering in acute respiratory distress syndrome,57 for the hemodynamic management of potential organ donors,58 and even for optimization of the pacemaker pacing mode in patients with cardiogenic shock.45 More recently, esophageal Doppler has been proposed as a tool for evaluating volume response in the cyclic changes induced by mechanical ventilation and with passive leg elevation maneuvering.59,60
Table 1 describes the different techniques available and the systems most commonly used in critical patients for estimating CO, with the advantages and limitations of each of them. Additional hemodynamic variables afforded by the different systems are also specified.
Techniques available for estimating cardiac output (CO) in the critical patient.
| System | Advantages | Disadvantages | Additional variables | |
| Static | Dynamic | |||
| Pulmonary artery catheter | Fully validated technique | Invasive system | CVP | |
| Information on flow variables (gold standard for CO), intrathoracic pressures and tissue perfusion (SvO2). | Less invasive alternatives for most data provided | PAP | ||
| Allows calculation of oximetric variables (DO2, VO2) | Lack of knowledge in interpreting the data | PCWP | ||
| Increased incidence of complications | ||||
| A) PiCCO® | Continuous information on multiple variables. | Invasive | CVP | SVV |
| Measurement of volumes | Requires larger caliber vascular accesses | GEDV | PPV | |
| Measures of lung edema and permeability | Requires recalibration in situations of instability | EVLW | ||
| Allows finer hemodynamic management | PVPI | |||
| GEF | ||||
| B) LiDCO® | Scantly invasive | Invasive system | ITBV | SVV |
| Any arterial or venous line | Interference of lithium salts and non-depolarizing muscle relaxants | PPV | ||
| Validated technique | ||||
| Continuous information on multiple variables | ||||
| C) FloTrac® | Continuous | Requires validation in patients with diminished systemic vascular resistance | SVV | |
| Requires no external calibration | Not validated in patients with ventricular assist devices or intraaortic counterpulsation balloon | |||
| Minimally invasive | Aortic insufficiency | |||
| Peripheral vascular access | ||||
| D) Volume View® | Continuous information on multiple variables | Few validation studies to date | EVLW | SVV |
| Measurement of volumes | PVPI | |||
| Measurement of lung edema and permeability | GEDV | |||
| GEF | ||||
| E) Most care® | Requires no manual calibration | Few validation studies to date | SVV | |
| Monitors cardiac cycle efficiency (CCE) | PPV | |||
| F) Nexfin® | Noninvasive (requires no arterial or venous access) | Scantly validated | ||
| Bioimpedance (BET) | Noninvasive | Scantly validated | ||
| Limited in critical patients, major surgery and chest alterations | ||||
| Susceptible to environmental changes (noise), patient movements and electrode placement | ||||
| Bioreactance NICOM® | Noninvasive | Erroneous values in presence of: | SVV | |
| Lesser operative costs and capacitation requirements | External and internal pacemakers, left ventricle assist devices (LVADs) | |||
| Good signal-to-noise ratio | Severe pulmonary hypertension | |||
| No variability in relation to body changes or electrode positioning | Severe aortic or tricuspid insufficiency and thoracic aortic alterations | |||
| Continuous, real time data | Intracardiac shunts | |||
| A) Esophageal Doppler | Minimally invasive | Habitual use (not exclusive) in patients on mechanical ventilation | ||
| Rapid placement and application of the technique | Few studies available in non-surgical critical patients | |||
| Continuous monitorization | Operator-dependent | |||
| Rapid learning curve | ||||
| B) USCOM® | Noninvasive | Not continuous | ||
| Rapid learning curve | Operator- and acoustic window-dependent | |||
| Requires no calibration | Not valid in severe valve disease | |||
| Rapid start of measurements | Pending validation studies | |||
| Partial CO2 re-inhalation (NICO®) | Minimally invasive | Not continuous | ||
| Simple | Requires intubated patient | |||
| Frequently repeatable | Artifacts with intrapulmonary shunt ↑ | |||
As we have seen, different monitorization tools can be used to help clinicians to evaluate a diagnosis and to guide them in different treatment strategies, particularly referred to the management of fluids and drugs. In turn, these instruments can improve the results obtained in terms of fewer complications, lesser time on mechanical ventilation, etc.–thereby improving morbidity-mortality and hospital stay.
Each system has its advantages and inconveniences. The choice of one monitorization technique or other is influenced by different factors, fundamentally the characteristics of the system, patient-related parameters, and factors related to the clinician using the system. Aspects referred to the monitoring system that must be taken into consideration are its availability, the setting in which it is to be used, and the cost of the device. In turn, aspects referred to the clinician are knowledge of the technique, operator experience, ease of use and interpretation of the results, and dependency or not upon the operator. In many cases the time factor leads to the choice of less invasive techniques that can be applied on an immediate basis. General considerations, in relation to the characteristics of the patient, include particularly the fact that the more serious the patient condition, the greater the required precision of the hemodynamic parameters obtained. At least for the time being, this requirement in the context of continuous monitorization is satisfied by the invasive techniques. The systems which we have described as being less invasive, without the need for central venous catheterization, could be more useful in the Emergency Department or in certain hospital areas for the initial management of patients, evaluation of their clinical course, and for deciding whether admission to the ICU is indicated or not. Likewise, they may prove useful in those patients in which admission to critical care is not considered necessary, in view of the underlying disease process, but who nevertheless need to be treated and stabilized. In other cases we resort to more invasive techniques, chosen according to the patient disease involved.
Thus, the PAC and measurements of PCWP appear to remain more useful in patients with different types of heart failure, in cardiogenic shock, or in patients with pulmonary hypertension. Provided operator skill is guaranteed, echocardiography and esophageal Doppler can be regarded as methods of choice during the evolution of the patients, and in those cases in which the implantation of an intracardiac catheter is contraindicated.
The transpulmonary dilution techniques that determine intrathoracic volumes (global end-diastolic volume and extravascular lung water) can be regarded as options of choice in guiding fluid management, improving lung function, and in reducing the time on mechanical ventilation in acute respiratory failure and acute respiratory distress syndrome.
In patients with severe sepsis and septic shock, it appears more advisable to use systems that obtain CO from analysis of arterial pulse wave morphology. These systems offer information on the phase of shock in which the patient is found, though frequent calibration is needed in the initial stages, due to the alterations in vascular tone.
The systems and parameters used to assess the response to fluid therapy include the cardiac volumes derived from transpulmonary dilution techniques (PPV and SVV are the parameters that can guide evaluation of the response to volume expansion), esophageal Doppler flow velocity, and the echocardiographic indices, as well as dynamic indices obtained from the methods based on the analysis of pulse wave morphology. The greatest controversy in recent years refers to volume expansion in the early phases of shock. Although central venous pressure is still used in daily practice for volume management, volumetric parameters are considered to be more useful than filling pressures, since they are not altered by the respiratory cycle. Likewise, the dynamic indices obtained from analysis of the pulse wave have limitations that should be taken into account when monitoring the patient, since they are only applicable in controlled mechanical ventilation and with a regular heart rhythm of normal frequency.61
Independently of the process involved, of the device employed, and of the variables used to guide our intervention, it must be remembered that our objective is to improve tissue perfusion, i.e., to restore physiological values referred to oxygen transport-consumption, through the assessment of lactate concentrations and SvO2/SvcO2.62
ConclusionsThe ultimate purpose of hemodynamic monitorization is reducing mortality in the critically ill patient. At present, we have a range of more or less invasive techniques that can be used to monitor different hemodynamic parameters. The choice of one device or other should be determined by the following factors: the experience of the operator in performing the technique, facility of use and interpretation of the results, the precision of the system, and its cost-effectiveness.
The setting in which the system is used, the seriousness of the patient condition, and the pursued objectives (both diagnostic and therapeutic) help choose among the systems and methods described in this review. In order for physicians to more effectively use any one of these devices, they must understand its functioning, its advantages and inconveniences, the scenario best suited to each system, and of course they must be able to interpret the data obtained.
It can be concluded that monitorization of the critical patient must be global, with multiparametric monitorization combining the hemodynamic parameters described in this review and the metabolic data referred to oxygen transport and consumption, with the purpose of optimizing tissue perfusion and improving survival of the critically ill patient.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Mateu Campos ML, et al. Técnicas disponibles de monitorización hemodinámica. Ventajas y limitaciones. Med Intensiva. 2012;36:434–44.