In recent years, technological improvements have reduced the complexity of extracorporeal membrane oxygenation devices. This has enabled the development of specific devices for the extracorporeal removal of CO2. These devices have a simpler configuration than extracorporeal membrane oxygenation devices and uses lower blood flows which could reduce the potential complications. Experimental studies have demonstrated the feasibility, efficacy and safety of extracorporeal removal of CO2 and some of its effects in humans.
This technique was initially conceived as an adjunct therapy in patients with severe acute respiratory distress syndrome, as a tool to optimize protective ventilation. More recently, the use of this technique has allowed the emergence of a relatively new concept called “TRA-protective ventilation” whose effects are still to be determined. In addition, the extracorporeal removal of CO2 has been used in patients with exacerbated hypercapnic respiratory failure with promising results.
In this review we will describe the physiological and technical fundamentals of this therapy and its variants as well as an overview of the available clinical evidence, focused on its current potential.
Recientemente las mejoras tecnológicas han permitido reducir la complejidad de los dispositivos de oxigenación por membrana extracorpórea, dando paso al desarrollo de dispositivos específicos para la eliminación extracorpórea de CO2. Estos dispositivos tienen un montaje más simple y utilizan flujos sanguíneos más bajos, lo que potencialmente disminuye las complicaciones vasculares y hemodinámicas. Estudios experimentales han demostrado la factibilidad, eficacia y seguridad de la eliminación extracorpórea de CO2 y algunos de sus efectos en humanos.
Esta técnica, que fue concebida como un tratamiento complementario en los pacientes con SDRA grave, permite la optimización de la ventilación protectora e incluso ha abierto el camino a nuevos conceptos, como lo que se ha denominado ventilación «ultraprotectora»”, cuyos beneficios aún están por determinarse. Además, la eliminación extracorpórea de CO2 se está implementando en pacientes con insuficiencia respiratoria hipercápnica agudizada con resultados prometedores.
En esta revisión describiremos los fundamentos fisiológicos y técnicos de esta terapia y sus distintas variantes, así como la evidencia clínica disponible hasta la fecha, enfocados en su potencial en el paciente con insuficiencia respiratoria.
In recent years, different strategies and technological improvements have made it possible to reduce the size and complexity of extracorporeal membrane oxygenation (ECMO) devices–thereby allowing a gradual increase in the use and safety of these systems.1,2
In concordance with these advances, and with the aim of contributing to this simplification process, Gattinoni and Kolobow were the pioneers in describing the need to dissociate oxygenation support from exclusive ventilation extracorporeal support, with the purpose of optimizing lung protection during mechanical ventilation. This gave rise to the extracorporeal CO2 removal (ECCO2R) devices,3 which extract CO2 from venous blood by passing it through a membrane similar to that used in ECMO. The main difference is that the blood flow rates used in this case are much lower, and the arterial and venous cannulas are therefore smaller.4 These systems were initially conceived mainly for patients with severe acute respiratory distress syndrome (ARDS), where the protective ventilation strategies produce important hypercapnia.5 More recently, however, the technique has also been used in patients with exacerbated chronic obstructive pulmonary disease (COPD).6
In ARDS, the strategy of the ARDSNet of using a low tidal volume (Vt) (6–8ml/kg ideal body weight) to reduce lung distension, and a high PEEP to improve oxygenation, resulted in a very important decrease in mortality.7 In addition, hyperinsufflation and alveolar opening and closure intrinsically lead to a condition known as ventilator-induced lung injury, which is minimized by using this strategy.8 In a post hoc analysis of the study of the ARDSNet, both the patients receiving low Vt and those receiving high Vt were seen to benefit from a plateau pressure (Pplat) of <30cmH2O, thus evidencing that additional reductions in Vt may be needed in order to maintain Pplat <30cmH2O.9 In this same line, it has been shown that the use of low Vt can prevent the development of ARDS in patients at risk.10 However, despite the described benefits, adherence to the protective ventilation strategy is still not adequate or uniform, and in some cases it may prove insufficient.11
Effects of hypercapniaThe effects of hypercapnia have been extensively studied in animals and have been corroborated in a number of observational clinical studies.12,13 While hypercapnic acidosis may cause vasodilatation in tissues such as the brain, at pulmonary level it causes vasoconstriction, with an increase in mean pulmonary artery pressure. This, added to the effects of positive pressure ventilation, leads to an important increase in right ventricle afterload.14 The pulmonary hypertension induced by hypercapnia can contribute to the appearance of cor pulmonale in patients with ARDS, with an associated increase in mortality.15 Likewise, hypercapnia and acidosis are established risk factors for the appearance of arrhythmias, which further complicate the management of these patients. In other tissues, hypercapnic acidosis can increase gastric secretion and induce a degree of systemic vasodilatation.
In contrast, some studies have found that high CO2 concentrations reduce lung injury through attenuation of the effects of the free radicals and a decrease in the activity of neutrophils16 and other immunological factors, and may even exert protective effects against endotoxin-induced lung damage.17 However, many of these changes might be explained not only by hypercapnia but also by acidosis, and may even be independent–as occurs in the alveolar epithelial monolayers, where hypercapnia with a compensated pH affords no benefit and may even cause damage.18
Different authors have proposed the use of ultra-protective mechanical ventilation (3–4ml/kg ideal body weight) combined with ECCO2R, with the ultimate aim of preventing acute ventilator-induced lung injury. In potential, this strategy would avoid the risks of hypercapnia and would reduce the needs for sedation.19,20
Technical and physiological fundaments of extracorporeal CO2 removalTechnical simplification has caused the development and potential applications of extracorporeal CO2 extraction (ECCO2R) to advance quickly, avoiding some of the initial problems associated to ECMO. In this respect, ECCO2R is theoretically simpler and has fewer logistic and personnel requirements. In fact, in the case of the low-flow devices, the complexity is similar to that of the continuous renal replacement techniques, which are now very widespread.21
The elimination of CO2 currently represents an intermediate step between conventional ventilatory support and total support with ECMO. This is so because the technique is able to replace more than 50% of ventilatory demand, and therefore allows a reduction of the conventional minute ventilation requirements. The membranes allowing gas exchange are generally made of hollow biocompatible material fibers (poly-4-methyl-1-pentene)22 with an exchange surface area ranging from 0.6 to 2.5m2, and in some cases they are coated with heparin or other components designed to improve biocompatibility. The fundamental technical difference with respect to ECMO is the reduced blood flow involved (300–500ml/min), which is enough for eliminating most of the CO2 produced by metabolism–all thanks to the increased solubility and linear kinetics of this gas in plasma.21 The main advantage of using a lower blood flow is that we can use smaller-caliber cannulas, with improved anticoagulation control.
This efficient elimination of CO2 can allow us to safely reduce the mechanical ventilation needs and lower hypercapnia–avoiding its effects upon the central nervous system, the right side of the heart, and other previously described effects.
The cannulas we can use range between 13 and 17Fr in caliber, and are usually placed at the patient bedside using the Seldinger technique. On the other hand, thanks to the cumulative technical experienced gained with ECMO, the blood pumps have also experienced important developments, and we can now use electromagnetic pumps in which heat and mechanical trauma are minimized. Mention also should be made of the availability of “pump-free” devices that use the arteriovenous gradient of the patient to make the blood flow across the CO2 extracting membrane.
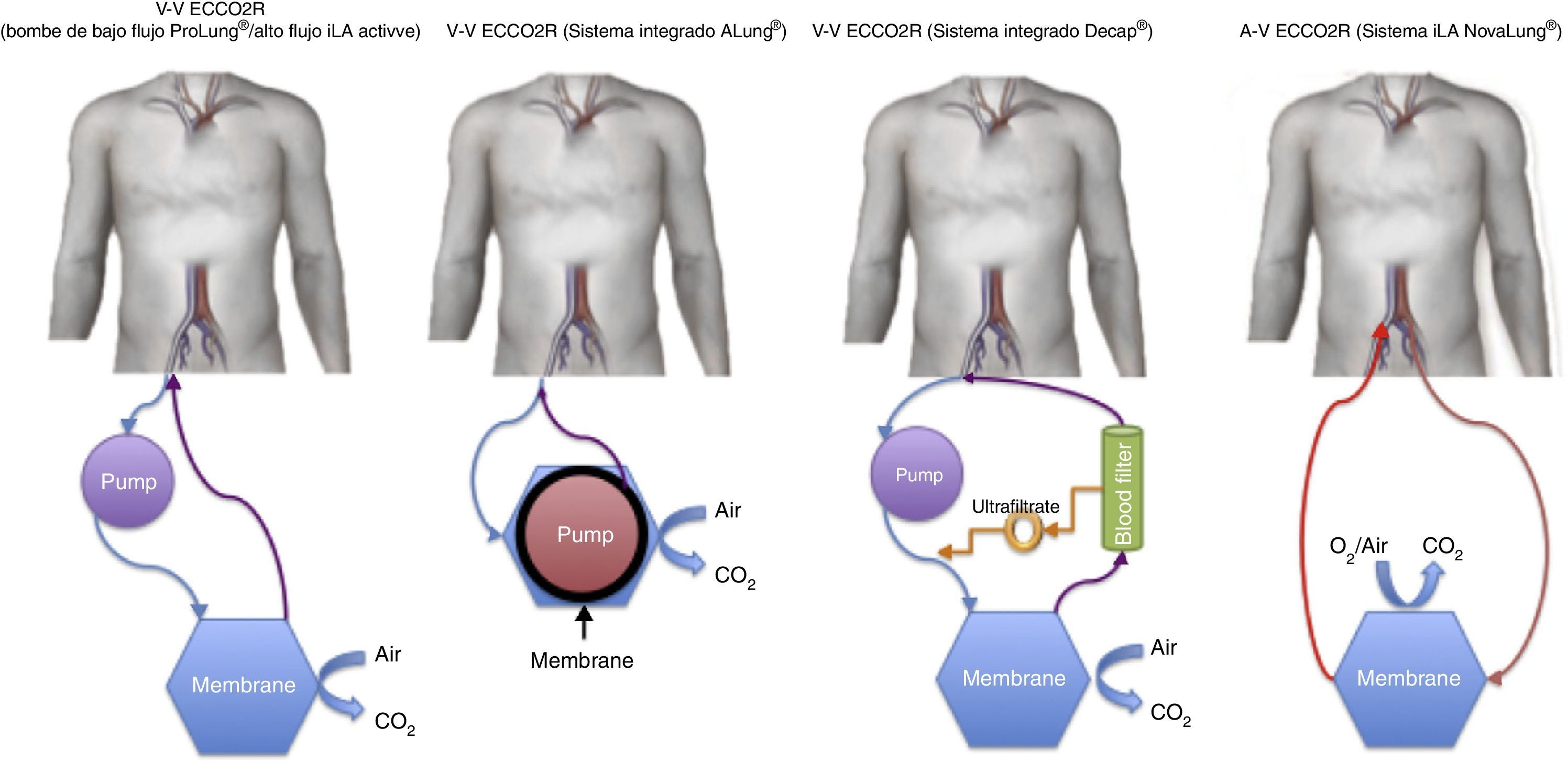
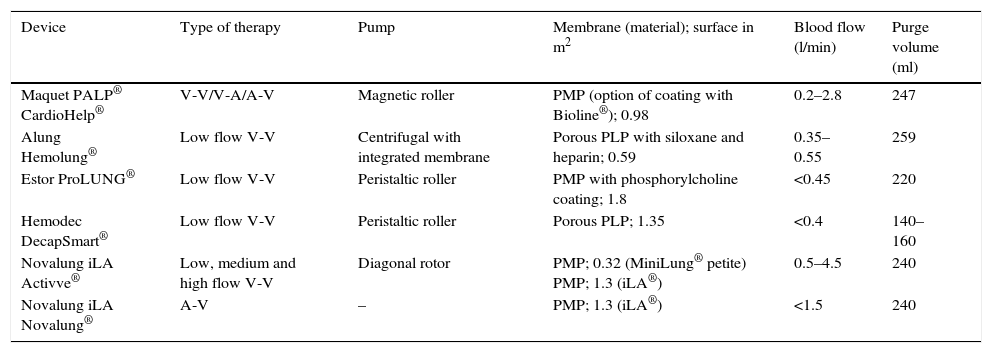
A brief description is provided below of the devices that are currently available (Table 1 and Fig. 1).
Technical characteristics of the different extracorporeal CO2 removal devices.
| Device | Type of therapy | Pump | Membrane (material); surface in m2 | Blood flow (l/min) | Purge volume (ml) |
|---|---|---|---|---|---|
| Maquet PALP® CardioHelp® | V-V/V-A/A-V | Magnetic roller | PMP (option of coating with Bioline®); 0.98 | 0.2–2.8 | 247 |
| Alung Hemolung® | Low flow V-V | Centrifugal with integrated membrane | Porous PLP with siloxane and heparin; 0.59 | 0.35–0.55 | 259 |
| Estor ProLUNG® | Low flow V-V | Peristaltic roller | PMP with phosphorylcholine coating; 1.8 | <0.45 | 220 |
| Hemodec DecapSmart® | Low flow V-V | Peristaltic roller | Porous PLP; 1.35 | <0.4 | 140–160 |
| Novalung iLA Activve® | Low, medium and high flow V-V | Diagonal rotor | PMP; 0.32 (MiniLung® petite) PMP; 1.3 (iLA®) | 0.5–4.5 | 240 |
| Novalung iLA Novalung® | A-V | – | PMP; 1.3 (iLA®) | <1.5 | 240 |
A-V: arteriovenous; PMP: poly-4-methyl-1-pentene; PLP: polypropylene; V-A: venoarterial (extracorporeal membrane oxygenation); V-V: venovenous.
Novalung iLA® (Novalung, Germany) and Affinity® NT (Medtronic, Minneapolis, MN, USA): The elimination of CO2 and partial oxygenation is achieved through the percutaneous or preferably surgical placement of a cannula occupying no more than 70% of the vascular lumen (usually the femoral artery), with another cannula placed in a large-caliber vein. In order to function correctly, the gradient must be ≥60mmHg, and a certain degree of patient hemodynamic stability is therefore required.
Venovenous devicesNovalung iLA Activve® (Novalung, Germany): This system uses the same iLA® membrane described above, but incorporated within a console with a diagonal pump capable of operating over a broad range of flows (0.5–4.5l/min)–thus making it possible to administer the entire spectrum of extracorporeal ventilatory support from low-flow ECCO2R to venovenous ECMO.
DecapSmart® (Hemodec, Salerno, Italy): This is a device fitted with a roller pump that uses an oxygenation membrane and a hemofilter in series. The hemofilter ultrafiltrate is returned to the circulation before the membrane, producing a plasma recirculation effect with the purpose of securing additional elimination of the CO2 dissolved in it. Likewise, the system allows the use of anticoagulation in a way similar to the renal replacement devices.
ProLUNG® (Estor SpA, Pero, Italy): This device is similar to that described above, but does not use a hemofilter in the circuit. Instead, it has a non-porous biocompatible poly-4-methyl-1-pentene membrane with a surface area of 1.8m2, making recirculation unnecessary. It also comes with a monitor that regulates the administered air flow and measures the elimination of CO2 in digital form (ProLUNG Meter®).
Hemolung® (Alung Technologies, Pittsburgh, USA): In contrast to the previous membranes, this system makes use of a cartridge which houses the pump and the membrane. The central core rotates to radially accelerate the blood toward the periphery where the membrane is located. Although the latter has a surface of 0.67m2, its efficacy in eliminating CO2 is similar to that of the devices described above.
Pump-Assisted Lung Protection or PALP® (Maquet, Rastatt, Germany): This is a compact system including the console, pump and membrane in a single small piece of equipment (CardioHelp®)–thus making it very portable. In addition, it allows us to replace the conventional membrane with a membrane designed for complete ECMO, for transfer purposes.
The elimination of CO2 using the different systems is mainly determined by the mean blood flow, and to a lesser extent by the air flow and membrane surface area and contact time. Therefore, correct catheter placement is crucial in order to optimize the treatment, avoid membrane coagulation problems, and maximize treatment efficiency.
Evidence for useDifferent experimental studies have shown ECCO2R to be feasible, effective and safe.23,24 Based on the results of such studies, the use of ECCO2R techniques in ventilation support has been suggested in clinical situations where they may be useful, such as acute respiratory distress syndrome and the exacerbation of chronic obstructive pulmonary disease.
In acute respiratory distress syndromeThe bases for the use of ECCO2R in acute respiratory distress syndrome (ARDS) have largely been extrapolated from studies with ECMO. The studies evaluating the use of ECCO2R in ARDS are heterogeneous and involve different devices, designs and primary outcomes. The different studies evaluate the use of ECCO2R as an adjuvant to protective ventilation, and even in what has been called “ultraprotective” ventilation.
Terragni et al. carried out a small study of 32 patients with less than 72h of ARDS. They selected those patients who despite protective ventilation presented a Pplat between 28 and 30cmH2O, lowering the Vt to 4ml/kg and connecting them to ECCO2R. In the mentioned group it proved possible to reach a Pplat of 25cmH2O with higher PEEP, and a decrease in proinflammatory cytokines was demonstrated in the bronchoalveolar lavage,19 thereby evidencing a biological effect indicating lesser ventilator-induced damage. Adopting a similar approach, the randomized Xtravent study20 compared protective ventilation (6ml/kg ideal body weight) versus ultraprotective ventilation (3ml/kg ideal body weight)+ECCO2R. In a post hoc analysis of the subgroup of patients with PaO2/FiO2 <150mmHg, treatment with ultraprotective ventilation+ECCO2R was found to significantly reduce the days without mechanical ventilation. Complications in the form of bleeding or local problems with the vascular cannulas were recorded in 7.5% of the patients treated with ECCO2R. In this case the arteriovenous technique was used.
ECCO2R integrated in a dialysis circuit demonstrated a decrease in the use of vasopressors and improvement of acidosis in patients with renal and respiratory failure mostly due to viral pneumonia.25
Lastly, a recent systematic review26 including 14 studies (two randomized controlled trials and 12 observational studies) found no global differences in mortality, stay in the UCI or days without mechanical ventilation, except in the subgroup corresponding to the more seriously ill patients. In relation to safety, a progressive decrease in the number of complications was observed. In the case of the arteriovenous techniques, the most frequent complication was ischemia of the extremity in which the cannula was placed–the problem being serious in 6 cases (5 compartmental syndromes and one amputation). In the venovenous techniques, the most frequent complication was coagulation of the membrane/circuit of the device. In both cases the patients assigned to ECCO2R had greater transfusion needs.
A number of studies are currently underway to determine the clinical usefulness of the ECCO2R devices combined with ultraprotective ventilation (such as the SUPERNOVA trial, endorsed by the European Society of Intensive Care Medicine). However, the evidence available to date is inconclusive, and the use of this treatment modality in ARDS must be individualized, based on physiological and clinical criteria.
In exacerbated chronic obstructive pulmonary diseaseExacerbations of chronic obstructive pulmonary disease (COPD) are the leading cause of hospital admission in these patients, and can manifest on different occasions in a short period of time (winter season). Although most such episodes can be treated on a medical basis, in some cases both noninvasive ventilatory support (NIMV) and invasive ventilatory support may prove necessary, depending on the severity of the condition and the predominant symptoms. Thus, in a fraction of these patients, despite the absence of major changes in oxygenation or in the symptoms, the exacerbation of COPD may sometimes lead to CO2 retention–with the inevitable consequences this has at central nervous system level. Furthermore, each exacerbation constitutes a risk factor for further exacerbations, with an increase in mortality.27 The mortality rate among patients with exacerbations of COPD is about 4.8% in individuals subjected NIMV, according to Lindenauer et al.,28 and 29.3% in those in which NIMV fails and conversion to invasive ventilation is required.29 Despite the advances in the application and refinement of the protocols regarding the use of NIMV, an important proportion of patients (19–40%) continue to fail and require intubation.30
The first clinical study on the efficacy and safety of ECCO2R in patients with hypercapnic respiratory failure was published in 2012.31 This was a retrospective, multicenter study in which 21 patients with exacerbated hypercapnia received treatment with arteriovenous ECCO2R before NIMV failure, defined as the need for intubation. This group of patients was then compared with a retrospective cohort of individuals requiring invasive ventilation. Ninety percent of the patients in the intervention group did not require invasive ventilation. However, no differences in mortality were demonstrated. As regards the complications, two cases of major bleeding and 7 cases of minor bleeding were reported in the course of treatment, as well as one femoral pseudoaneurysm and one case of heparin-induced thrombocytopenia.
Burki et al.6 conducted a pilot study involving venovenous ECCO2R and, as in the previous study, used the technique in a group of patients with hypercapnia and with an important risk of requiring intubation. Furthermore, in another arm of the study they included patients who had suffered two failed NIMV weaning attempts and who rejected intubation, while a third group was subjected to invasive ventilation, with the evaluation of ECCO2R in those individuals who could not be weaned from ventilation. Intubations and invasive ventilation were avoided in both the first and the second group. In the third group the authors recorded a decrease in dyspnea and in the degree of ventilatory support, allowing satisfactory weaning of three of 11 patients.
More recently, Del Sorbo et al.32 conducted a similar clinical (paired cohort) study in which the intervention group was subjected to NIMV plus venovenous ECCO2R. The risk of intubation was seen to increase three-fold in the patients with NIMV alone compared to those patients in which NIMV was combined with ECCO2R. The observed difference was not significant, however. The most frequent complication associated to ECCO2R was coagulation of the circuit, as has been reported by other authors.
Other indicationsThere is clinical experience with the use of ECCO2R in patients with bronchopleural fistula, primary graft dysfunction in lung transplantation, intracranial hypertension and hypercapnia, as well as in other clinical situations in which the physiopathology suggests to a potential beneficial effect of ECCO2R. However, such uses are merely anecdotal, with a lack of sufficient cumulative clinical experience.
ConclusionsThe technical advances in recent years have allowed ECCO2R to become simple and feasible, and it now represents a further option for the support of patients with serious respiratory diseases and high mortality. As a treatment option, ECCO2R is positioned between conventional ventilatory support and total respiratory support (ECMO). We therefore feel that its place in clinical practice could be the population of patients with ARDS presenting PaO2/FiO2 >80 and <150mmHg, in which treatment and conventional ventilation support have been maximally optimized, and in which it is considered advisable to minimize pulmonary distension and/or attenuate the effects of hypercapnia and acidosis–since ECCO2R has been found to be effective in this setting. Likewise, in patients with exacerbated COPD without indications of intubation or in which NIMV is contraindicated, ECCO2R represents a new management option that should be considered on an individualized basis, in coherence with the risk/benefit ratio.
Due to their lower complications rate, we consider the venovenous techniques to be the best option. However, ECCO2R is neither a definitive solution nor a perfect device, and its effects upon other clinical indicators remain to be determined.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Romay E, Ferrer R. Eliminación extracorpórea de CO2: fundamentos fisiológicos y técnicos y principales indicaciones. Med Intensiva. 2016;40:33–38.