Cardiopulmonary support with extracorporeal membrane oxygenation (ECMO) support is a more and more common practice of Intensive Care Units (ICU) these days.1 According to the ELSO (Extracorporeal Life Support Organization) registry conducted back in 2016, ECMO support was used in more than 2000 adult patients with cardiac conditions and with an average survival of 42%.2 Since ECMO support se is implemented when all the other usual therapeutic approaches have been tried, having this level of cardiopulmonary support available is a very important leap forward in the management of patients with refractory cardiogenic shock.
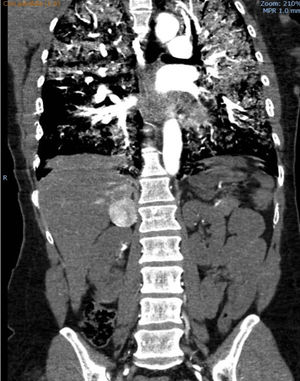
We hereby present the case of a 41-year-old woman without any prior significant clinical history who presented to the ER with clinical manifestations of general discomfort, headache and palpitations of 24-h duration. Upon arrival, she was having a hypertensive emergency (180/100), hypoperfusion with hyperlactacidemia and hypoxemic respiratory failure. The thoraco-abdominal CT scan conducted confirmed the presence of bilateral alveolar occupation and one 4cm-hyperuptaking lesion in the arterial phase in her right suprarenal gland (Fig. 1). The echocardiogram showed the presence of severe left ventricular (LV) hypokinesia that spared the apical segments and one ejection fraction (EF) of 17% (video 1).
Being the situation cardiogenic shock and multi-organ dysfunction with need for invasive mechanical ventilation and support with noradrenaline and dobutamine at high doses, the patient was transferred to our center, where axillary–femoral veno-arterial (VA) ECMO support was provided immediately. This improved multi-organ dysfunction, the amines where completely removed, and the patient showed a tendency toward arterial hypertension. The catecholamines in urine test was conducted (metanephrine and normetanephrine) that confirmed levels 10–20 times higher than the value of reference; also, the CT scan was reviewed and confirmed the diagnosis of pheochromocytoma (PHC).
After six (6) days of ECMO support and clinical improvement, the severe ventricular dysfunction was still there. Since ventricular device support weaning was not an option, the PHC surgical approach was decided, through multidisciplinary approach, without ECMO removal.
Prior to the intervention, only conditioning therapy during 4 days with phenoxybenzamin and esmolol was possible and a slight improvement of ventricular function was observed (LVEF 25%). The surgery conducted was one right suprarenalectomy procedure but while the tumor was being manipulated, the patient suffered from a new adrenergic crisis that worsened the LVEF (15%) and after the resection of the tumor, she suffered a new episode of serious hypotension that required complete ECMO support in order to keep optimal perfusion. During the following days, the patient's left ventricular function improved and ECMO support was withdrawn twelve (12) days later.
As associated complications, the patient suffered heparin-induced thrombopenia that lead to argatroban anticoagulant therapy during the ECMO support therapy; respiratory infection due to Escherichia coli and Aspergillus spp. that resolved with targeted antimicrobial therapy; and serious tetraparesis with motor-axonal damage that slowly recovered with physical therapy. Forty days after hospital admission, the patient was transferred to her floor, and forty-nine days later she was discharged from the hospital. The echocardiographic study conducted looked normal and the anatomopathological study conducted confirmed the diagnosis of PHC.
Stress cardiomyopathy (SC), also known as Tako-Tsubo syndrome, has widely been reported in the medical literature; its onset is usually an acute one imitating an acute coronary syndrome (ACS) and is usually associated with emotional stress.3 For its diagnosis in the echocardiography, segmental alterations of its contractility beyond the territory of a coronary artery can be found, making it necessary to rule out the presence of coronary disease, myocarditis or PHC.4 It usually has good prognosis, only 10% of the patients develop cardiogenic shock and less than 5% die from it.5
The onset of PHC as a SC is a rare entity in the medical literature, even more rare with an inverted Tako-Tsubo syndrome.6,7 If we take a look at the cases reported so far, we will see that PHC-induced SC is more common among young patients, is not usually related to stress factors as factors triggering this condition, and is associated with much more complications (cardiogenic shock, arrhythmias and even cardiac arrest).6
The use of ECMO as a life support measure in patients with PHC-induced cardiogenic shock is scarce and, often, only based on case series.8,9 In the multicenter registry conducted in France by Sauneuf et al.10 from January 2000 through December 2015, 34 cases were reported, out of which only 14 (41%) required VA-ECMO support for hemodynamic stabilization. These patients were in a more serious condition, their hospital stays were longer, and they required more support therapy (amines and mechanical ventilation), and in up to five (5) cases complications associated with ECMO support were reported. Yet despite all this, 75% of the patients survived and all of them recovered their ventricular function without the need for a transplant or any other ventricular support.10
In this group of patients, it is recommended that PHC surgery is postponed for a few weeks, once prior conditioning therapy has been implemented correctly, being exeresis with ECMO mechanical circulatory support exceptional. In our case, an urgent surgical approach was decided given the slight improvement of cardiac dysfunction and the complications occurred during ECMO that would not allow an optimal pharmacological protection. The surgery with ECMO controlled the hemodynamic alterations anticipated with the manipulation of the tumor, both in the catecholaminergic discharge and in the vasoplegia-induced profound posterior shock and new profound decline of myocardial contractility.
The clinical course of arterial hypertension and systolic dysfunction, and the atypical echocardiographic presentation lead us to review the findings of the CT scan that was the diagnostic modality of PHC. The catecholamines in urine test usually takes a few days or even weeks, which is an inviable delay in extreme cases like ours.
In patients with PHC-induced SC, the use of ECMO support has some risks too, it is a measure of optimal circulatory support that allows myocardial and organ recovery and helps hemodynamically during tumor resection.
Conflicts of interestThe authors declare no conflicts of interests associated with this article whatsoever.
Please cite this article as: Martin-Villen L, Corcia-Palomo Y, Escalona-Rodriguez S, Roldan-Reina A, Acosta-Delgado D, Martin-Bermudez R. Soporte con membrana de oxigenación extracorpórea en paciente con miocardiopatía de estrés secundaria a feocromocitoma. Med Intensiva. 2018;42:566–568.