The present study explores the possible factors related to severe cases of pandemic flu.
DesignA retrospective cohort study was conducted in patients hospitalized with Influenza A/H1N1 2009 during the pandemic period.
SettingRamon y Cajal University Hospital (Madrid, Spain).
PatientsAll hospitalized patients with positive RT-PCR (real-time polymerase chain reaction) for Influenza A/H1N1 2009 virus.
Main variablesThe main variables collected were: history of risk factors for severe Influenza, history of immunization, clinical presentation, laboratory tests, chest X-ray report, administration of antiviral treatment, and hospital stay.
ResultsThe median age of the 100 cases was 38 years (range 4 months to 80 years). Seventy-seven percent of the patients had at least one risk factor. Asthma was the most common factor among patients younger than 18 years, versus smoking in the older subjects. Antiviral therapy was initiated a median time of three days (range 0–18 days) after the onset of illness. Nineteen percent of the patients were admitted to Intensive Care, and 2% died. Metabolic disease and abnormal chest X-ray findings were factors associated to admission to the ICU.
ConclusionAs in other studies, abnormal chest X-ray findings upon admission and metabolic disease were related to poor outcomes of 2009 pandemic Influenza A (H1N1) infection in our patients.
Determinar los factores de riesgo para ingreso en la unidad de cuidados intensivos (UCI) en pacientes con infección por virus pandémico (H1N1) 2009.
DiseñoEstudio de cohorte retrospectivo en pacientes ingresados por Influenza A/H1N1 2009 durante el periodo pandémico.
ÁmbitoHospital Universitario Ramón y Cajal.
PacientesTodos los pacientes ingresados con reacción en cadena de la polimerasa en transcripción inversa (RT-PCR) positiva para virus de Influenza A/H1N1 2009.
Variables de interésHistoria de factores de riesgo para Influenza grave, vacunación para Influenza estacional 2008–2009, síntomas y signos clínicos, pruebas de laboratorio, hallazgos en la radiografía de tórax, tiempo en la administración de antiviral y estancia hospitalaria.
ResultadosLa mediana de edad de 100 casos fue 38 años (mínimo: 4 meses, máximo: 80 años). El 77% tuvo al menos un factor de riesgo, siendo el asma la comorbilidad más frecuente en los menores de 18 años y el hábito tabáquico en los mayores. La mediana de tiempo entre el comienzo de los síntomas y el inicio de antiviral fue 3 días (mínimo: 0 días, máximo: 18 días). El 19% de los pacientes fueron ingresados en UCI y el 2% fallecieron por gripe. En el análisis multivariable, enfermedad metabólica y presencia de infiltrados en la radiografía de tórax se asociaron de forma significativa a ingreso en la UCI.
ConclusiónUna radiografía de tórax anormal en el momento del ingreso, junto con la presencia de ciertas comorbilidades, especialmente enfermedades metabólicas, sugieren la posibilidad de peor pronóstico de gripe pandémica (H1N1) 2009.
Between late March and early April 2009, the Mexican health authorities detected an increase in the number of influenza cases three times greater than that recorded in the same period of 2008.1,2 On April 17, the United States Centers for Disease Control and Prevention (CDC) confirmed the first cases of human infection caused by a new influenza viral strain typified as A/H1N1, in two pediatric patients living in California.3 In Spain, circulation of the new influenza virus was first detected in the week between 24 and 30 May 2009, by the Sentinel Physicians Network of the Community of Madrid, and two weeks later by the Sentinel Physicians Network of Navarre – followed by spread of the infection to the rest of the country.4 On 11 June, the World Health Organization (WHO) raised its pandemic alert to the highest level, declaring phase 6 or pandemic phase, indicative of extensive community spread on at least two continents.5
In most cases, influenza A/H1N1 2009 infection produced mild symptoms, with higher incidence rates among children and young adults, and exhibiting a mortality rate of less than 0.6%.6–12 However, the fatalities were recorded mainly in a population younger than that affected by seasonal flu.13,14 In the United States and the United Kingdom, hospitalization due to pandemic flu was more frequent among individuals under 18 years of age – particularly infants under one year of age – and less common among people over age 65 years. Over one-half of the hospitalized patients suffered some background disorder, and between 13 and 44% had been admitted to the Intensive Care Unit (ICU).15–17
Different studies carried out in Spain and in other countries have identified the factors associated to mortality risk in cases of infection due to pandemic flu. In this context, obesity, cancer, immune deficiencies and chronic respiratory illnesses appear as the main risk factors in children and hospitalized adults.15,18,19 Other authors have also reported an association between these factors and the risk of admission to Intensive Care.16,20,21 In Spain, several series involving patients admitted to the ICU have shown that certain populations – such as obese individuals and subjects with chronic pulmonary disease – may be at a higher risk of suffering serious illness.22,23
The present study summarizes the clinical findings in patients admitted to Ramón y Cajal University Hospital (Madrid, Spain) for the treatment of influenza A/H1N1 2009 infection, and explores its possible associations to the risk of admission to Intensive Care.
Patients and methodsRamón y Cajal University Hospital is a third-level center with 1090 functioning beds that serves as reference hospital for Healthcare Area IV of the Community of Madrid, with a recruitment population of 592,576 inhabitants.
The study comprises all patients admitted to this hospital with clinical manifestations of flu syndrome (temperature 37.8°C or higher, with cough and sore throat) or acute respiratory infection (sudden onset in under 12h) during at least 24h, with positive reverse transcription polymerase chain reaction (RT-PCR) findings for influenza virus A/H1N1 2009 in pharyngeal exudate, during the pandemic period.
The Department of Preventive Medicine reviewed the case histories of the patients admitted during the pandemic period, using a standardized form covering demographic information, history of risk factors (as determined by the Spanish Ministry of Health), 2008–2009 seasonal influenza vaccination, clinical signs and symptoms, laboratory test findings, chest X-rays, time from symptoms onset to the administration of antiviral medication, and length of hospital stay. A bivariate analysis was performed to compare the clinical characteristics of the patients and the severity of the disease between those individuals not requiring admission to the ICU and those who required admission to the ICU or died as a result of flu. The nonparametric Mann–Whitney U-test was used in the case of the continuous variables, while the chi-squared or Fisher exact test was used in the case of the categorical variables. All factors found by the analysis to yield p<0.10 were in turn considered in a multivariate logistic regression model to determine the associations between the clinical characteristics and the severity of the disease. Statistical significance was assumed for p<0.05, and all analyses were made using the SPSS version 15 statistical package for Microsoft Windows.
ResultsIncidence of hospitalizationThis study describes 100 cases admitted due to flu syndrome or acute respiratory infection.
The global hospitalization rate was 16.8 cases per 100,000 inhabitants. The specific rates by age groups showed patients under 10 years of age to have the highest hospitalization rate attributable to the disease, particularly infants under two years of age, with 69.8 cases per 100,000 inhabitants, while the lowest rate corresponded to patients between 10 and 17 years of age, with 7.5 cases per 100,000 inhabitants.
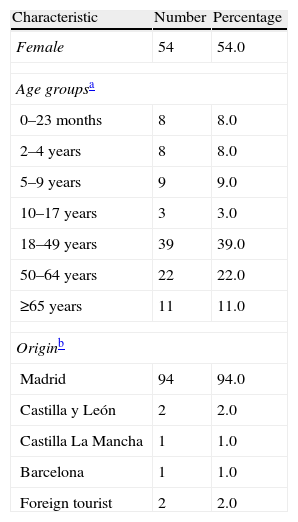
Clinical characteristicsThe 100 cases included in the study were admitted to this hospital between 7 July and 29 December 2009. The median age of the patients was 38 years (minimum: 4 months, maximum: 80 years). Most of the patients resided in the Community of Madrid (94%) (Table 1).
Characteristics of the 100 patients admitted due to A/H1N1 2009 influenza infection in Ramón y Cajal University Hospital (July–December 2009).
| Characteristic | Number | Percentage |
| Female | 54 | 54.0 |
| Age groupsa | ||
| 0–23 months | 8 | 8.0 |
| 2–4 years | 8 | 8.0 |
| 5–9 years | 9 | 9.0 |
| 10–17 years | 3 | 3.0 |
| 18–49 years | 39 | 39.0 |
| 50–64 years | 22 | 22.0 |
| ≥65 years | 11 | 11.0 |
| Originb | ||
| Madrid | 94 | 94.0 |
| Castilla y León | 2 | 2.0 |
| Castilla La Mancha | 1 | 1.0 |
| Barcelona | 1 | 1.0 |
| Foreign tourist | 2 | 2.0 |
The median patient age was 38 years (minimum: 4 months, maximum: 80 years).
Usual place of residence.
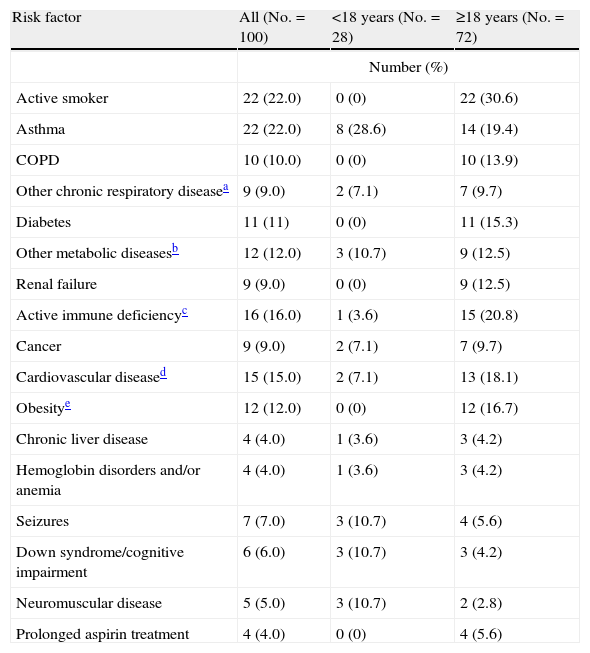
Full information on the initial symptoms of the disease was available for 25 of the 100 patients included in the study (25.0%). Fever and general discomfort were present in over 90% of the cases, followed by cough in 88%, dyspnea in 48%, and symptoms such as sore throat, rhinorrhea and headache, in 20–40% of the cases. The median time from onset of the symptoms to admission was three days (minimum: 0 days, maximum: 17 days). Of the 100 patients, 77 (77%) had at least one risk factor for severe influenza (Table 2), including 60.7% of the children (<18 years) and 83.3% of the adults (≥18 years); in turn, 46% presented at least two factors, and all the patients aged 65 years or older suffered some background disorder. Asthma was the most common disease among the children (28.6%), while the adults showed a predominance of smoking (30.6%). Seizures, cognitive dysfunction and neuromuscular diseases were more common among the younger patients (17.9%) than in the adults (8.3%). None of the patients were pregnant at the time of admission (Table 2).
Risk factors stratified by age.
| Risk factor | All (No.=100) | <18 years (No.=28) | ≥18 years (No.=72) |
| Number (%) | |||
| Active smoker | 22 (22.0) | 0 (0) | 22 (30.6) |
| Asthma | 22 (22.0) | 8 (28.6) | 14 (19.4) |
| COPD | 10 (10.0) | 0 (0) | 10 (13.9) |
| Other chronic respiratory diseasea | 9 (9.0) | 2 (7.1) | 7 (9.7) |
| Diabetes | 11 (11) | 0 (0) | 11 (15.3) |
| Other metabolic diseasesb | 12 (12.0) | 3 (10.7) | 9 (12.5) |
| Renal failure | 9 (9.0) | 0 (0) | 9 (12.5) |
| Active immune deficiencyc | 16 (16.0) | 1 (3.6) | 15 (20.8) |
| Cancer | 9 (9.0) | 2 (7.1) | 7 (9.7) |
| Cardiovascular diseased | 15 (15.0) | 2 (7.1) | 13 (18.1) |
| Obesitye | 12 (12.0) | 0 (0) | 12 (16.7) |
| Chronic liver disease | 4 (4.0) | 1 (3.6) | 3 (4.2) |
| Hemoglobin disorders and/or anemia | 4 (4.0) | 1 (3.6) | 3 (4.2) |
| Seizures | 7 (7.0) | 3 (10.7) | 4 (5.6) |
| Down syndrome/cognitive impairment | 6 (6.0) | 3 (10.7) | 3 (4.2) |
| Neuromuscular disease | 5 (5.0) | 3 (10.7) | 2 (2.8) |
| Prolonged aspirin treatment | 4 (4.0) | 0 (0) | 4 (5.6) |
Tuberculosis, bronchiectasia, obstructive sleep apnea syndrome.
Hyperparathyroidism, hypothyroidism, inappropriate secretion of ADH syndrome, hyperuricemia.
HIV, chemotherapy, corticosteroids, immune disorders.
Excludes systemic arterial hypertension.
BMI≥percentile 95 (<18 years) and BMI≥30 (≥18 years).
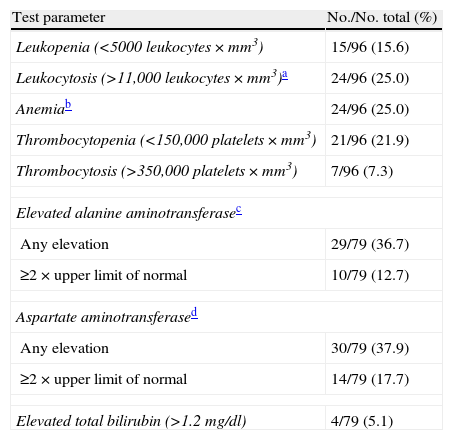
Upon admission, 15 out of 96 patients with information corresponding to the complete blood count (15.6%) presented leukopenia, 24 (25.0%) anemia, and 21 (22.3%) thrombocytopenia (Table 3). Of the 48 patients diagnosed with pneumonia, 11 (22.0%) yielded positive cultures (4 blood cultures and 7 sputum or other respiratory sample cultures). Streptococcus pneumoniae was the most frequent microorganism (36.3%), followed by Pseudomonas aeruginosa (27.2%), and each of the following: coagulase-negative Staphylococcus, Haemophilus influenzae, Escherichia coli and Stenotrophomonas maltophilia, in 9.0% of the samples. In the remaining 37 patients, no microorganisms were isolated from the analyzed samples.
Clinical laboratory test findings.
| Test parameter | No./No. total (%) |
| Leukopenia (<5000 leukocytes×mm3) | 15/96 (15.6) |
| Leukocytosis (>11,000 leukocytes×mm3)a | 24/96 (25.0) |
| Anemiab | 24/96 (25.0) |
| Thrombocytopenia (<150,000 platelets×mm3) | 21/96 (21.9) |
| Thrombocytosis (>350,000 platelets×mm3) | 7/96 (7.3) |
| Elevated alanine aminotransferasec | |
| Any elevation | 29/79 (36.7) |
| ≥2×upper limit of normal | 10/79 (12.7) |
| Aspartate aminotransferased | |
| Any elevation | 30/79 (37.9) |
| ≥2×upper limit of normal | 14/79 (17.7) |
| Elevated total bilirubin (>1.2mg/dl) | 4/79 (5.1) |
Newborn infants <28 days of age were excluded from this analysis.
Anemia was assessed according to hematocrit: ≥19 years (<41% in males and <36% in females), 12–18 years (<36% in boys and <37% in girls), 6–12 years (<35% in both sexes), 2–6 years (<34%), 6 months to 2 years (<33%), 6 months (<31%), 2 months (<28%) and 1 month (<33%).
>30U per liter in >1 year and >54U per liter in <1 year.
>35U per liter in >1 year and >65U per liter in <1 year.
Of the 98 patients with chest X-rays upon admission, 47 (48.0%) showed some infiltration; the median age of these patients was 36 years (minimum: 1 year, maximum: 80 years), and 66.0% had at least one risk factor. The radiological findings included bilateral infiltrates in 22 patients (46.8%) and infiltrates confined to a single lobe in 25 (53.2%).
TreatmentOf the 100 patients, 94 (94.0%) received antiviral treatment. In all cases the medication consisted of oseltamivir, except in two cases (2.0%), where oseltamivir was followed by zanamivir. In 80 patients with information on the starting date of antiviral treatment, the median time from onset of the symptoms to the start of therapy was three days (minimum: 0 days, maximum: 18 days); 36 (45.0%) received the antiviral agent in the first 48h after symptoms onset, 5 (6.3%) received the treatment before admission to hospital, 70 (87.5%) received therapy in the first 48h after admission, and 5 (6.3%) received it more than 48h after admission. The median duration of treatment with oseltamivir was 5 days (minimum: 5 days, maximum: 22 days), versus 36 days for zanamivir (minimum: 6 days, maximum: 67 days).
Analysis of disease severityOf the 100 patients analyzed, 19 were admitted to the ICU and two died of the flu. Seventy-nine percent of those admitted to Intensive Care had at least one risk factor. Ten of these subjects (52.6%) required mechanical ventilation, 7 (36.8%) developed acute respiratory distress syndrome (ARDS), and three (15.8%) had a clinical diagnosis of sepsis. All the patients received antiviral treatment with a dosing regimen that did not differ from that described above.
The median hospital stay of the patients admitted to the ICU was 7 days longer than in the case of the ward patients; this difference proved statistically significant (p<0.001).
The first death due to pandemic flu occurred in a 14-year-old girl with Lennox-Gastaut syndrome who developed septic syndrome with consumption coagulopathy secondary to acute respiratory infection with influenza A/H1N1 2009 and possible bacterial overinfection. The second fatality corresponded to a 60-year-old male without known risk factors for flu complications, admitted to the ICU due to acute respiratory failure secondary to influenza A/H1N1 2009 pneumonia, with severe hypoxia, oliguria and hypotension. Both patients received antiviral treatment upon admission to hospital – the girl within 48h after symptoms onset, and the adult 7 days after symptoms onset.
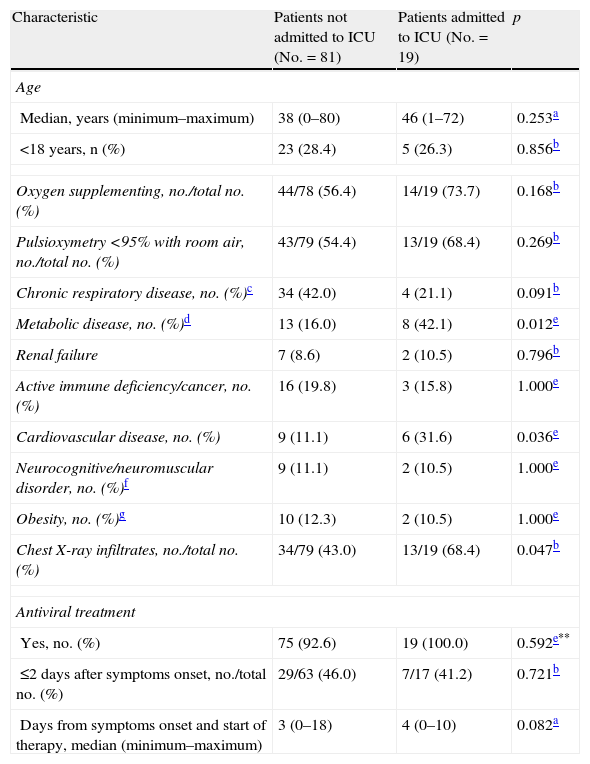
In the bivariate analysis, the patients admitted to the ICU showed a greater probability of suffering metabolic disease, including diabetes, and some infiltration on the chest X-rays upon admission, than those patients not admitted to the ICU (Table 4). In contrast, no difference was observed in the median time from symptoms onset to hospital admission between these patients. Likewise, no differences were observed in relation to vaccination against seasonal influenza 2008–2009. In a multivariate analysis considering modeled upon maximum metabolic disease, the presence of infiltrates in the chest X-rays upon admission, and cardiovascular disease, the first two variables were significantly correlated to admission to the ICU.
Bivariate analysis of the clinical characteristics and risk factors between patients not admitted to the ICU and those admitted to the ICU.
| Characteristic | Patients not admitted to ICU (No.=81) | Patients admitted to ICU (No.=19) | p |
| Age | |||
| Median, years (minimum–maximum) | 38 (0–80) | 46 (1–72) | 0.253a |
| <18 years, n (%) | 23 (28.4) | 5 (26.3) | 0.856b |
| Oxygen supplementing, no./total no. (%) | 44/78 (56.4) | 14/19 (73.7) | 0.168b |
| Pulsioxymetry <95% with room air, no./total no. (%) | 43/79 (54.4) | 13/19 (68.4) | 0.269b |
| Chronic respiratory disease, no. (%)c | 34 (42.0) | 4 (21.1) | 0.091b |
| Metabolic disease, no. (%)d | 13 (16.0) | 8 (42.1) | 0.012e |
| Renal failure | 7 (8.6) | 2 (10.5) | 0.796b |
| Active immune deficiency/cancer, no. (%) | 16 (19.8) | 3 (15.8) | 1.000e |
| Cardiovascular disease, no. (%) | 9 (11.1) | 6 (31.6) | 0.036e |
| Neurocognitive/neuromuscular disorder, no. (%)f | 9 (11.1) | 2 (10.5) | 1.000e |
| Obesity, no. (%)g | 10 (12.3) | 2 (10.5) | 1.000e |
| Chest X-ray infiltrates, no./total no. (%) | 34/79 (43.0) | 13/19 (68.4) | 0.047b |
| Antiviral treatment | |||
| Yes, no. (%) | 75 (92.6) | 19 (100.0) | 0.592e** |
| ≤2 days after symptoms onset, no./total no. (%) | 29/63 (46.0) | 7/17 (41.2) | 0.721b |
| Days from symptoms onset and start of therapy, median (minimum–maximum) | 3 (0–18) | 4 (0–10) | 0.082a |
Mann–Whitney U-test.
Chi-squared test.
Includes asthma, COPD or other chronic respiratory disease.
Includes diabetes.
Fisher exact test.
Includes seizures, Down syndrome or other cognitive dysfunction and neuromuscular disease.
BMI≥percentile 95 (<18 years) and BMI≥30 (≥18 years).
In contrast to seasonal flu epidemics which are associated with higher hospital admission rates among individuals over 65 years of age and infants under two years of age, the incidence of hospitalization due to pandemic flu in this study was greater among subjects under 10 years of age.14 The hospital admission rate in patients over 65 years age was comparable to that of the group with the lowest incidence (10–17 years of age). The estimated rates could underestimate the true hospital admission rates due to pandemic flu, considering that the numerator does not include those patients with flu residing in Healthcare Area IV but admitted to a hospital pertaining to some other healthcare area in the Community of Madrid. The absence of pregnant women in this study is due to the lack of an Obstetrics Department in the hospital – those pregnant patients requiring admission being referred to another reference center.
In the same way as in other published series,17,18,24 the largest proportion of admissions due to pandemic flu in our center involved young individuals between 18 and 49 years of age – those under age 65 representing close to 90% of all admissions. This discrepancy between the incidence of hospitalization and the proportion of admissions by age intervals is explained by the population distribution of Healthcare Area IV, where 47% of the recruitment population is between 18 and 49 years of age, and only 10% is under 10 years of age.
Over 80% of the adults and 60% of the children had some risk factor for severe seasonal influenza. The prevalence of these factors in our series is higher than in other studies, in both adults and children admitted to hospital because of seasonal flu.15,25–27 This difference can be explained by the reasonable tendency among physicians to preferentially decide admission of those patients with risk factors for flu complications. In the same way as in seasonal flu, chronic respiratory disease, chronic obstructive pulmonary disease (COPD) and asthma were globally the most frequent risk factors among the admitted patients.
A review of the literature shows that the early administration of oseltamivir in patients with pandemic (H1N1) 2009 viral infection can shorten hospital stay and reduce the risk of progression to serious illness requiring admission to the ICU or potentially capable of causing death.13 There is evidence that antiviral treatment started within 48h after symptoms onset affords increased benefit.13 In our analysis, the patients admitted to the hospital ward did not show a greater probability of having received oseltamivir in the first 48h of disease onset than those patients admitted to the ICU because of flu.
A body mass index of ≥28kg/m2 been cited as a risk factor for severe pandemic flu.12,15,17–19,22,23 The latter is also associated to other comorbidities that are known risk factors for complicated flu disease, such as diabetes, metabolic disorders, and cardiovascular and pulmonary diseases (obstructive sleep apnea and hypoventilation syndrome and obesity). In this study, the absence of a statistically significant association between obesity and severe disease could be explained by the limited number of event analyzed. However, the results of the multivariate analysis are concordant with those studies that point to metabolic disorders as risk factors for severe influenza.12,28,29 The presence of chest X-ray infiltrates has also been cited as an independent risk factor for severe pandemic flu in the medical literature.17 In our series, 98% of the patients with such radiological findings were diagnosed with pneumonia, and 75% of those admitted to the ICU presented this complication.
In our patients, as in other series, abnormal X-ray findings at the time of admission, together with the presence of certain comorbid conditions – particularly metabolic diseases–suggested a more serious course of the illness.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: González-Vélez AE, et al. Factores asociados a ingreso en unidad de cuidados intensivos en pacientes hospitalizados por Influenza pandémica A/H1N1 2009. Med Intensiva. 2011;35:463–9.