The use of noninvasive mechanical ventilation was evaluated in our series of patients admitted to our ICU with pneumonia due to influenza A virus H1N1, assessing the need for intubation, arterial blood gases and clinical improvement, the development of complications and ICU and hospital stay.
DesignRetrospective and observational study.
SettingICU of Castellón University General Hospital (Castellón, Spain).
PopulationPatients admitted to ICU with pneumonia due to influenza A virus H1N1 and acute hypoxemic respiratory failure.
InterventionsBoussignac CPAP, Helmet system and BiPAP Vision® were used.
ResultsFive of 10 patients with pneumonia and hypoxemia were analyzed, showing 100% effectiveness of noninvasive mechanical ventilation in terms of clinical and arterial blood gas improvement, and avoiding intubation in all cases. There were no patient deaths in ICU or in hospital. The duration (median) of ventilation was 6 (4–11) days, with an ICU stay of 9 (7–11) days. The number of complications was low (except for urinary tract infection due to Pseudomonas aeruginosa), and only the noise produced by CPAP was underscored. There were no infections among the staff.
ConclusionsBased on our results, increased use of noninvasive mechanical ventilation in future epidemics could be proposed.
Análisis del empleo de la VMNI en nuestra serie de pacientes ingresados en la unidad de cuidados intensivos (UCI) afectados por nuevo virus de la gripe A (H1N1), en especial aquellos afectados por neumonía con insuficiencia respiratoria aguda (IRA) hipoxémica grave, observando la necesidad de intubación, mejoría clínico-gasométrica, desarrollo de complicaciones, mortalidad, estancia en UCI y hospitalaria.
DiseñoEstudio retrospectivo observacional.
ÁmbitoUCI del Hospital General de Castellón.
PacientesPacientes ingresados en la unidad con neumonía primaria o secundaria, con IRA de predominio hipoxémico.
IntervencionesSe empleó CPAP de Boussignac, sistema Helmet y BiPAP Vision.
ResultadosDe un total de 10 pacientes ingresados con infección por gripe A H1N1, se empleó la VMNI en 7 (70%) pacientes con un fracaso del 28% (una agudización de asma y otra insuficiencia ventilatoria con obstrucción de vía aérea). Dentro del grupo hipoxémico analizado (5 pacientes), la efectividad de la VMNI fue del 100% en cuanto a mejoría gasométrica y clínica, evitando la intubación de todos estos pacientes. Asimismo, no se produjo ninguna muerte tanto en UCI como en el hospital. La duración (mediana) de la ventilación fue de 6 (4–11) días y la estancia en UCI, de 9 (7–11) días. La tasa de complicaciones fue pequeña (una infección de orina). La tolerancia de la VMNI fue aceptable, destacando el ruido producido por la CPAP. No se produjo ningún contagio en el personal sanitario.
ConclusionesA la luz de los resultados, se podría plantear un mayor empleo de la VMNI ante futuras epidemias.
The recent pandemic produced by the new influenza A (H1N1) virus has been an important test for healthcare systems. Despite the initial alarm, the infection has had less of an impact than initially feared. Nevertheless, the most seriously ill patients have required admission to Intensive Care. The literature1–3 shows that patients admitted to the Intensive Care Unit (ICU) with probable or confirmed infection developed multiorgan dysfunction, with severe hypoxemic acute respiratory failure (ARF) requiring respiratory support in between 71.8 and 93% of the cases.1–3 In this context, invasive mechanical ventilation (IMV) was the most frequent first management option (76.2%). Among the patients subjected to noninvasive mechanical ventilation (NIMV), the failure rate was between 75 and 85%.1,2
NIMV involves a lesser complications rate than IMV (particularly due to the lesser incidence of ventilator associated pneumonia), and offers greater tolerability, since the patient is able to speak, eat, eliminate secretions, etc. The use of NIMV in patients with hypoxemic ARF has shown good results, with improved oxygenation, lessened fatigue, the avoidance of intubation in an important percentage of patients, and a reduction in mortality.4–6 Despite such benefits, however, no indication for the use of NIMV in Hypoxemic ARF has been established.7,8
The present study analyzes the use of NIMV in subjects with pneumonia and severe hypoxemia belonging to our series of patients infected with the new influenza A (H1N1) virus requiring admission to the ICU. To this effect we analyzed the incidence of NIMV, failure and mortality in the noninvasive ventilation group, as well as the clinical (heart rate and respiratory frequency) and blood gas evolution of the patients (based on the pO2/FiO2 ratio).
Patients and methodsType of study: A retrospective observational study was carried out.
PatientsWe included those patients admitted to our Unit between August 2009 and January 2010 with a presumed, suspected or confirmed diagnosis of infection with the new influenza A (H1N1) virus, and who developed primary or secondary pneumonia (i.e., after prior bacterial pneumonia) with hypoxemic ARF.
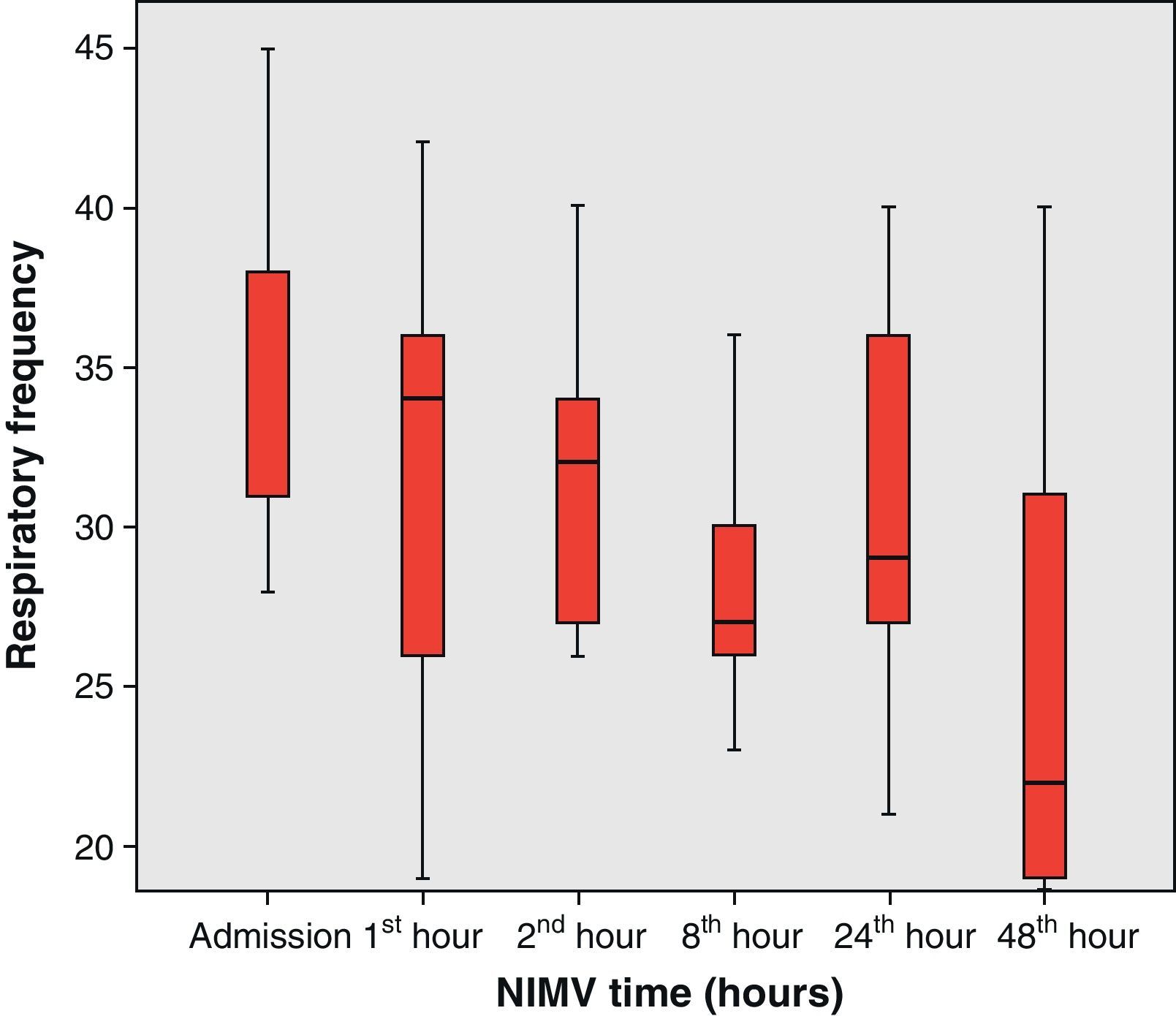
Community-acquired pneumonia was defined as lower respiratory tract infection with the presence of opacities on the chest X-rays, and signs and symptoms of respiratory infection (fever, cough, pleuritic pain, leukocytosis or leukopenia, and the presence or absence of secretions).9 Primary pneumonia was considered in those cases with confirmed new influenza A (H1N1) virus infection and without the isolation of Legionella spp. or Streptococcus pneumoniae in blood or lung samples, or of their antigens in urine. Cases failing to meet these criteria were regarded as secondary pneumonia.10 Pneumonia was considered severe when the criteria of the ATS/IDSA were met.9 Use was made of the classical definition of acute respiratory failure (ARF), i.e., respiratory frequency>30rpm, partial oxygen pressure (PaO2)<60mmHg, or partial carbon dioxide pressure (PaCO2)>45mmHg. The group of patients with hypoxemic ARF (PaO2<60mmHg) in turn met the criteria of acute lung injury (ALI) or adult respiratory distress syndrome (ARDS).
We therefore analyzed those patients with hypoxemic ARF presenting primary or secondary pneumonia, and who in the absence of contraindications for NIMV7 were considered amenable to noninvasive ventilation.
Patients presenting predominantly hypercapnic ARF upon admission, with no X-ray findings compatible with diffuse alveolar infiltrates or severe hypoxemia (PaO2<60mmHg) were excluded from the final analysis.
MonitorizationUpon admission of the patient to the Unit, a central venous and arterial line was placed for less invasive hemodynamic monitorization using the PiCCO system (PULSION Medical Systems, Munich, Germany). Transcutaneous oxygen saturation (SatcO2) was monitored using an Oxisensor Nellcor II D-25 pulse oximeter (Nellcor® Puritan Bennet Inc., Decasanton, CA, USA) connected to an Infinity Delta head monitor (Dräger Medical System, Danver, USA). The arterial blood samples for blood gas determinations were processed with an ABL560 co-oximeter (Radiometer Medical A/S®, Copenhagen, Denmark). Sputum and urine samples were collected, together with blood culture samples for identifying the causal agents of the pneumonic process. Nasal and pharyngeal swab samples were obtained, with the determination of antigenemia and polymerase chain reaction (PCR) testing as protocol in application to patients with suspected H1N1 viral infection.
Upon admission and during stay in the unit, data were collected relating to personal information, diagnosis, stay in the ICU and in hospital, the duration of mechanical ventilation, the evaluation of severity based on the Acute Physiology and Chronic Health Evaluation (APACHE II) and Sequential Organ Failure Assessment (SOFA) scores, and hemodynamic, respiratory and blood gas parameters (including at baseline and 1, 2, 8, 24 and 48h after the start of ventilation). We documented the complications occurring during stay in the Unit, such as orotracheal intubation, pneumothorax, acute renal failure (with or without hemofiltration), situation of multiorgan failure, nosocomial infections and mortality. The patients were questioned about complications that could be attributed to NIMV (claustrophobia, aerophagia, insomnia, headache, noise).
NIMV was based on continuous positive airway pressure (CPAP) using either the Helmet CaStar (StarMed; Mirandola, Modena, Italy) or the Boussignac-Vygon® system. The Helmet system was connected to a CPAP Whisperflow® with a high oxygen flow generator (up to 150 l/min), while in the other Helmet port we fitted a positive end-expiratory pressure (PEEP) device to secure supra-atmospheric pressure during the entire respiratory cycle.
In both CPAP systems, the initial values used were FiO2 1 and PEEP 5–15cmH2O until clinical improvement was achieved (reduction of respiratory frequency and heart rate), and/or SatcO2 94–96%.
The NIMV device used was the BiPAP Vision (Respironics Inc.®, Pennsylvania, USA) fitted to an orofacial or complete facial mask or Total face® (Respironics Inc.®, Pennsylvania, USA), and connected to an active humidification system (Fischer and Payckel Healthcare, Ltd.).
Procedure: The patients were moved to individual closed boxes in which all healthcare personnel members adopted the measures against contagion established by the Hospital Epidemics Control Committee (gloves, coat, cap and N95 mask).
The patients were explained the procedure to be carried out (NIMV or CPAP), and each device was positioned by several team members. The mask was selected according to the clinical condition of the patient, with a size adapted to the facial anatomy in each case. After starting ventilation, we gradually increased ventilatory support with PEEP and pressure support above PEEP (PS), until achieving volumes of 5–7ml/kg and a respiratory frequency of 25–28rpm, so that in the first hour of support we reached a minimum PS of 10–15cmH2O and a minimum PEEP of 5–8cmH2O. The oxygen concentration was increased until clinical improvement (reduction of respiratory frequency and heart rate) and/or SatcO2 94–96% was achieved. Once patient cooperation and sufficient adaptability was obtained, the mask was adjusted. Posteriorly, patient tolerability (both clinical and psychological) was evaluated.
The indication of orotracheal intubation in patients subjected to NIMV was based on the presence of evident labored breathing (tachypnea, accessory muscle use, thoracoabdominal dissociation, encephalopathy), progressive metabolic lactic acidosis or multiorgan dysfunction. We did not exclusively consider hypoxemia as intubation criterion, since these patients presented severe hypoxemia due to their background illness.
The patients admitted to our Unit were reported as cases to the Spanish National Influenza A Registry, promoted by the Infectious Diseases Work Group (Grupo de Trabajo de Enfermedades Infecciosas, GTEI) of the Spanish Society of Intensive and Critical Care Medicine and Coronary Units (Sociedad Española de Medicina Intensiva, Crítica y Unidades Coronarias, SEMICYUC).10
Informed consentThe study protocol was approved by the Clinical Research Ethics Committee, and written informed consent was requested from the patients included in the study.
Statistical analysisThe SPSS version 11.0 statistical package was used for the statistical analysis. The Student t-test or Mann–Whitney U-test was used for the comparison of quantitative variables, depending on whether the sample followed a normal or non-normal distribution, respectively. In the case of qualitative variables, use was made of the chi-squared test or two-tailed Fisher exact test, when the number of cases was under 5. Statistical significance was considered for p<0.05.
Repeated measures analysis of quantitative variables was carried out using the Friedman test, evaluating the effect of NIMV upon the hemodynamic and respiratory variables.
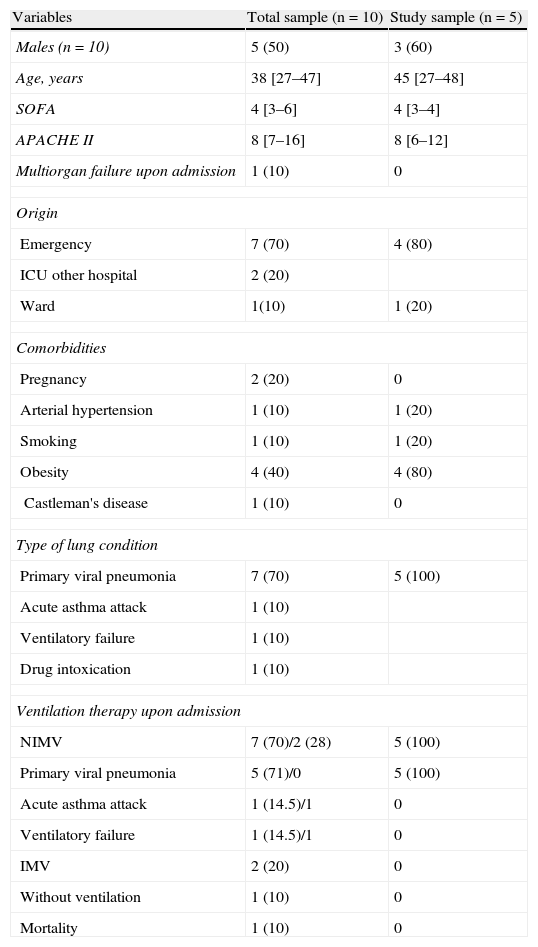
ResultsDuring the period between August 2009 and January 2010, a total of 10 patients were reported to the Spanish National Influenza A Registry. Of these subjects, 7 suffered primary viral pneumonia, two suffered ventilatory failure due to asthma exacerbation – one in the context of Castleman's disease – and the last presented drug intoxication with an initially low level of consciousness that required intubation at home (Table 1). Most of the patients were young, with no background disease. Obesity and pregnancy were recorded as antecedents in two patients. Seven patients (70%) were referred from the Emergency Department, two came from other hospitals, and another had been admitted from a hospital ward. Following admission, we initially applied noninvasive ventilatory support in 7 patients (70%), while two patients were already intubated upon admission, and in another case the optimum respiratory condition of the patient allowed us to omit any type of ventilatory support.
Baseline characteristics of the patients upon admission to the ICU.
| Variables | Total sample (n=10) | Study sample (n=5) |
| Males (n=10) | 5 (50) | 3 (60) |
| Age, years | 38 [27–47] | 45 [27–48] |
| SOFA | 4 [3–6] | 4 [3–4] |
| APACHE II | 8 [7–16] | 8 [6–12] |
| Multiorgan failure upon admission | 1 (10) | 0 |
| Origin | ||
| Emergency | 7 (70) | 4 (80) |
| ICU other hospital | 2 (20) | |
| Ward | 1(10) | 1 (20) |
| Comorbidities | ||
| Pregnancy | 2 (20) | 0 |
| Arterial hypertension | 1 (10) | 1 (20) |
| Smoking | 1 (10) | 1 (20) |
| Obesity | 4 (40) | 4 (80) |
| Castleman's disease | 1 (10) | 0 |
| Type of lung condition | ||
| Primary viral pneumonia | 7 (70) | 5 (100) |
| Acute asthma attack | 1 (10) | |
| Ventilatory failure | 1 (10) | |
| Drug intoxication | 1 (10) | |
| Ventilation therapy upon admission | ||
| NIMV | 7 (70)/2 (28) | 5 (100) |
| Primary viral pneumonia | 5 (71)/0 | 5 (100) |
| Acute asthma attack | 1 (14.5)/1 | 0 |
| Ventilatory failure | 1 (14.5)/1 | 0 |
| IMV | 2 (20) | 0 |
| Without ventilation | 1 (10) | 0 |
| Mortality | 1 (10) | 0 |
NIMV, noninvasive mechanical ventilation; IMV, invasive mechanical ventilation.
The data express n (%), n total (%)/n total failure (%) or median [interquartile range 25–75].
Regarding the rest of the physiological parameters under baseline conditions (Table 2), mention must be made of the important initial hypoxemia, marked chest X-ray involvement, and the presence of alterations in liver function, with increased levels of enzymes indicative of cytolysis. Upon admission to the Unit, all patients received (or had previously received) empirical antibiotic treatment with ceftriaxone, clarithromycin and oseltamivir (at doses of 150mg/12h), except the patient with bronchoaspiration due to drug intoxication, where there was no suspicion of influenza a infection.
Hemodynamic, ventilatory and laboratory test parameters of the patients upon admission to the ICU.
| Physiological parameters | Total sample (n=10) | Study sample (n=5) |
| MBP, mmHg | 98 [87–117] | 110 [85–119] |
| Heart rate, bpm | 127 [95–141] | 105 [78–141] |
| Respiratory frequency, rpm | 35 [33–38] | 38 [29–41] |
| pH, mmHg | 7.42 [7.27–7.48] | 7.47 [7.42–7.51] |
| pCO2, mmHg | 40 [35–51] | 37 [34–46] |
| Base excess, mmHg | 1 [–2–2] | 2 [0–11] |
| HCO3, mmHg | 25 [23–26] | 26 [24–33] |
| pO2/FiO2 | 87 [65–113] | 63 [58–87] |
| Chest X-rays, number quadrants (%) | ||
| 1/4 | 3 (30) | 0 |
| 2/4 | 2 (20) | 1 (20) |
| 3/4 | 2 (20) | 2 (40) |
| 4/4 | 3 (30) | 2 (40) |
| LDH, IU/l | 934 [448–2503] | 934 [772–2503] |
| GOT, IU/l | 48 [28–61] | 53 [48–626] |
| GPT, IU/l | 63 [14–76] | 72 [45–406] |
| Leukocytes/μl | 13,150 [4375–18,125] | 4400 [4150–9750] |
| Platelets/μl | 185,500 [128,750–274,500] | 165,000 [131,000–238,000] |
| Urea, mg/dl | 29 [16–52] | 26 [18–67] |
| Creatinine, mg/dl | 0.9 [0.7–1.3] | 1 [0.7–1.2] |
| CRP, mg/l | 93 [35–260] | 95 [54–360] |
| PCT, ng/ml | 2.4 [0.4–14.8] | 5.1 [0.3–17.4] |
The data express median [interquartile range 25–75] if not stated otherwise.
The global mortality rate was one patient (10%) admitted to our Unit from another center, under conditions of multiorgan failure, and who died after 24h. Two patients (30%) subjected to NIMV failed during their stay in the Unit; one of them suffered asthma exacerbation which in a few hours progressed towards severe ventilatory failure with respiratory acidosis secondary to bronchospasm, while the other patient (with Castleman's disease) suffered upper airway obstruction due to supraglottic inflammation (as a result of the background illness) requiring emergency intubation three days after starting NIMV. Of note in both of these cases was the presence of respiratory acidosis overlying hypoxemia, together with minimal lung infiltrates on the chest X-rays. For these reasons we decided to exclude them from the study of the hypoxemic patients proper.
Comparison of the physiological variables between the global sample and the group of 5 patients with hypoxemic respiratory failure revealed no significant differences (Tables 1 and 2). In the global sample, the median time from symptoms onset to hospital admission and from symptoms onset to ICU admission was 3 (2–6) days and 4 (2–8) days, respectively. In the study group, the median was 5 days in both cases, without differences between the two groups (Table 3).
In the hypoxemic group, three patients received CPAP with the Helmet® system, another received NIMV, and the fifth received a combination of CPAP with the Boussignac® system, followed by NIMV due to the absence of significant improvement. It should be noted that none of them required orotracheal intubation (100% success) – this leading to excellent results in that none of them died in the ICU or at hospital discharge.
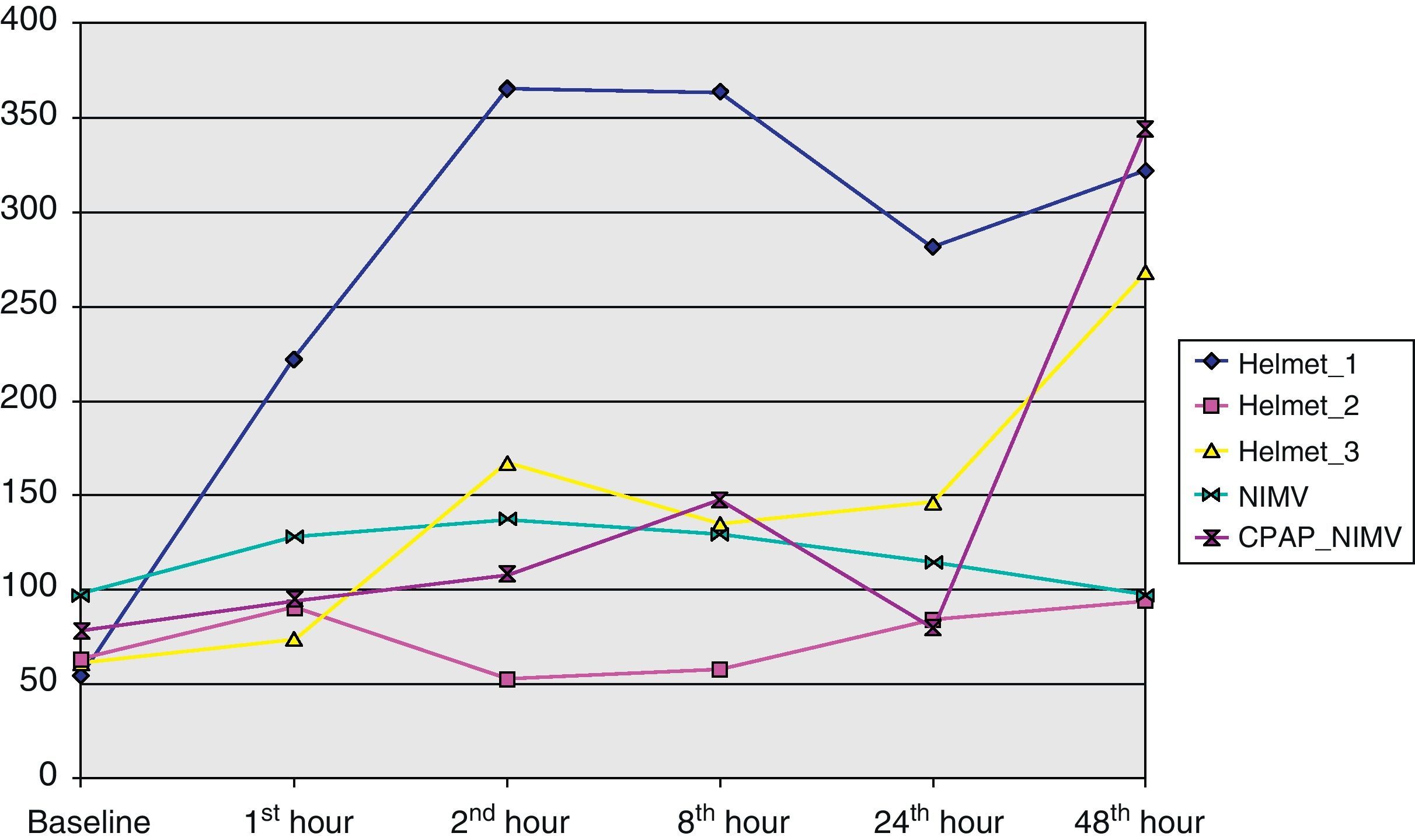
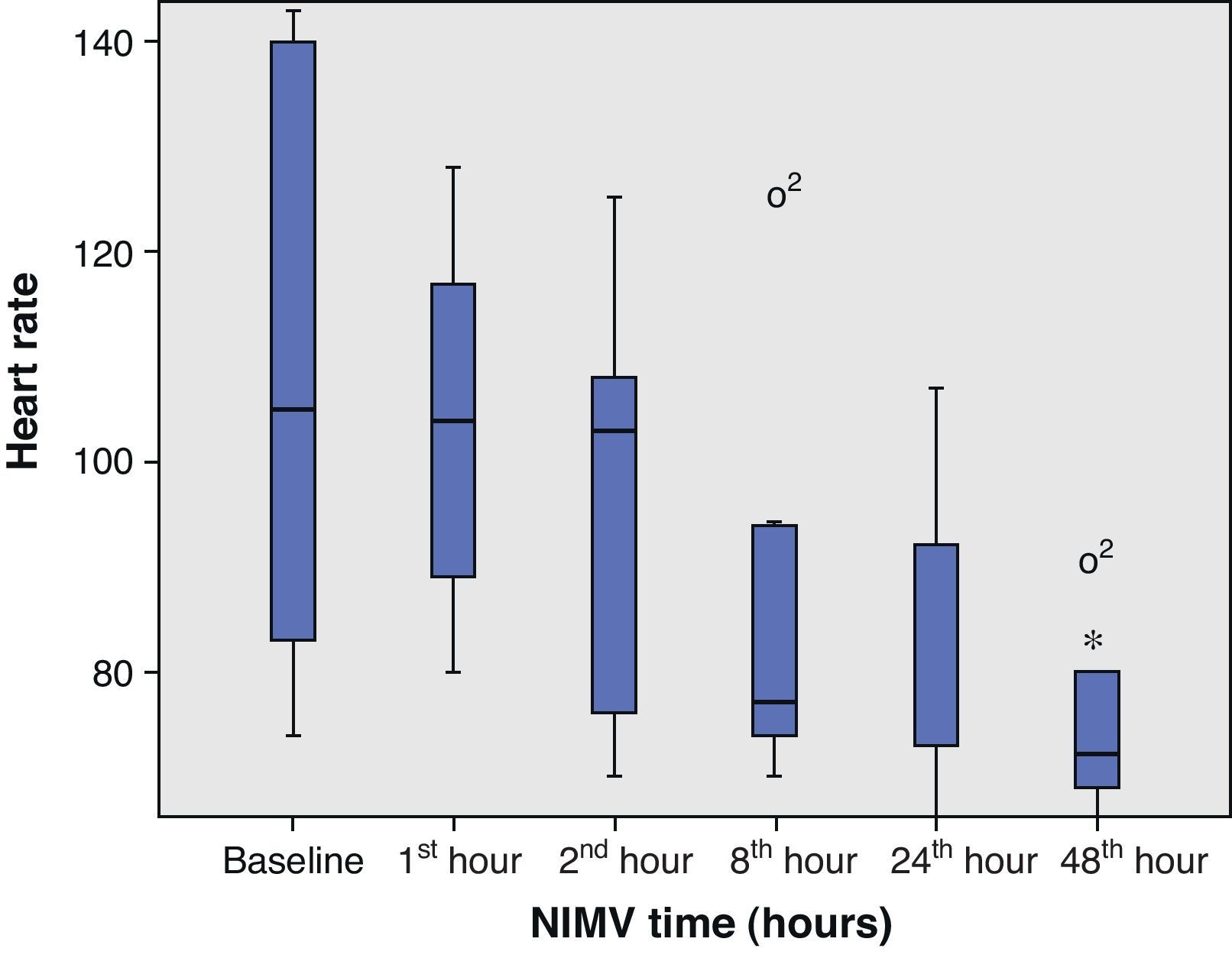
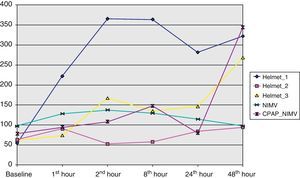
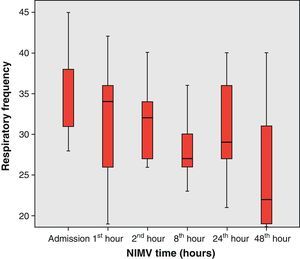
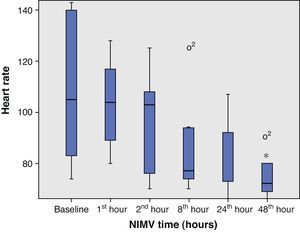
From the clinical prospective, progressive and statistically significant improvement was observed in gas exchange (Fig. 1) and in the clinical situation of the patient as measured by the respiratory frequency (Fig. 2) and heart rate (Fig. 3) (p<0.05).
The durations of ventilatory support and of ICU and hospital stay are reported in Table 4 – no differences being observed between the two groups. Following resolution of the acute phase condition, the patients were administered a questionnaire addressing the complications associated to noninvasive ventilatory support; most subjects made mention of the presence of noise with the CPAP system, though this problem was comparatively more pronounced with the Helmet® system (Table 5), and prevented them from sleeping. As other complications, one patient suffered urinary infection due to Pseudomonas aeruginosa.
Despite the limited size of the analyzed sample, the results indicate that the application of NIMV in its different modalities – but particularly CPAP with the Helmet® system – has been effective in improving hypoxemic ARF in this group of patients, thereby making it possible to avoid orotracheal intubation and its associated complications.
The situation of single organ dysfunction (where hypoxemic ARF predominated) led us to choose NIMV as the initial option, since in our setting the latter constitutes a first management procedure for all patients suffering ARF, clinical conditions allowing. Another factor that decided the balance in favor of NIMV was the extraordinary clinical tolerance of hypoxemia shown by these patients – this indicating dichotomization between the clinical picture and the blood gas and radiological findings. Despite the severe hypoxemia produced by the diffuse infiltrates in several lung fields, the patients showed clinical tolerance of the condition far greater than that seen in patients with the same degree of hypoxemia but produced by pneumonia or ARDS, since subjects with processes of this kind tend to suffer markedly labored breathing.
Finally, the absence of evident signs of muscle fatigue led us to use continuous positive airway pressure (CPAP) systems instead of noninvasive devices with two pressure levels (except in 2 cases). Through the production of supra-atmospheric pressure along the respiratory cycle, the CPAP systems increase residual functional capacity and displace the fluid filling the alveoli towards the periphery, thereby increasing the surface for gas exchange – which in turn results in a reduction of the hypoxemia. Although CPAP is not in truth a NIMV system, it is usually viewed as such because it offers most of the properties of NIMV.
The use of NIMV as first therapeutic option is in contraposition to the literature,1,2,10–12 where the percentage of patients ventilated with NIMV is relatively small (6–33%), and involves a failure rate of over 85%.2 This disparity of results may be due to the greater number of failed organs in these patients at the start of NIMV, in contrast to the situation of almost exclusive respiratory failure found in our patients at the time of starting noninvasive support (measured with the SOFA scale), together with the fact that they were referred directly from the Emergency Department, with immediate initiation of NIMV.
Although some articles have reported satisfactory results with NIMV in hypoxemic patients,4–6 and even in patients with ARDS, the poor results obtained as a consequence of experience gained with NIMV in both Canada2 and the United States have generated skepticism towards the use of NIMV, and this is presently reflected in the recommendations relating to ventilatory support.13 The other factor influencing the rejection of NIMV is the added risk of viral spread through the microdroplets and contagion of the healthcare personnel,13 as occurred in the 2003 epidemic registered in Canada, where 72% of the healthcare personnel members became infected. In our experience there has been no case of such contagion, due to the extreme safety measures adopted in the management of these patients.
A number of interfaces or masks are available for performing NIMV: face masks, orofacial masks, or helmets. The latter are closed systems made of transparent polyvinyl, and share the benefits of NIMV, but are much more comfortable, since they do not harm the bridge of the nose.14 Different publications have demonstrated the success of the helmet device in patients with hypoxemic ARF,14–16 in both NIMV modality and with the use of continuous flow systems (CPAP). Taking into consideration that prolonged ventilation was expected in hypoxemic patients, and the fact that there were no evident signs of labored breathing or hypercapnia, we decided to use these devices, which moreover were found to be effective. The main inconvenience of the helmet system using CPAP is carbon dioxide retention,17 though this problem was not observed in any of our patients, since high air-oxygen flows were used.17 On the other hand, the complications rate was low – the main problem being the noise produced by the CPAP continuous flow system.
Is NIMV a panacea? The answer to this question is clearly no, since cohort-based studies have shown that in ARDS patients subjected to NIMV in specialized units, percentage failure is in the order of 50%.6 The extraordinary course of our patients offers food for thought: if these individuals had been directly intubated in the presence of a radiological picture of ARDS together with a pO2/FiO2 ratio of <200, would the results have been the same? Perhaps not. And if further NIMV had been used, would the results have been those published? This is an issue for debate.
In what patients would we consider noninvasive support from the start? Perhaps in conscious and cooperative patients, without situations of shock and with almost exclusive respiratory failure, without evident signs of labored breathing (which may require immediate orotracheal intubation) or presenting metabolic lactic acidosis which would be indicative of tissue hypoperfusion. The time we should wait before considering intubation in a patient failing to show clear blood gas improvements has not been clearly established, though a delay in intubation has been shown to increase mortality.18 As predictive factors of NIMV failure, Antonelli et al.6 established the presence of increased patient severity (SAPS II>34) and the absence of improvement of the pO2/FiO2 ratio to >175 1h after starting NIMV. Perhaps one hour for suspending NIMV is too brief to assess its effect in these patients; it therefore may be extended to several hours (no time limit has been established), though in all cases evaluating the rest of the ventilatory, hemodynamic and metabolic parameters.
Our results support the use of NIMV (and particularly CPAP with the helmet system) in patients with hypoxemic ARF due to the new influenza A (H1N1) virus. Accordingly, and with a view to dealing with future pandemics, the use of these devices could be encouraged as an effective treatment option in selected cases.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Belenguer-Muncharaz A, et al. Utilización de la ventilación mecánica no invasiva en neumonía grave por virus H1N1. Med Intensiva. 2011;35:470–7.