Acute respiratory failure is the leading cause of hospitalization in pediatrics. High-flow nasal cannulas (HFNCs) offer a new alternative, but the evidence and indications are still debated. The performance of HFNCs at high altitude has not been described to date.
ObjectiveTo describe the use of HFNCs in pediatric patients admitted with respiratory failure and explore the factors associated with treatment failure.
MethodologyA prospective cohort study was carried out in patients between 1 month and 18 years of age managed with HFNCs. The demographic and treatment response data were recorded at baseline and after 1, 6 and 24h. The number of failures was determined, as well as the length of stay, complications and mortality. Patients with treatment failure were compared with the rest.
ResultsA total of 539 patients were enrolled. Infants (70.9%) of male sex (58.4%) and airway diseases such as asthma and bronchiolitis (61.2%) were more frequent. There were 53 failures (9.8%), with 21 occurring in the first 24h. The median length of stay was 4 days (IQR 4); there were 5 deaths (0.9%) and 13 adverse events (epistaxis) (2.2%). Improvement was observed in vital signs and severity over time, with differences in the group that failed, but without interactions. The final logistic model established an independent relationship of failure between the hospital (OR 2.78, 95%CI 1.48–5.21) and the initial respiratory rate (OR 1.56, 95%CI 1.21–2.01).
ConclusionsHFNCs afford good clinical response, with few complications and a low failure rate. The differences found between institutions suggest a subjective relationship in the decision of therapy failure.
El fallo respiratorio agudo es la principal causa de hospitalización en pediatría. Las cánulas nasales de alto flujo (CNAF) ofrecen una nueva alternativa, pero sigue existiendo debate en torno a la evidencia e indicaciones. No se ha descrito su comportamiento en gran altitud.
ObjetivoDescribir el uso de CNAF en pacientes pediátricos que ingresan con insuficiencia respiratoria y explorar los factores asociados al fracaso de la terapia.
MetodologíaEstudio de cohortes prospectivo. Pacientes entre un mes y 18 años manejados con CNAF. Se describieron datos demográficos y se evaluó la respuesta al inicio, 1.a, 6.a y 24.a horas. Se determinó el número de fracasos, así como estancia, complicaciones y mortalidad. Se compararon los pacientes con fracaso al tratamiento.
ResultadosIngresaron 539 pacientes. Fueron más frecuentes los lactantes (70,9%) de sexo masculino (58,4%) con afecciones respiratorias como asma y bronquiolitis (61,2%). Se presentaron 53 fracasos (9,8%), 21 en las primeras 24 horas. La mediana de estancia fue de 4 días (RIQ 4), hubo 5 éxitus (0,9%) y 13 eventos adversos –epistaxis– (2,2%). Se observó mejoría de signos vitales y gravedad en el tiempo con diferencias en el grupo que fracasó, pero sin interacciones. El modelo logístico final estimó una relación independiente del fracaso, entre el hospital (OR 2,78; IC95% 1,48-5,21) y la frecuencia respiratoria inicial (OR 1,56; IC95% 1,21-2,01).
ConclusiónLa CNAF es un sistema con buena respuesta clínica, pocas complicaciones y una baja tasa de fracasos. Las diferencias entre las instituciones sugieren una relación subjetiva de la decisión del fracaso.
Respiratory conditions are the most common cause for consultation in the routine pediatric practice, and are responsible for the high rates of hospitalization and mortality reported, especially in developing countries.1 The statistics from the World Health Organization (WHO) show that in 2016 the leading cause of death in children under 5 were respiratory infections.2 Actually, these are cause for admission in nearly 70% of the causes for admission to the pediatric intensive care unit (PICU).3 Back in 2027, Colombia reported 11286 PICU admissions due to respiratory infections in children under 5, which amounts to 54.5% of all PICU admissions reported regardless of the age group.4
The management of these patients consists of oxygen therapy with or without ventilation assistanceance (invasive or non invasive).3 This is achieved using different techniques and interfaces based on their different requirements and risk-benefit ratio.1,3 Since the beginning of the present century, the use of machines capable of providing conditioning (both heated and fully humidified) gas mixtures at high-flow oxygen through nasal cannulas has become very popular.5,6 The different hypotheses based on which high-flow nasal cannulas (HFNC) generate ventilation assistance have already been discussed by other authors.7,8
Although studies have not come up with a definitive definition yet on the role that this system plays, some pediatric surveys already show the high level of acceptance in the routine clinical practice9,10 despite the low evidence established regarding its use.11,12 There seems to be a clinical perception of effectiveness and easiness of use13; also, there has been a change of paradigm away from the use of invasive systems to the use of non-invasive ones, thus reducing the impact of conditions like bronchiolitis.14,15 Some studies back up its effectiveness (clinical response to therapy) among pediatric patients for early ventilation assistance,16–19 post-extubation,20 and for the management of different types of conditions that present with ventilatory failure.18,21,22 However, when compared to other non-invasvie ventilatory support devices, its effectiveness is not that high.11,16,21
Bogotá, the capital of Colombia, is 2600m above sea level (ASL), which has important implications to the oxygen pressure available. In altitudes over 1500m ASL, carotid body stimulation provides higher respiratory rates and tidal volume to compensate for the atmospheric inspired oxygen pressure fall.23 These changes complicate diagnosis,24 monitorization thresholds,25 and the response to the management of patients at high altitudes.26 Our group has recently published the experience on the use of HFNC in newborn babies and found high fractions of oxygen being used and few signs of severity due to work of breathing.27 Descriptions of use in middle-income countries with several cities located at moderate and high altitudes have not been reported yet.
Some studies describe factors associated with this therapy failure based on physiological variables upon admission,28–30 response to these variables,18,30 severity scales,28,31 and disease factors.29 There is even a subjective component in the decisions made based on the type of center.18 Up to this moment, no study has analyzed these factors in cities at high altitude.
This study will describe the use of HFNC in pediatric patients admitted to pediatric critical care units due to respiratory failure and explore the predictive factors of this therapy failure in 2 units of Bogotá between 2013 and 2016.
MethodologyProspective, observational study of patients between 1 month and 18 years of age admitted to the PICUs of the Sociedad de Cirugía de Bogotá Hospital de San José and Clínica Infantil Colsubsidio Intermediate Care Unit (IMCU), both located in Bogotá, Colombia. These units are exclusively pediatric are located at teaching general hospitals and have 8/13 beds with an average 300/1000 admissions-year. The ratios of health professionals per patient are: 1 nurse for every 6/8 patients, 1 patient care technician for every 3 patients, and 1 respiratory therapist for every unit. Everybody is coordinated by a pediatric intensivist during daytime and pediatricians on call at night and on national holidays. These units receive medical and surgical populations including cancer patients in one of the units. Also, they assist they population of Bogotá, Colombia. However, these units are not capable of performing cardiovascular surgery, extracorporeal circulation or transplant therapies. Both units use specifically designed high-flow therapy machines bought between 2012 and 2015 with certified and disposable materials. These machines can provide high-flow oxygen rates (OptiflowTM; €40 to €70), respiratory circuits (€50 to €150) with integrated heating (50–150 €), and servo-assisted humidifier (F&P 850™ or myAIRVO™ 2; €2500 to €3000). Both units include other non-invasive ventilation systems in conventional machines and interfaces for different ages although with limited variability. From November 2013 through December 2016, all patients with clinical signs of respiratory failure according to the treating physician who prescribed ventilatory support through HFNC were studied. The study only included the first moment of support with the HFNC since some patients required more uses during their hospital stay. Patients referred to a different center 24h prior to the administration of HFNC or those whose forms had not been properly filled in by their treating physician at the beginning of the follow-up were excluded. Data from each patient was collected at the beginning of therapy and 1, 6, and 24h into therapy including: demographic parameters like age, weight, and sex, respiratory diagnosis defined by its main pathophysiological mechanism (small airway like bronchiolitis and asthma, occupational lung disease like pneumonia or edema, upper airway like obstructive laryngitis, and neuromuscular conditions like brain related motor failure), and the radiological pattern at admission as described by the treating physician and, when in doubt, by assistant radiologists. Physiological variables like respiratory rate (RR), heart rate (HR), hemoglobin saturation levels measured by pulse-oximetry (SpO2), fraction of inspired oxygen (FiO2) and its ratio (SpO2/FiO2), and flow rate administered by kilogram of weight were measured. The degree of severity of the clinical signs was measured using the variables of the Modified Wood's clinical asthma severity scale (M-WCAS) and the correlation between the SpO2/FiO2 ratio, and HR or the Respiratory rate-OXygenation (ROX) index.32 Also, the SpO2/FiO2 ratio was grouped in ranges of 100, the M-WCAS scale in categories of severity while both the HR and the RR were categorized as abnormal if outside the 10th and 90th percentiles of age using the data published by Fleming et al.33 As the well-being measure no scales were used. Instead, the difficulties feeding and the possibility of crying or taking according to age were used as indirect surrogate markers. The continuous variables were expressed as means and standard deviations or means and interquartile ranges (IQR) based on their distribution. The categorical variables were expressed as absolute values and percentages. The changes seen in the variables over time were compared by analyzing repeated measures with the mixed model technique to determine the variance of data. P values <.05 were considered statistically significant. Mortality, the PICU stay, and the frequency of occurrence of all adverse events found were measured as well. The «failed case» definition was used to define cases where the HFNC was withdrawn and invasive mechanical ventilation was started; cases were divided into early (first 24h of use) and late cases (>24h of use). A bivariate comparison was made based on the type of failure reported. Then, a multivariate analysis was conducted using a logistics regression model with the relevant or significant variables seen in the bivariate analysis. All data were verified and tabulated in an Excel spreadsheet and processed using the statistical software package Stata 13. Data are presented in tables for better understanding. This study was presented and approved by every center research ethics committees (Sociedad de Cirugía de Bogotá Hospital de San José Human Research Ethics Committee [IRB#00011307], September 16, 2013; Clínica Infantil Colsubsidio Human Research Ethics Committee, April 4, 2017 [CEI-36-15]). Both authorized an exception to the informed consent since there was no risk involved in the study.
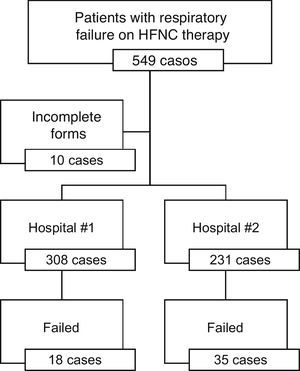
ResultsA total of 549 patients were hospitalized. Ten of these patients were excluded from the study since their data had not been properly collected. A total of 308 patients were hospitalized in hospital #1 (57.1%) and 231 in hospital #2 (42.9%) (Fig. 1).
The overall clinical and demographic characteristics of the admissions are shown in Table 1. Younger newborn babies were more prevalent compared to preschoolers and schoolers. The most prevalent diagnosis was small airway disease followed by occupational lung disease. The radiological pattern seen more often was the presence of opacities followed by signs of lung entrapment. The therapy was more often used for primary support rather than as a post-extubation system. The average flow rate was 1.3±0.63mL/kg/min with an average FiO2 ratio of 0.68±0.19.
Demographic and diagnostic description and outcomes of patients on high-flow nasal cannula therapy.
| Variable | Overall | ||
|---|---|---|---|
| n | % | ||
| Center | Hospital #1 | 308 | 57.1 |
| Hospital #2 | 231 | 42.9 | |
| Sex | Masculine | 315 | 58.4 |
| Feminine | 224 | 41.6 | |
| Age | Younger newborn baby | 300 | 55.6 |
| Older newborn baby | 82 | 15.2 | |
| Preschooler | 103 | 19.1 | |
| Schooler | 44 | 8.2 | |
| Teen | 10 | 1.9 | |
| Disease pattern | Small airway | 330 | 61.2 |
| Lung occupation | 149 | 27.6 | |
| Upper airway | 30 | 5.6 | |
| Neuromuscular | 9 | 1.7 | |
| Non-pulmonary | 21 | 3.9 | |
| Radiological pattern (n=481) | Opacity | 266 | 55.3 |
| Entrapment | 114 | 23.7 | |
| Mixed | 68 | 14.1 | |
| Normal | 33 | 6.9 | |
| Post-extubation cannula | 47 | 8.6 | |
| Failures | Overall | 53 | 9.8 |
| Early | 21 | 3.9 | |
| Late | 32 | 5.9 | |
| Deaths | 5 | 0.9 | |
| Adverse events | Epistaxis | 12 | 2.2 |
| Other | 0 | 0.0 | |
%: percentage; n: subjects studied; preschooler: 2 to 5 years; older newborn baby: 13 to 24 months; schooler: 6 to 12 years; teen: 13 to 18 years; younger newborn baby: <12 months.
Table 2 and Fig. 2 show the evolution of variables at the follow-up. Within the first hour 2 cases of failed therapy were reported, another 12 cases 6h later and another 7 within the next 24h. The cannula was withdrawn due to epistaxis (11 cases) or patient improvement (defined as a positive change in his general state of health) in 16 cases after 6h and in 48 cases at 24h. In total, 84.23% of the patients were still using the system at the 24-h follow-up. Both the heart rate and the respiratory rate improved significantly with the passing of the hours and the SpO2/FiO2 ratio was abnormal in most cases with a tendency toward improvement (Fig. 2). A study was conducted to look for indicators of poor FiO2 titration. It found that in 33.3% of the data, the SpO2 was >97%; still, the SpO2/FiO2 ratio analyzed in these groups was low (SpO2 ≤97%: median 138, IQR 66 vs. SpO2 >97%: median 152, IQR 64). After studying respiratory severity using the M-WCAS scale some scores, mostly mild and moderate, improved statistically with the passing of the hours, an improvement remained with the use of HFNC. The ROX index started with an average score of 3.5±1.5 and improved up to 4.1±1.9 with a statistically significant difference; however, the cut-off value of 4.8 (reported in the medical literature for adults) was seen in 9.43% of all failed therapies and in 16.2% of the remaining ones. Within the well-being measures, it was found that 95% of the patients can speak or cry with the active system. Significant feeding differences occurred in a variable range (20% to 30%) that were eventually treated with a feeding probe.
Well-being follow-up within the first 24h of follow-up in patients on high-flow nasal cannula therapy.
| Variable | Beginning | 1h | 6h | 24h | P* | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | n | % | N | n | % | N | n | % | N | n | % | ||
| Speaks (cries) with the system | 538 | 525 | 97.6 | 530 | 508 | 95.8 | 504 | 486 | 96.4 | 443 | 419 | 94.6 | .014 |
| Mouth feeding (vs. feeding probe) | 521 | 424 | 81.4 | 514 | 388 | 75.5 | 498 | 370 | 74.3 | 448 | 322 | 71.9 | .000 |
| Appearance** | |||||||||||||
| Good | 531 | 374 | 70.4 | 520 | 374 | 71.9 | 484 | 337 | 69.6 | 430 | 275 | 64.0 | .008 |
| Acceptable | 95 | 17.9 | 115 | 22.1 | 122 | 25.2 | 135 | 31.4 | |||||
| Poor | 62 | 11.7 | 31 | 6.0 | 25 | 5.2 | 20 | 4.7 | |||||
N: subjects studied; n: subjects affected; %: percentage of studied patients.
Follow-up of patients on high-flow nasal cannula therapy in relation to time. Comparison in relation to failed therapy cases. Chart showing the average behavior and variance of every physiological variable by subgroups of overall success and failure within the first 24h of follow-up. Comparison between variable and time, failure, and time-failure interaction using linear mixed models with random effects (individual). P values < .005 were considered statistically significant. Failure differences are shown with an asterisk [*]; time differences are shown with the pound sign [#]; interacion differences are shown with the dollar sign [$]. (A) Inspired oxygen fraction (FiO2) ratio. (B) Oxygen saturation through pulse-oxymetry (SpO2) and FiO2 ratio (SpO2/FiO2 ratio). (C) Heart rate (HR) ratio. (D) Respiratory rate (RR) ratio. (E) ROX index ratio (correlation between the SpO2/FiO2 ratio and the HR). (F) M-WCAS, Modified Wood's clinical asthma severity scale.
A total of 53 failed cases were presented (9.8%), 21 of which (3.9%) failed before 24h (early) and the remaining 32 (5.9%) after this timeframe. The average hospital stay was 4 days (IQR 4). A total of 5 deaths were reported, 1 due to early failure, 2 due to late failure, and 2 without failure with limitation of therapeutic effort due to the baseline condition. A total of 12 epistaxes were reported as adverse events that required specific treatment (5 within the first hour, 4 after 6h, and 3 after 24h) and the withdrawal of therapy in 11 cases. These events happened in 1 of them (0.33%) in a young newborn baby, 7 (8.54%) in older newborn babies, 3 (2.91%) in preschoolers, and 1 (2.27%) in a schooler.
Comparison by type of failed therapy reportedWhen the types of failed therapy and the variables reported were compared, overall differences per hospital, younger age of the patients, higher heart and respiratory rates, and lower ROX index scores were found (Table 3). Regarding these variables, it was studied whether this difference occurred with early or late failures. We saw that, except for the ROX index, the remaining factors were more relevant in late compared to early failed therapies. The multivariate comparison only confirmed the hospital effect and the respiratory rate for overall and late failures (Table 4).
Bivariate analysis comparing the study variables to the different types of therapy failure in patients on high-flow nasal cannula therapy.
| Variable | Overall failure | Early failure | Late failure | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | OR | 95%CI | OR | 95%CI | |||||
| Center | Hospital #1 | Ref | ||||||||
| Hospital #2 | 2.88 | 1.58 | 5.22 | 1.22 | 0.51 | 2.93 | 5.22 | 2.22 | 12.29 | |
| Sex | Feminine | Ref | ||||||||
| Masculine | 1.09 | 0.61 | 1.95 | |||||||
| Disease pattern | Small airway | Ref | ||||||||
| Lung occupation | 0.93 | 0.48 | 1.80 | |||||||
| Radiological pattern | Normal | Ref | ||||||||
| Opacity | 1.32 | 0.38 | 4.58 | |||||||
| Entrapment | 0.75 | 0.19 | 3.02 | |||||||
| Mixed | 1.53 | 0.38 | 6.05 | |||||||
| Age, years | 0.74 | 0.59 | 0.92 | 0.79 | 0.59 | 1.07 | 0.71 | 0.52 | 0.96 | |
| FIO2 | 1.41 | 0.30 | 6.69 | |||||||
| Flow mL/min | 1.00 | 0.93 | 1.07 | |||||||
| HR/10 | 1.18 | 1.04 | 1.34 | 1.18 | 0.97 | 1.43 | 1.16 | 0.99 | 1.36 | |
| RR/10 | 2.13 | 1.13 | 4.02 | 1.75 | 0.80 | 3.82 | 1.99 | 1.03 | 3.87 | |
| SpO2/FiO2 ratio | 1.00 | 0.99 | 1.01 | |||||||
| ROX index | 0.72 | 0.56 | 0.93 | 0.63 | 0.41 | 0.96 | 0.80 | 0.60 | 1.07 | |
| M-WCAS | 1.00 | 0.80 | 1.24 | |||||||
Bivariate analysis using the logistics regression model, only by categories if the overall failure rate was statistically significant.
95%CI, 95% confidence interval; FiO2, inspired oxygen fraction; HR/10, heart rate grouped by 10 beats; M-WCAS, Modified Wood's clinical asthma severity scale; OR, odds ratio; ref., reference category; ROX index, correlation index between the SpO2/FiO2 ratio and the RR; RR/10, respiratory rate grouped by 10 breathings; SpO2/FiO2 ratio, correlation between pulse-oximetry and FiO2.
Multivariate model comparing significant variables based on the different types of therapy failure in patients on high-flow nasal cannula therapy.
| Variable | Overall failure | Early failure | Late failure | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | OR | 95%CI | OR | 95%CI | ||||
| Hospital #2 | 2.82 | 1.50 | 5.29 | 1.10 | 0.45 | 2.72 | 5.58 | 2.25 | 13.88 |
| Age, years | 0.79 | 0.62 | 1.00 | 0.81 | 0.59 | 1.10 | 0.77 | 0.55 | 1.09 |
| HR/10 | 0.99 | 0.86 | 1.15 | 1.08 | 0.87 | 1.33 | 0.94 | 0.78 | 1.12 |
| RR/10 | 1.42 | 1.03 | 1.95 | 0.95 | 0.59 | 1.55 | 1.78 | 1.20 | 2.64 |
| ROX index | 0.87 | 0.64 | 1.17 | 0.63 | 0.37 | 1.09 | 1.03 | 0.73 | 1.45 |
Multivariate logistics regression analysis with statistically significant variables in the bivariate analysis.
95%CI, 95% confidence interval; HR/10, heart rate grouped by 10 beats; OR, odds ratio; ROX index, correlation index between the SpO2/FiO2 ratio and the RR; RR/10, respiratory rate grouped by 10 breathings; SpO2/FiO2, correlation between pulse-oximetry and FiO2.
This study shows the management of a cohort of patients from 2 centers located at high altitudes. It found greatly compromised oxygenation and lower ventilation severity according to the M-WCAS scale with clinical variables response and a low percentage of failure within the first 24h, although with great diversity of patients and outcomes in the study centers.
The demographic make-up by sex, age, and disease pattern is similar to that reported by other cohorts that have been using the HFNC system. For example, back in 2017, Coletti et al. published a cohort of patients monitored in a polyvalent pediatric unit. They confirmed an incidence rate of cases of 44% among females, newborn babies in 49% of the cases, being small airway disease the most prevalent conditions of all (65%).34 However, there is still great variability of use depending on ages and disease patterns, which is consistent with the differences found between the two centers studied and other cohorts reported in the medial literature that also change over time.35,36
The study patients showed much more serious oxygenation disorders compared to the ones reported by other groups: Oto et al. reported a mean FiO2 of 0.39±0.08,26 the TRAMONTANE clinical trial on bronchiolitis reported mean values of 0.31±0.1,37 while Pilar et al. reported mean FiO2 values of 0.6% and a 0.4% IQR in patients with asthma. Also, Er et al. found early SpO2/FiO2 ratios of 160±57, and failed therapies with SpO2/FiO2 ratios of 195.
These patients’ work of breathing was less serious compared to the one reported by Oto et al.38 based on the M-WCAS scale (8.8±2.5) or by Pilar21 regarding asthma (median 8; IQR 2), which was consistent with significant respiratory compromise, as reported by Er et al. that described mRAID (modified respiratory distress assessment instrument) severity scores of 9.3±1.4 and also severe respiratory compromise.22 Other studies like the TRAMONTANE trial37 on bronchiolitis (M-WCAS score of 4±1) report values that are similar to this study, rather suggesting a different indication (oxygenation due to high altitude) or due to over-use (therapy was withdrawn in 16% of our patients before the 24h-mark), which may be adding extra-costs for the healthcare system (between €100 and €250 in consumables per patient), worries already expressed by other authors.39–41
These differences regarding oxygen and ventilation changes can partially explain the low frequency of failed therapies found in this study conducted at high altitudes (2640m ASL) compared to the variable failed therapy rate reported [40% (21) the highest one and 15% to 20% the lowest one22,38] in studies conducted in countries located at low altitudes that used similar or lower FiO2, but higher severity scores. We believe that in children who live at sea level hypoxia is indicative of a major respiratory compromise. However, at high altitudes it is rather an indication of a low inspiratory oxygen content available. This is why they look more hypoxemic, but with lower severity scores in the work of breathing.
The clinical response seen in the vital signs is similar to the one reported by other groups.22,38 However, the oxygen baseline parameters are much lower compared to other series studied at lower altitudes, suggestive that the cut-off values for abnormality for patients at high altitudes in terms of risk or response to the use of HFNC should be different. This should be studied and confirmed comparatively with centers located at different altitudes.
The ROX index was assessed to test its predictive capabilities regarding clinical response. It showed that the cut-off values of the original study32 were not good for this cohort because here we are dealing with a pediatric population with higher respiratory rates. Also, because this cohort lives at high altitudes where the critically ill patients’ SpO2/FiO2 ratios tend to be much lower. However, values change parallel to the clinical response, which is why additional studies should be conducted to validate these cut-off values in pediatric cohorts living at high altitudes.
Regarding the flow rate used for support purposes, it is consistent with what has been reported by other groups (between 1mL/kg/min and 2mL/kg/min). Recently, at least in newborn babies, it is clear what flow rate should be used according to two different studies: one physiological study42 that has been analyzing responses in the work of breathing of children <3. This study found that flow rates between 1.5mL/kg/min and 2mL/kg/min produce the best effect on the work of breathing as determined by the pressure-rate product (PRP). And another randomized clinical trial that showed no differences in the group that used flow rates at 3mL/kg/min vs. 2mL/kg/min in patients with bronchiolitis.17
The improvement of well-being has not been objectively confirmed or standardized in the pediatric population yet, especially in preverbal patients who are the main users. However, scales like the modified Comfort have confirmed improved scoring.43 This study found that a great percentage of patients could communicate, speak or cry, which we believe backs up the hypothesis that HFNC systems provide greater comfort to these patients, which is consistent with what has been reported in studies with adults.44,45 Regarding feeding, many were still being fed through the mouth or probe in case of fatigue, that happened to be a little bit more intense at the end of the follow-up. Still, enteral feeding was secured during the entire therapy without any adverse events being reported. These findings are consistent with those reported by a different study of patients with bronchiolitis that analyzed this aspect of cannulas and found a low rate of adverse events (mostly emesis) unrelated with the intensity of support, but rather with disease onset that is predictive of longer hospital stays.46
In the search for failure predictors only the hospital and the early respiratory rate were confirmed as independent predictors. The early respiratory rate is a well-known parameter of risk of respiratory failure among the pediatric population1; however, the role that the hospital may be playing suggests that some unmeasured variables, subjective at times, determined the treating physician's declaration of a case of failed therapy.
The strength of this study is that it was the first large pediatric cohort ever to analyze clinical response in patients treated in cities located at high altitudes. Its multicenter nature and multiple respiratory diagnoses achieved support its findings. Its weakness is that it did not include comparative cohorts at different altitudes for result comparison purposes. Also, the overall great heterogeneity of diseases and ages analyzed.
In conclusion, the use of HFNC in this study at high altitudes behaved clinically different compared to other studies conducted at lower altitudes: we are talking about patients whose FIO2 requirements are much higher and whose oxygenation improved significantly after the implementation of the HFNC system; ventilation was less compromised than other studies conducted at lower altitudes reported, which should be confirmed in other cohorts studied at different altitudes in order to determine if there is overuse and the associated extra-costs. The factors associated with failure were an initially high respiratory rate and the treating center, suggestive of a not-well defined subjective component in the decision to escalate treatment to invasive ventilation. More comparative studies are needed to define clear criteria of failure. Also, clinical guidelines aimed at pediatric populations living in cities located at high altitudes should be elaborated as well.
FundingThis study received personal funding from the authors of Fundación Universitaria de Ciencias de la Salud, Universidad Nacional de Colombia, Hospital de San José, and Clínica infantil Colsubsidio.
Authors/collaboratorsAll authors contributed to the project and deserve to be included as actual authors for their work:
- (1)
Designing the study, data mining or analyzing and interpreting the data obtained.
- (2)
Writing the draft of the manuscript or doing the critical review of the study intellectual content.
- (3)
Approving the final version of the manuscript that was presented here.
None declared.
We wish to thank the entire personnel from the Hospital de San José y la Clínica Infantil de Colsubsidio Respiratory Therapy Unit for their collaboration collecting and handling the patients's clinical histories.
Please cite this article as: Vásquez-Hoyos P, Jiménez-Chaves A, Tovar-Velásquez M, Albor-Ortega R, Palencia M, Redondo-Pastrana D, et al. Factores asociados al fracaso de la terapia con cánulas nasales de alto flujo en pacientes pediátricos con insuficiencia respiratoria en dos unidades de cuidados críticos pediátricos a gran altitud. Med Intensiva. 2021;45:195–204.









![Follow-up of patients on high-flow nasal cannula therapy in relation to time. Comparison in relation to failed therapy cases. Chart showing the average behavior and variance of every physiological variable by subgroups of overall success and failure within the first 24h of follow-up. Comparison between variable and time, failure, and time-failure interaction using linear mixed models with random effects (individual). P values < .005 were considered statistically significant. Failure differences are shown with an asterisk [*]; time differences are shown with the pound sign [#]; interacion differences are shown with the dollar sign [$]. (A) Inspired oxygen fraction (FiO2) ratio. (B) Oxygen saturation through pulse-oxymetry (SpO2) and FiO2 ratio (SpO2/FiO2 ratio). (C) Heart rate (HR) ratio. (D) Respiratory rate (RR) ratio. (E) ROX index ratio (correlation between the SpO2/FiO2 ratio and the HR). (F) M-WCAS, Modified Wood](https://static.elsevier.es/multimedia/21735727/0000004500000004/v1_202104220744/S2173572721000175/v1_202104220744/en/main.assets/thumbnail/gr2.jpeg?xkr=1dZuESKpnCAWr3yCSGZ24A==)


