To assess the diagnostic accuracy of the criteria used to detect patients carrying multiresistant microorganisms (MRMs).
DesignA prospective observational study was carried out from May 2014 to May 2015.
SettingPolyvalent Intensive Care Unit.
PatientsA cohort of consecutively admitted patients meeting the following criteria for preventive isolation according to the “Zero Resistance” project: hospital length of stay >4 days in the last three months (“hospital”); antibiotherapy during one week in the last month (“antibiotic”); institutionalized patients or recurrent contact with healthcare (“institution or care”); MRM carrier in the last 6 months (“previous MRM”).
VariablesDemographic data, culture results and isolation time. A multivariate analysis was performed using multiple logistic regression between each of the risk factors and patient MRM carrier status.
ResultsDuring the study period, 575 patients were admitted, of which 28% met the isolation criteria (162). Fifty-one (31%) were MRM carriers. Of the patients who did not meet the criteria, 29 (7%) were carriers. In the multivariate analysis, the only variable independently associated to carrier status was “previous MRM”, with OR=12.14 (95% CI: 4.24–34.77).
ConclusionsThe only criterion independently associated with the ability to detect patients with MRMs upon admission to the ICU was the existence of “previous MRM”.
Evaluar la precisión diagnóstica de los criterios empleados para detectar al paciente realmente portador de microrganismos multirresistentes (MMR).
DiseñoEstudio prospectivo, observacional de mayo de 2014 a mayo de 2015.
ÁmbitoUnidad de cuidados intensivos polivalente.
PacientesCohorte de pacientes ingresados de forma consecutiva que cumplían los siguientes criterios de aislamiento preventivo basados en el proyecto «Resistencia Zero»: hospitalización de más de 4 días en los últimos 3 meses («hospital»); antibioterapia durante una semana en el último mes («antibiótico»), pacientes institucionalizados o en contacto con cuidados sanitarios («institución o cuidado»); portador de MMR los últimos 6 meses («MMR previo»).
VariablesVariables demográficas, resultados de los cultivos obtenidos con presencia o no de MMR y tiempo de aislamiento. Se realizó un análisis multivariable con regresión logística múltiple entre cada uno de los factores de riesgo y el que el paciente fuera portador de MMR.
ResultadosDurante el periodo de estudio ingresaron 575 pacientes y cumplieron los criterios de aislamiento un 28%. De los 162 pacientes con criterios 51 (31%) eran portadores de MMR y de los que no cumplían criterios 29 (7%) sí que eran portadores. En el análisis multivariable la única variable asociada de forma independiente con el ser portador fue «MMR previo», con una OR 12,14 (IC 95%: 4,24–34,77).
ConclusionesEl único criterio que se asoció de forma independiente con la capacidad de detectar los pacientes con MMR al ingreso en la UCI fue haber presentado un «MMR previo».
Infections produced by multiresistant microorganisms (MRMs) are a growing concern and are among the leading causes of mortality.1 This situation has been recognized by the World Health Organization (WHO), which has defined antimicrobial drug resistances as one of the greatest public health threats worldwide.1,2
Contact isolation measures (washing of hands, the wearing of single-use gloves and gowns, and – where possible – individual rooms for patients) applied on a preventive basis in individuals with risk factors for MRMs constitute a fundamental strategy in order to prevent spreading.3–6 In the Intensive Care Unit (ICU), due to the characteristics of the patients and the use of complex apparatuses that may act as vectors or reservoirs, there is a high risk of MRM cross-transmission, and for this reason the mentioned preventive isolation measures may be even more important in this specific setting.7–9
However, observational studies suggest that preventive isolation measures are expensive, reduce interaction between the healthcare professionals and patients, and increase patient anxiety and depression.10 For this reason, we consider it necessary to minimize the number of patients subjected to unnecessary preventive isolation.
Our working hypothesis is that some of the criteria used result in unnecessary preventive isolation. The present study was carried out to evaluate the diagnostic precision of the criteria used to detect MRM carriers and establish the number of unnecessary isolations in our population.
Patients and methodsA prospective observational study was made to assess the capacity of the different preventive isolation criteria to detect MRM carriers in the 10-bed medical-surgical ICU of a 250-bed second-level hospital center.
Calculation was made of the recruitment time needed to reach at least 150 isolations based on the preventive isolation data of the Unit in the previous years. We analyzed a cohort of patients consecutively admitted during a period of one year (May 2014 to May 2015) and who met the following preventive isolation criteria contemplated by the “Zero Resistance” project11: a hospital stay of over four days in the last three months (“hospital”); intravenous or oral antibiotic treatment for at least one week in the last month (“antibiotic”); institutionalized patients or individuals with recurrent contact with healthcare (oncology, dialysis, etc.) (“institution or care”); and MRM carrier status at some time during the last 6 months (“previous MRM”). In the case of patients in the hospital ward at the time of admission to the ICU, and with “previous MRM” as isolation criterion, preventive isolation was only considered if there had been some negative sample between the time of the positive sample and admission to the Unit. If this were not the case, non-preventive contact isolation was considered. The microorganisms regarded as MRMs were methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus sp., extended spectrum beta-lactamase (ESBL) producing enterobacteria, carbapenemase-producing enterobacteria, enterobacteria resistant to three or more antibiotic families commonly used for their treatment; Pseudomonas spp. resistant to three or more antibiotic families commonly used for their treatment; Acinetobacter spp. independently of their sensitivity; and Stenotrophomonas spp. independently of their sensitivity.
As part of the routine protocol of the Unit, surveillance samples were collected in all patients from nasal, pharyngeal, rectal and ostomy (colostomy, ileostomy, tracheotomy, etc.) exudates upon admission and once a week until discharge from the ICU. Only patient SARM carrier status was analyzed in the case of nasal exudates. The clinical samples were left to the criterion of the physician in charge of the patient.
The isolation measures included mandatory hand hygiene at 5 different timepoints: (a) before contact with the patient; (b) before performing an aseptic procedure; (c) after risk of exposure to body fluids; (d) after contact with the patient; and (e) after contact with the surroundings of the patient,12 using water and antiseptic soap if the hands were stained, or employing an alcoholic solution if the hands were apparently clean.3,13 Upon entering the individual patient room, the staff were required to wear a gown and gloves, and a surgical mask was only required in the event of coming into contact with the patient airway.3 Isolation was suspended if the first surveillance sample (or clinical sample, if any) of admission proved negative. If the first sample was found to be positive, suspension was postponed until two negative samples were obtained. The readmission of a given patient was regarded as a new case.
The following demographic variables were analyzed: patient age and gender, Simplified Acute Physiology Score 3 (SAPS-3) severity status upon admission to the ICU, type of patient (medical versus surgical, considering surgical patients to be those coming from the operating room after elective or urgent surgery), ICU stay, ICU mortality, hospital mortality and isolation time.
Unnecessary preventive isolation was defined in the absence of MRMs in the initial samples.
The study was approved by the local Clinical Research Ethics Committee.
Normal data distribution was assessed by means of the Kolmogorov test. Continuous variables were reported as the median and interquartile range (IQR; p25–p75), since most of them exhibited a non-normal distribution. Categorical variables were reported as the total number and percentage. A sensitivity and specificity analysis was made of each of the variables, together with multiple logistic regression analysis involving a cutoff point of 0.5, α 5% and p<0.05 for entry or not to the model between each of the risk factors and confirmed patient positive MRM carrier status.
ResultsA total of 575 patients were admitted during the study period, of which 162 met the preventive isolation criteria (28% of the total patients admitted during that period). The demographic data of the patients subjected to preventive isolation are reported in Table 1. The MRMs detected in the carriers, with or without preventive isolation, are shown in Table 2.
Demographic variables of the patients requiring preventive isolation.
| Demographic variables, n=162 | |
|---|---|
| Median age, years (p25–p75) | 66 (56–75) |
| Female gender, n (%) | 71 (44) |
| Median SAPS 3 (p25–p75) | 50 (41–61) |
| Medical patients, n (%) | 79 (49) |
| Median ICU stay, days (p25–p75) | 4 (3–8) |
| ICU mortality, n (%) | 7 (4) |
| Hospital mortality, n (%) | 17 (11) |
| Median total isolation time, days (p25–p75) | 4 (3–5) |
| Median stay prior to ICU admission, days (p25–p75) | 1 (1–6) |
ICU: Intensive Care Unit; SAPS 3: Simplified Acute Physiology Score 3.
Multiresistant microorganisms isolated in carrier patients.
| MRMs (n) | |
|---|---|
| With preventive isolation | Without preventive isolation |
| Acinetobacter spp. (1) | ESBL-producing enterobacteria (21) |
| ESBL-producing enterobacteria (26) | MRSA (6) |
| Carbapenemase-producing Klebsiella pneumoniae (1) | Stenotrophomonas maltophilia (1) |
| Escherichia coli MRMs (2) | |
| Proteus mirabilis MRMs (1) | |
| Pseudomonas aeruginosa MRMs (4) | |
| Stenotrophomonas maltophilia (1) | |
| MRSA (19) | |
ESBL: extended spectrum betalactamase; MRMs: multiresistant microorganisms; MRSA: methicillin-resistant Staphylococcus aureus.
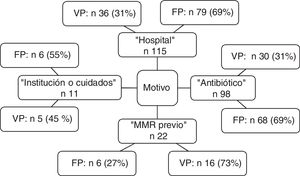
Of the 162 patients with preventive isolation criteria, 51 (31%) were finally confirmed as MRM carriers upon admission, independently of whether confirmation was based on a clinical sample, surveillance sample, or both. Of the patients that did not meet the preventive isolation criteria (n=413), a total of 29 were found to be colonized and/or infected with MRMs upon admission (7%) (Fig. 1).
The distribution of criteria of positive MRM status leading to preventive isolation is shown in Fig. 2. Of note is the observation that the criterion “hospital” was the reason for isolation in 115 patients, of which 79 were subjected to unnecessary isolation (69%). In the case of “antibiotic”, 68 of the 98 patients (69%) isolated based on this criterion were not MRM carriers. The sensitivity and specificity, as well as the positive and negative predictive values of each criterion are shown in Table 3.
Analysis of sensitivity and specificity.
| Isolation criteria | PPV (95% CI) | NPV (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|---|
| All | 0.31 (0.24–0.39) | 0.93 (0.90–0.95) | 63 (53–74) | 78 (74–81) |
| Hospital | 0.31 (0.23–0.41) | 0.91 (0.88–0.93) | 46 (35–57) | 84 (81–87) |
| Antibiotic | 0.31 (0.22–0.41) | 0.90 (0.87–0.92) | 38 (27–49) | 86 (83–89) |
| Institution or care | 0.45 (0.17–0.77) | 0.86 (0.83–0.89) | 6 (1–11) | 99 (99–100) |
| Previous MRM | 0.73 (0.50–0.89) | 0.88 (0.85–0.91) | 20 (11–29) | 99 (97–100) |
95% CI: 95% confidence interval; NPV: negative predictive value; PPV: positive predictive value.
In the multivariate analysis, the only variable independently associated to carrier status was “previous MRM”, with an odds ratio (OR) of 12.14 (95% confidence interval [95%CI]: 4.24–34.77) (Table 4).
DiscussionThe present study found that application of the criteria used to estimate the risk that a patient admitted to the ICU is a multiresistant microorganism carrier results in a 69% incidence of unnecessary preventive isolations. In contrast, 7% of the patients who did not meet these criteria were found to be colonized and/or infected with MRMs upon admission.
Our Unit, which previously had already applied preventive isolation measures based on a local protocol, adopted the preventive isolation criteria contemplated by the “Zero Resistance” project.11 This project is also integrated within the recommendations for the standardization of critical patient care established by a panel of experts of the 13 working groups of the Spanish Society of Intensive and Critical Care Medicine and Coronary Units (Sociedad Española de Medicina Intensiva, Crítica y Unidades Coronarias [SEMICYUC]), including the infectious diseases and sepsis working group.14
Preventive isolation measures, based on criteria of high MRM carrier risk, have been well established and are fundamented upon cross-transmission mainly through healthcare workers.15 Such measures are recommended by the European Center for Disease Prevention and Control,4 and have been seen to be effective in a number of studies. In this regard, An et al.16 found active surveillance followed by isolation and contact measures of caution to be inversely correlated to acquisition and consequent infection by carbapenem-resistant Acinetobacter baumannii in an ICU. In this same line, Zhou et al.,17 in a neonatal ICU with an outbreak of carbapenemase-producing Klebsiella, applied neonatal isolation (among other measures) to effectively control the outbreak. Matsumisha et al.,7 after noting that ICU patients have an 8-fold higher risk of becoming MRSA carriers, adopted contact preventive measures in all intubated patients and established comparisons versus a previous period in which isolation was only decided if carrier status was confirmed. The authors found that this change in protocol resulted in a significant decrease in the incidence of MRSA infection (from 12.2% to 5.6%).
However, other studies have found no association between isolation and the control of MRM transmission – considering that correct application of the basic measures of caution would be sufficient. As an example, McKinnell et al. compared contact isolation measures versus chlorhexidine washing alone for the prevention of MRSA transmission, and observed no increase in MRSA acquisition among the ICU patients. In turn, Ho et al.18 evaluated the efficacy of universal contact caution policies on antimicrobial resistance in relation to MRM colonization or infection before and after adopting the measures in burn patients, and recorded no differences. One study19 involving a cost-effectiveness analysis of three prevention strategies – universal decolonization, targeted decolonization, and screening and isolation – even reported that targeted decolonization and universal decolonization are less expensive and more effective than screening and isolation for preventing MRSA infection.
Apart from the controversy regarding the effectiveness of preventive isolation in avoiding the spread of MRMs, it must be underscored that contact isolation of patients suspected to be MRM carriers is not without adverse effects. In a recent study, Searcy et al.20 found that although isolated patients did not receive greater sedation, they had longer ICU stays and required longer periods of mechanical ventilation. Morgan et al. in turn showed contact isolation to be associated with less frequent healthcare staff entry to the patient room, poorer patient satisfaction, and a greater incidence of avoidable adverse effects – including falls, pressure ulcers and hypoglycemia.21–23
All this causes us to ponder whether isolation measures are really necessary, and whether the observation of adequate basic measures such as universal chlorhexidine washing and particularly hand washing – which reduces cross-transmission and is clearly useful – would be sufficient.24 However, in view of the contradictory findings, we considered it necessary to ensure the minimization of unnecessary preventive isolations, and to establish the best criteria for making this possible. Álvarez de Lerma et al. studied the impact of a consensus-based protocol for preventive isolation upon admission to an ICU, and recorded a significant decrease in incorrectly indicated preventive isolations.25 Individual examination of the criteria shows that although they are significantly able to detect MRM carriers, they also generate a very large number of false-positive indication – thereby resulting in the isolation of a significant number of patients that actually do not require isolation. Of the patients in our series, such “unnecessary” isolation occurred in 111 cases (19% of the patients admitted during the study period), with a mean isolation time of 5 days, and representing 555 stays with unnecessary isolation measures. These figures are relevant, considering the possible adverse effects associated to isolation commented above. This situation could be corrected through the use of early detection laboratory tests allowing early isolation suspension in non-carrier patients.26 It should be noted that the criteria “hospital” and “antibiotic” accounted for 69% of the false-positive results – this figure being considerable taking into account the negative effects associated with unnecessary isolation.
Based on the above, we consider it very important to adjust the criteria in order to ensure that preventive isolation is only applied to patients at a high risk of being MRM carriers. In our study, the only criterion found to be independently associated to MRM carrier risk was “previous MRM”, with a true-positive rate of 73%.
As weak points of our study, mention must be made of its single-center design, with a limited number of patients and specific local ecological characteristics. On the other hand, we included MRM carriers identified from clinical samples, surveillance samples or both. This circumstance on one hand increases the detection capacity, but bias may result from the fact that the clinical samples are dependent upon the physician in charge of patient care. At the time of suspending isolation, and thus its duration in the case of carriers identified from clinical samples and not from surveillance samples, we waited for negative readings from two surveillance samples in order to not subject the patient to further punctures, as occurs in the case of blood cultures – and this could constitute a confounding factor. Thus, based on our results, adjustment of the criteria could reduce both the total number and the stays of patients subjected to unnecessary isolation. It would be useful to conduct a study in this respect to ensure that reducing the isolation criteria does not significantly increase the number of patients that do not meet the criteria but who are effectively MRM carriers.
ConclusionsThe applied preventive isolation protocol resulted in unnecessary isolation in 69% of the cases, and failed to detect the presence of MRMs in 7% of the patients without isolation criteria. The only criterion found to be independently associated to the capacity to detect patients with MRMs upon admission to the ICU was the presence of MRMs in the last 6 months.
AuthorshipFederico Gordo and Ana Abella designed the study. All the authors contributed to data compilation. Federico Gordo, Ana Abella and David Varilla analyzed the results. Ana Abella and Federico Gordo wrote the manuscript. All the authors contributed to critical review of the manuscript.
Conflicts of interestNone.
Please cite this article as: Abella Álvarez A, Janeiro Lumbreras D, Lobo Valbuena B, Naharro Abellán A, Torrejón Pérez I, Enciso Calderón V, et al. Análisis del valor predictivo de los criterios de aislamiento preventivo en una unidad de cuidados intensivos. Med Intensiva. 2021;45:205–210.