To synthesize the evidence about diagnostic accuracy of inferior vena cava collapsibility (IVCc) in prediction of fluid responsiveness among spontaneously breathing patients.
DesignSystematic review of diagnostic accuracy studies.
SettingIntensive care units or emergency departments.
Patients and participantsspontaneously breathing patients with indication for fluid bolus administration.
InterventionsA search was conducted in MEDLINE and EMBASE. We included studies assessing IVCc accuracy for fluid responsiveness assessment with a standard method for cardiac output measure as index test.
Main variables of interestGeneral information (year, setting, cutoffs, standard method), sensitivity, specificity, and area under the receiving operator characteristics curve (AUROC). Risk of bias was assessed with QUADAS 2 tool. We obtained the pooled sensitivity, specificity and summary ROC curve, with estimated confidence intervals from a bivariate model. We also calculated positive and negative likelihood ratios and developed a Fagon nomogram.
ResultsEight studies were included with 497 patients. Overall, the studies presented a high risk of bias. IVCc sensitivity was 63% (95% CI – 46–78%) and specificity 83% (95% CI – 76–87%). Despite moderate accuracy of IVCc (SROC 0.83, 95% CI – 0.80–0.86), post-test probability of being fluid responsive based on a 50% pre-test probability led to considerable misclassification.
ConclusionsIVCc had moderate accuracy for fluid responsiveness assessment in spontaneously breathing patients and should not be used in isolation for this purpose.
Sintetizar la evidencia sobre la precisión diagnóstica de la colapsabilidad de la vena cava inferior (cVCI) en la predicción de la respuesta a los líquidos en pacientes que respiran espontáneamente.
DiseñoRevisión sistemática de estudios de precisión diagnóstica.
ÁmbitoUnidades de cuidados intensivos o servicios de urgencias.
Pacientes o participantesPacientes con respiración espontánea con indicación de administración de bolos de líquidos.
IntervencionesSe realizó una búsqueda en MEDLINE y EMBASE. Se incluyeron los estudios que evaluaban la precisión de la cVCI con un método estándar para medir el gasto cardíaco como prueba índice.
Variables de interés principalesInformación general (año, entorno, puntos de corte, método estándar), sensibilidad, especificidad y área bajo curva. El riesgo de sesgo se evaluó con la herramienta QUADAS2. Obtuvimos la sensibilidad combinada, la especificidad y la curva ROC resumida, con intervalos de confianza (IC) estimados a partir de un modelo bivariado.
ResultadosSe incluyeron 8 estudios con 497 pacientes. La sensibilidad de la cVCI fue del 63% (IC95%: 46-78%) y la especificidad del 83% (IC95%: 76-87%). A pesar de la precisión moderada de cVCI (SROC: 0,83; IC95%: 0,80-0,86), la probabilidad posterior a la prueba de responder a los fluidos basada en una probabilidad anterior al 50% dio lugar a una clasificación errónea considerable.
ConclusionesLa cVCI tuvo una exactitud moderada para la evaluación de la respuesta a los líquidos en pacientes que respiran espontáneamente y no debe usarse de forma aislada para este propósito.
Fluid infusion is often the first intervention given to patients with shock. However, only a half of patients respond with a significant increase in cardiac output after administration of a fluid bolus.1–3 Moreover, excessive fluid resuscitation may have adverse effects and is associated with worse outcomes in intensive care unit (ICU) patients.4–6
The assessment of fluid responsiveness has the potential to discriminate patients more prone to benefit of fluid infusion, avoiding unnecessary fluid administration to non-responders.1 Static parameters like central venous pressure (CVP), although often used in clinical practice are not reliable estimators of volume response and thus should not be used for fluid responsiveness assessment. On the other hand, dynamic parameters have been developed and validated and are suggested as the main way to assess volume response.7
Tools like pulse pressure variation have demonstrated good accuracy in fluid responsiveness assessment in ventilated patients under certain situations, but there are applicability issues that preclude its use in many ICU patients.7–9
Inferior vena cava collapsibility (IVCc) is usually measured with point-of-care echocardiography and may identify fluid-responsive patients.10 Some previous meta-analyses have shown good accuracy of inferior vena cava variation as a predictor of fluid responsiveness, especially in patients under controlled ventilation without inspiratory efforts.2,3,11 In spontaneously breathing patients, inspiratory effort reduces pleural pressure and may reduce inferior vena cava diameter irrespective of volume status, which could hamper the interpretation of IVCc results.12
Recently, several studies have evaluated the role of IVCc as a tool to assess fluid responsiveness in spontaneous breathing patients. However, most of those trials are single center studies with small samples sizes. Thus, the aim of this systematic review is to synthesize the available evidence about the diagnostic accuracy of inspiratory variation in inferior vena cava diameter in prediction of fluid responsiveness among spontaneously breathing patients.
Patients and methodsThis systematic review protocol was developed a priori and registered at PROSPERO International Registry of Systematic Reviews before the beginning of the search (registration number CRD42020149827). This study is reported following the recommendations of Preferred Reporting Items for Systematic review and Meta-Analysis of Diagnostic Test Accuracy studies (PRISMA-DTA).13
Search strategyThe search for relevant studies was conducted in virtual databases of MEDLINE and EMBASE, using the terms “fluids” or “fluid responsiveness” or “fluid therapy” or “fluid resuscitation” and “inferior vena cava” or “caval index” (or adequate synonyms, according to the database). Furthermore, references of selected studies and other reviews about this theme were revised for additional studies. Search and selection of studies was carried by two independent investigators (LCMCJ and GSDL). Disagreements were solved by a third investigator (BAMPB). The search for studies ended in September 2021.
Study selectionThe following criteria were used for inclusion in this systematic review:
- 1)
Prospective studies, with adults, in ICU or emergency departments, assessing accuracy of IVCc with point-of care echocardiography as a tool for fluid responsiveness evaluation.
- 2)
Studies with spontaneously breathing patients (not on mechanical ventilation), including both spontaneous and standardized respiratory maneuver assessment.
- 3)
Studies comparing the accuracy of IVCc against a standard method for cardiac output measure (such as VTI measure with echocardiogram or arterial pulse waveform monitors) before and after infusion of a bolus of fluid.
Exclusion criteria were: studies with children, studies in which it is not clear whether the patient is on spontaneous or controlled ventilation, studies without a reference test, studies without calculation of sensitivity, specificity, area under curve or with incomplete data and retrospective studies.
Data extractionThe primary outcome of this systematic review is the accuracy of IVCc in predicting fluid responsiveness, defined by sensitivity, specificity, pooled area under de curve and positive and negative likelihood ratios. After selection of studies, data extraction was made through a standardized form. In case of disagreements, the final decision was defined by consensus. Methodological quality and risk of bias assessment of the included studies was carried with the QUADAS 2 tool.14
The following data were extracted from each study: author name, year of publication, country where the study was realized, sample size, setting (ICU or emergency department), method of cardiac output measure, variation on cardiac output considered meaningful, number of fluid responders, sensitivity and specificity, area under curve, IVCc threshold, collapsibility in responders and non-responders, cardiac rhythm (regular or irregular), reason for fluid expansion, volume of fluid previously used and type and volume of fluid used in the test.
If any included study measured IVCc with both spontaneous and standardized respiratory maneuver, only spontaneous measure was used for the primary analysis. Posteriorly, we planned to make a sensitivity analysis including only studies with standardized respiratory maneuver. Whenever the study used the grey zone approach, we also extracted sensitivity and specificity of other cutoffs.
Statistical analysisCategorical variables were expressed as count and percentage and quantitative variables were expressed as mean (± standard deviation) or median (25th and 75th percentiles), as appropriate.
We obtained pooled sensitivity, specificity and summary receiving operator curve (SROC), with estimated confidence intervals using a mixed-effects bivariate regression model. Heterogeneity was explored visually in ROC space, since I2 measures are not adequate for heterogeneity evaluation in diagnostic accuracy studies. We also performed a post-hoc analysis as suggested by a reviewer to assess the presence of threshold effects. We assessed it visually through the sensitivity and specificity forest plots ordered by the individual study threshold and we present a Spearman correlation between sensitivity and specificity among the included studies, although this test needs to be interpreted with caution since it could be underpowered to detect this correlation, which is the rule, rather than the exception, in diagnostic accuracy studies.
Positive and negative likelihood ratios were also calculated. To better understand implications for practice we used an a priori defined 50% pre-test probability of fluid-responsiveness and developed a Fagan diagram to demonstrate graphically this analysis. All tests were conducted with software Stata SE, version 16 and the midas Stata package was used to conduct diagnostic accuracy meta-analysis.
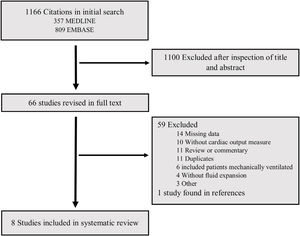
ResultsThe process of search and selection of studies is described in Fig. 1. Were identified 1166 citations in databases, of which 67 were selected for full review based on title and abstract. Of those only 7 studies fulfilled all inclusion and exclusion criteria, with one additional study identified in references of another systematic review. A list of all fully reviewed studies can be found in the online supplement (List S1). At the end, 8 articles were included in this systematic review, totaling 497 patients.15–22 The general characteristics of these studies are presented in Table 1.
Description of included studies.
| Author and year | Country | N | Population | Local | Gold standard | Criteria for fluid responsiveness | Type and volume of fluid given |
|---|---|---|---|---|---|---|---|
| Mcgregor, 2020 | England | 30 | Patients requiring IV fluid | ED | Echocardiogram | 10% increase in SV | Crystalloid, 250–500ml |
| Corl, 2019 | USA | 85 | Acute circulatory failure | ICU | NICOM™ | 10% increase in CI | 0.9% saline, 500ml |
| Bortolotti, 2018 | France | 55 | Infection and acute circulatory failure | ICU | Echocardiogram | 10% increase in VTI | 4% gelatin, 500ml |
| Corl, 2017 | USA | 124 | Acute circulatory failure | ICU | NICOM™ | 10% increase in CI | 0.9% saline, 500ml |
| Preau, 2017 | France | 90 | Acute circulatory failure | ICU | Echocardiogram | 10% increase in SVI | 4% gelatin, 500ml |
| Airapetian, 2015 | France | 59 | Acute circulatory failure or AKI or dehydration | ICU | Echocardiogram | 10% increase in CO | 0.9% saline, 500ml |
| Lanspa, 2013 | USA | 14 | Septic shock (within 6h of admission) | ICU | Echocardiogram | 15% increase in CI | Crystalloid, 10ml/kg |
| Muller, 2012 | France | 40 | Acute circulatory failure | ICU | Echocardiogram | 15% increase in VTI | HES 6% 130/0.4, 500ml |
Legend – USA: United States of America; AKI: acute kidney injury; ED: emergency department; ICU: intensive care unit; NICOM™: Non invasive cardiac output monitoring29; VTI: velocity-time integral measured with pulsed doppler on left ventricular outflow; SV: stroke volume; CO: cardiac output; SVI: stroke volume index; CI: cardiac index; HES: hydroxyethyl starch.
Most of the studies were single or bicentric, most of them performed in France. Most of the included studies were performed in the ICU and the most used criteria to start volume expansion were signs of poor peripheral perfusion and shock.
The gold standard in most studies was the assessment of cardiac output with echocardiography, but the criteria for defining fluid responsiveness varied among different studies, both in relation to the cut-off point (10% vs. 15%) and in relation to the reference being a measure of cardiac output or only stroke volume.
Table 2 describes IVCc performance in assessment of fluid responsiveness. About half of the patients were fluid responsive across all studies, and high heterogeneity was observed in definition of IVCc cutoffs, which ranged from 15% to 42%.
Data extracted from included studies assessing accuracy of IVCc as a predictor of fluid responsiveness.
| Author and year | N | Fluid responders | IVCc cut-off | IVCc – responders | IVCc – non responders | Sensitivity | Specificity | AUC (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Mcgregor, 2020 | 30 | 63.3% | >40% | NA | NA | 47% | 63% | 0.46 (0.26–0.67) |
| Corl, 2019 | 85 | 52% | >25% | 38.2% | 12.9% | 86% | 78% | 0.82 (0.74–0.88) |
| Bortolotti, 2018 | 55 | 53% | >37% | 49% | 11% | 66% | 85% | 0.82 (0.70–0.93) |
| Corl, 2017 | 124 | 49.2% | >25% | NA | NA | 87% | 81% | 0.84 (0.76–0.81) |
| Preau, 2017 | 90 | 55% | >31% | 47% | 14% | 76% | 88% | 0.82 (0.73–0.91) |
| Airapetian, 2015 | 59 | 49% | >42% | 35% | 27% | 31% | 97% | 0.62 (0.49–0.74) |
| Lanspa, 2013 | 14 | 35% | >15% | 52% | 11% | 100% | 66% | 0.83 (0.58–1.00) |
| Muller, 2012 | 40 | 50% | >40% | 64% | 19% | 70% | 80% | 0.77 (0.60–0.88) |
Legend – IVCc: inferior vena cava collapsibility; AUC: area under curve; 95% CI: 95% confidence interval; NA: not available.
The grey zone approach has been explored in three studies.15,16,18 It was observed that a cutoff between 11% and 22% of IVCc was associated with sensitivity greater than 90%, while a cutoff between 37% and 43% was associated with specificity greater than 90% for fluid responsiveness detection (Table S1 in online supplement).
Two studies evaluated IVCc with standardized respiratory maneuver,16,18 which consisted of deep, short-term inspiration (<5s) with continuous intensity that did not reach maximum inspiratory capacity. In general, the standardized respiratory maneuver increased the sensitivity of IVCc to 84–93%, maintaining a specificity of 88–90% (Table S2 in online supplement).
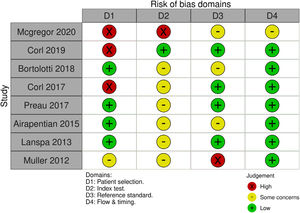
Risk of bias assessmentFig. 2 describes the assessment of methodological quality and the risk of bias. Most of the studies showed a high risk of bias and in all of them there were concerns in at least one of the characteristics evaluated by the QUADAS-2 tool. The main reasons for these results were selection of patients by convenience criterion (not consecutively), definition of IVCc cut-off a posteriori and non-blinding of the responsible for the gold standard test in relation to the IVCc results.
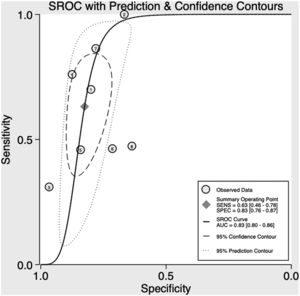
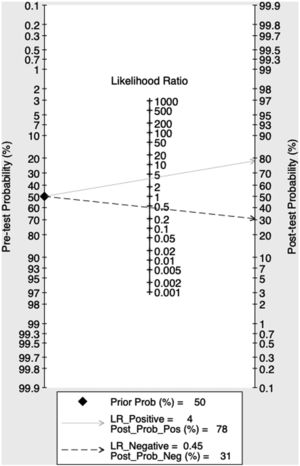
Quantitative synthesisFig. 3 illustrates the combined sensitivity, specificity, and AUC of IVCc in fluid responsiveness assessment. We observed a combined sensitivity of 63% (95% CI – 46–78%), a combined specificity of 83% (95% CI – 76–87%) and AUC of 0.83 (95% CI – 0.80–0.86). It was observed that the post-test probability of being fluid responsive based on a 50% pre-test probability was about 80% if the test was positive and 30% if the test was negative, as can be seen from Fagan nomogram (Fig. 4).
There was significant heterogeneity between studies, as can be seen by the wide distance between accuracy estimates of individual studies in the SROC curve as well as a 95% prediction region much larger than the 95% confidence region. Despite the visual analysis of the SROC curve and the sensitivity/specificity forest plot (Fig. S1) showing an inverse relationship between the sensitivity and specificity of the studies, Spearman's correlation showed only a weak correlation (ρ=−0.43) with a p value of 0.29, suggesting that a threshold effect is not the main reason for heterogeneity of the SROC.
DiscussionMain findingsIn this systematic review including eight studies and 497 patients, IVCc had moderate accuracy for fluid responsiveness assessment in spontaneously breathing patients, with sensitivity of 63% and specificity of 83%. Most of the included studies presented high risk of bias and high heterogeneity was observed. If used alone, IVCc modify post-test probability to 30% if negative and to 80% if positive. Moreover, this method was more useful when in extreme values and, if possible, with standardized respiratory maneuver.
Relationship with the literaturePreviously, several meta-analyses have assessed the accuracy of IVCc in fluid-responsiveness assessment, but the majority included both patients on mechanical ventilation and non-intubated patients and the number of studies, as well as the number of patients was low and methodological quality was poor.
Seccombe and collaborators conducted a review focused on the assessment of fluid-responsiveness in non-ventilated patients with sepsis.23 In this study, the authors found similar estimates of accuracy as in our study, with AUC close to 0.80, but quantitative synthesis was not performed due to the small number of patients and great heterogeneity between the studies. Since then, new studies have been published, motivating this review. In addition, no prior meta-analysis has assessed the impact of IVCc on post-test probability, which we believe to be more informative than the isolated values of sensitivity and specificity.
Although the observed accuracy is considered moderate, compared to other methods of assessing fluid-responsiveness in patients out of mechanical ventilation, IVCc has lower accuracy. Stroke volume variation greater than 13% after passive leg raising (PLR), for example, presents AUC of 0.96 and the pulse pressure variation greater than 52% after Valsalva maneuver presents AUC 0.98 for the assessment of fluid-responsiveness in spontaneously breathing patients.24 However, such methods are more laborious, while the evaluation of the inferior vena cava proves to be simpler and faster.
Another limitation of IVCc is related to the high variability of cutoff points within the studies. The variation in the diameter of the inferior vena cava during respiratory cycle is related to changes in chest and abdominal pressure, depending on intensity of respiratory efforts, venous compliance, blood volume and cardiac function. Some factors may increase heterogeneity in IVCc cutoffs, including irregular respiratory effort (both in frequency and amplitude), changes in the breathing pattern according to gender and age or with the type of breathing (diaphragmatic or thoracic), movement of the vena cava itself during inspiration, impairing the achievement of reliable images, presence of pulmonary hyperinflation and auto-PEEP, dysfunction of the right ventricle or conditions that generate restriction to cardiac filling (e.g., tamponade) and increased intra-abdominal pressure.12,25,26
Furthermore, the presence of different cut-off points between studies can lead to a threshold effect, in which those different cut-offs justify the difference in accuracy and the observed heterogeneity among studies.27 Our results suggest that although different cut-offs may contribute to observed heterogeneity, they alone cannot explain all the heterogeneity. Other factors such as differences between populations, differences in the method of patient selection, different inclusion timing, differences in standard test, among other confounding factors may justify the observed heterogeneity.28
Moreover, it was observed a wide grey zone between the cut-off point with optimal sensitivity and the cut-off with optimum specificity. Thus, the observation of extreme IVCc values (less than 10% or greater than 50%) is more useful, in excluding or confirming the presence of fluid-responsiveness. On the other hand, in patients with intermediate IVCc values, the probability of classifying the patient inappropriately is higher.
The risk of additional fluid infusion is also a factor to be considered in the analysis of IVCc. As suggested by Corl,15 in patients at high risk associated with fluid overload it would be interesting to use a higher cutoff point (IVCc>40%), maximizing specificity and positive predictive value, while in patients with lower risk of fluid overload it would be acceptable to use a more liberal cutoff point (IVCc>15–20%).
Strengths and limitationsThis review presents several strengths, including a comprehensive search of the literature, inclusion of several recent studies in the field, registration in PROSPERO, use of QUADAS-2 tool to assess the risk of bias and adherence to good practices for reporting systematic reviews (according to PRISMA-DTA). In addition, an assessment of clinical applicability was performed using a Fagan nomogram, which makes the data presented more informative than isolated analysis of sensitivity and specificity, as well as inclusion of evaluation of grey zone approach and standardized respiratory maneuver.
However, this review also has several limitations. The included studies present small numbers of patients, generating inaccurate estimates and with a high degree of heterogeneity; none of the studies included an assessment of the effect of the intervention on clinical outcomes, as well as none of the studies considered patient demographic characteristics such as age and gender, and most of the studies had a high risk of bias assessed by the QUADAS-2 tool.
There was no search for studies in some other relevant databases, as well as we did not search the grey literature. In addition, despite the absence of language restrictions, we admit that most of the references searched were in the English language. Finally, most of the studies included in this review were carried out in the ICU, and it is not possible to extrapolate the observed results for the assessment of fluid-responsiveness in patients in other settings.
Implications for practiceIn clinical practice, the question of whether a patient is fluid-responsive is quite common in the early treatment of patients with sepsis, shock or acute kidney injury. There are many possible ways to assess fluid-responsiveness. On one extreme, one may give a fluid bolus and assess whether cardiac output increased or not while observing for increased venous pressure (i.e., fluid challenge). On the other hand, one may assess fluid-responsiveness with the current standard of care, the passive leg raising approach.
However, PLR may be seen as cumbersome by clinicians because one needs to have real-time cardiac output measurements and doing so with critical care echocardiography is subject to unavailability (due to windowing issues) and to errors in assessing the velocity-time integral (VTI) when changing bed positioning. In this context, IVCc may be a useful alternative due to its simplicity. However, after a 50% pre-test probability of being fluid-responsive, clinicians must be aware that it changes post-test probability to 80% when positive and only to 30% when negative.
Furthermore, our results show more imprecision in sensitivity and clinicians should be aware of this when using this technique to assess fluid-responsiveness. Since clinicians are usually biased towards giving fluids, a more sensitive approach (to exclude with certainty patients who would not benefit from fluid boluses) is preferred to a more specific approach (which is the case of IVCc). Taken together with the totality of evidence, one should probably prefer PLR to IVCc, given its superior test performance and should rely on IVCc only when PLR is not testable or prone to error.
Implications for further researchThe use of IVCc in clinical practice needs further research before widespread utilization, considering that it is relatively easy to undertake but at the cost of a moderate discriminatory power. Particularly, multicenter studies validating the best cut-offs and evaluating test performance are necessary even before clinical impact studies. After validation of best cut-offs and reasonable test performance is confirmed, clinical trials to assess the impact of using this tool to assess fluid-responsiveness on clinical outcomes in critically ill patients are necessary.
ConclusionsIn this systematic review we observed that IVCc had moderate accuracy for fluid responsiveness assessment in spontaneously breathing patients, with pooled sensitivity of 66%, specificity of 83% and SROC of 0.85. Overall, the included studies presented high risk of bias and high heterogeneity not attributable only to the threshold effects. Further studies are necessary to validate the best cut-offs, evaluate test performance and the clinical impact of this method in clinical practice.
Authors’ contributionConceptualization: all authors.
Data collection: GSDL, LCMCJ.
Writing: LCMCJ, BAMPB.
Data analysis: LCMCJ, BAMPB.
Review and editing: BAMPB.
Final version approval: all authors.
Conflict of interestsThe authors declare that they have no conflict of interest.