The concept of mechanical power in mechanical ventilation is intrinsically derived from the equation of respiratory motion. The equation of motion in mechanical ventilation is a fundamental tool for understanding the interaction between the forces applied by the ventilator and the biomechanical properties of the respiratory system in a one-dimensional plane. This equation describes how ventilatory pressures must be adjusted to generate the necessary airflow and overcome the elastic and resistive forces of the lungs and the thoracic cavity.1
However, mechanical power goes a step further by integrating these forces on a cyclical and dynamic basis, multiplying the work of breathing by the respiratory rate to obtain the total rate of energy transfer. This is crucial, as the repetitive forces applied to the lungs during mechanical ventilation can accumulate and lead to micro-injuries and structural damage, a phenomenon that cannot be fully captured by simply measuring static pressures or volumes.2
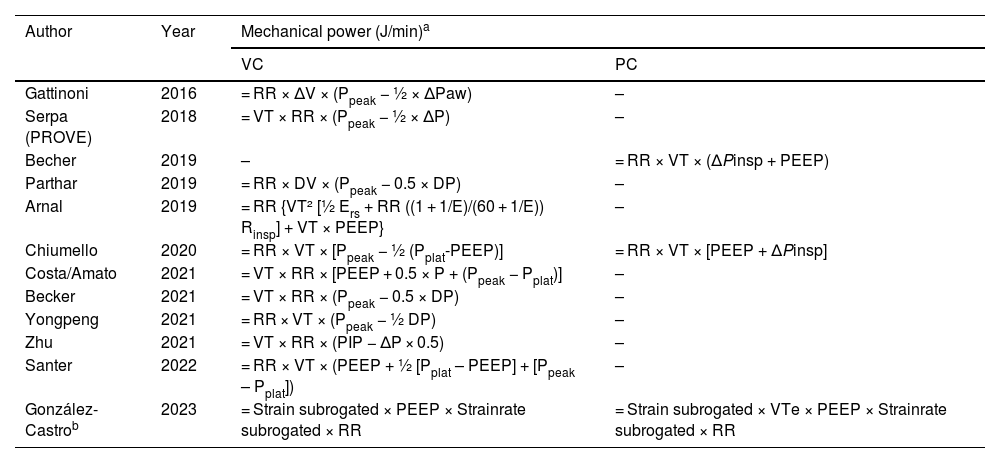
The concept was first formalized in the scientific medical literature by Gattinoni et al. in 2016. In its basic formula, it included parameters such as tidal volume (VT), respiratory rate (RR), peak pressure (Ppeak), and driving pressure, with a predominantly static focus. The idea was that integrating all these variables better represented the overall risk of ventilator-associated lung injury (VALI) than measuring each parameter in isolation (Table 1). Both the study conducted by Gattinoni and that of subsequent researchers focused on quantifying the mechanical energy applied to the lung from a simplified perspective, previously referred to as volutrauma and barotrauma or traditionally known as volutrauma and barotrauma, optimizing ventilation by limiting peak inspiratory pressures and driving pressure (ΔP), without explicitly considering the dynamics of lung tissue.3
Main equations proposed since 2016 to estimate mechanical power in both volume and pressure control.
| Author | Year | Mechanical power (J/min)a | |
|---|---|---|---|
| VC | PC | ||
| Gattinoni | 2016 | = RR × ΔV × (Ppeak − ½ × ΔPaw) | – |
| Serpa (PROVE) | 2018 | = VT × RR × (Ppeak − ½ × ΔP) | – |
| Becher | 2019 | – | = RR × VT × (ΔPinsp + PEEP) |
| Parthar | 2019 | = RR × DV × (Ppeak − 0.5 × DP) | – |
| Arnal | 2019 | = RR {VT² [½ Ers + RR ((1 + 1/E)/(60 + 1/E)) Rinsp] + VT × PEEP} | – |
| Chiumello | 2020 | = RR × VT × [Ppeak − ½ (Pplat-PEEP)] | = RR × VT × [PEEP + ΔPinsp] |
| Costa/Amato | 2021 | = VT × RR × [PEEP + 0.5 × P + (Ppeak − Pplat)] | – |
| Becker | 2021 | = VT × RR × (Ppeak − 0.5 × DP) | – |
| Yongpeng | 2021 | = RR × VT × (Ppeak − ½ DP) | – |
| Zhu | 2021 | = VT × RR × (PIP − ΔP × 0.5) | – |
| Santer | 2022 | = RR × VT × (PEEP + ½ [Pplat – PEEP] + [Ppeak – Pplat]) | – |
| González-Castrob | 2023 | = Strain subrogated × PEEP × Strainrate subrogated × RR | = Strain subrogated × VTe × PEEP × Strainrate subrogated × RR |
In contrast, Santer et al. (2022) and González-Castro et al. (2024) advance toward a more dynamic biomechanical approach. They incorporate the concept of pulmonary strain (the relationship between tidal volume and lung capacity) and its rate of change (strain rate), addressing cyclic deformation and its impact on tissue damage in volume- and pressure-controlled modes. This perspective reflects a more complex understanding of the ventilation-induced cumulative damage, through which not only the size of the pressures exerted is relevant but also the frequency and speed of tissue deformation. By integrating these components, they aim to offer a more accurate predictive model to assess the risk of VALI, assuming a framework that potentially captures both the transferred mechanical energy and the underlying mechanisms of tissue deformation.4,5
Future perspectives in the field of mechanical ventilation may focus on advancing biomechanical models that integrate strain and strain rate, allowing for more precise monitoring of pulmonary deformation and tissue damage in real-time, while optimizing ventilation. This will eventually lead to a more dynamic approach by adjusting ventilatory parameters based on individual pulmonary resilience rather than solely relying on traditional pressures and volumes.
All signing authors meet the authorship requirements, and all declared no conflicts of interest whatsoever. This work is in full compliance with the standards of good clinical practice. No AI tools have been used in its preparation. All copyright rights are granted to REVISTA MEDICINA INTENSIVA if accepted for publication.
Alejandro González-Castro: Conception and drafting of the manuscript.
Aurio Fajardo: Drafting and proofreading of the manuscript.
FundingNone declared.