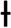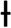Hemodynamic resuscitation is considered a cornerstone of the initial treatment of septic shock. However, there is growing concern about its side effects. Our objective was to assess the relationship between fluid administration and norepinephrine infusion and the development of lung injury.
DesignRandomized in vivo study in rabbits.
SettingUniversity animal research laboratory.
PatientsEighteen New Zealand rabbits. Control group (SHAM, n=6), Sepsis group with or without hemodynamic resuscitation (ETX-R, n=6; ETX-NR, n=6).
InterventionsSepsis was induced by intravenous lipopolysaccharide administration and animals were followed-up for 4h. Hemodynamic resuscitation with Ringer lactate (20mL·kg−1) was administered and later norepinephrine was initiated 3h after sepsis induction. At the end, the left lung was excised.
Main variables of interestAn indwelling arterial catheter and an esophageal Doppler were placed. Lung mechanics were monitored with side stream spirometry. Lung damage was analyzed by histopathological examination.
ResultsThe SHAM group did not show hemodynamic or respiratory changes. Lipopolysaccharide administration aimed an increase in cardiac output and arterial hypotension. In the ETX-NR group, animals remained hypotensive until the end of the experiment. Resuscitation with fluids and norepinephrine reversed arterial hypotension. Compared to the ETX-NR group, the remaining lung of the ETX-R group showed greater accumulation of neutrophils and reactive type-II pneumocytes, thicker alveolar wall, alveolar hemorrhage and non-aerated pulmonary areas. Lung injury score was larger in the ETX-R group.
ConclusionsIn our experimental study, following a strategy with bolus fluids and late norepinephrine used in the early phase of endotoxic septic shock has a negative influence on the development of lung injury.
La resucitación hemodinámica es considerada piedra angular en el tratamiento inicial del shock séptico. Sin embargo, existe creciente preocupación sobre sus efectos indeseables. Nuestro objetivo fue evaluar la relación entre la administración de fluidos e infusión de noradrenalina y el desarrollo de lesión pulmonar.
DiseñoEstudio aleatorizado en animales vivos.
ÁmbitoLaboratorio universitario de investigación.
ParticipantesDieciocho conejos de raza New Zealand White. Grupo control (SHAM, n=6), grupo séptico con o sin resucitación hemodinámica (ETX-R, n=6; ETX-NR, n=6).
IntervenciónLa sepsis fue inducida tras administración intravenosa de lipopolisacárido, y los animales fueron seguidos durante 4h. La resucitación hemodinámica mediante suero Ringer lactato (20ml·kg-1) y posterior noradrenalina fue iniciada a las 3h de ser inducida la sepsis. Al final del estudio, el pulmón izquierdo fue extraído.
Principales variables de interésFueron empleados catéter arterial y doppler esofágico. La mecánica pulmonar fue monitorizada con sensor de flujo. El daño pulmonar fue analizado mediante examen histopatológico.
ResultadosEl grupo control no mostró cambios hemodinámicos ni respiratorios. La administración del lipopolisacárido produjo un incremento del gasto cardíaco e hipotensión arterial. En el grupo ETX-NR, los animales permanecieron hipotensos hasta el final del estudio. La resucitación con fluidos y noradrenalina revirtió la hipotensión arterial. Comparados con el grupo ETX-NR, en el grupo ETX-R el estudio histopatológico mostró mayor acumulación de neutrófilos, así como mayor presencia de neumocitos activados tipo II, engrosamiento de la pared alveolar, hemorragia alveolar y zonas pulmonares no aireadas. La escala final de daño pulmonar fue mayor en el grupo ETX-R.
ConclusionesEn nuestro estudio experimental, la estrategia basada en la administración de fluidos y posterior infusión de noradrenalina en la fase precoz del shock séptico tiene una influencia negativa sobre el desarrollo de la lesión pulmonar.
Severe sepsis and septic shock are the most frequent causes of admission in intensive care units, with a high mortality rate around 30%.1 Recently reviewed definitions characterize sepsis as life-threatening organ dysfunction, while septic shock is a subset of sepsis in which particularly profound circulatory, cellular and metabolic abnormalities substantially increase mortality.2 Hemodynamic resuscitation, by means of fluid bolus (30mL·kg−1) and subsequent norepinephrine (NE), has once again been considered by the Surviving Sepsis Campaign (SSC) as a cornerstone of the initial treatment of septic shock.3 The rationale for these recommendations is that, in the early phase of severe sepsis and septic shock, restoring intravascular volume and maintaining end-organ perfusion are the top priorities.3,4 However, the optimal strategy of hemodynamic resuscitation in the early hours of severe sepsis and septic shock is still controversial. Given the evidence of harm associated with positive fluid balance accumulated in septic patients from hospital admission to Intensive Care Unit (ICU) discharge,5–7 there is a growing concern about the undesirable effects of this strategy, such as acute respiratory distress syndrome (ARDS).8–10
ARDS is a devastating complication of sepsis that influences its clinical management and outcomes.11,12 On the one hand, studies have established a strong relationship between fluid administration and the appearance of ARDS.13–18 On the other hand, the cardiovascular effects of NE on the development of lung injury have not been adequately investigated. NE, due to its ability to increase venous return and myocardial contraction in septic shock patients, increases pulmonary flow and perfusion,19–22 which would likely increase the severity of lung injury.
Because up to 40% of sepsis patients develop ARDS,23 and given that sepsis is a systemic inflammation state with high capillary permeability, it is possible that the effects of hemodynamic resuscitation in the early phase of septic shock could lead to enhaced pulmonary edema and increased the risk for ARDS development. The purpose of this experimental study is to investigate the effects of bolus fluid administration and infusion of NE on the development of lung injury. We hypothesized that this strategy used in the early phase of septic shock leads to lung injury development.
Patients and methodsThis study was approved by the Ethics Committee of the University of Cadiz (license 07-9604) and the Junta de Andalucía. Animal care and use procedures conformed to national and European Union regulations and guidelines (Spanish Royal Decree 53/2013 and EU Directive 2010/63/EU).
Anesthesia and instrumentationEighteen New-Zealand rabbits (weight 2.51±0.13kg) were anesthetized with an intramuscular dose of xylazine hydrochloride (10mg·kg−1) and ketamine (40mg·kg−1). The adequacy of anesthesia throughout the experiment was assessed by the absence of any significant blood pressure and/or heart rate change, either spontaneous or in response to a noxious stimulus (tail clamping). The rabbits were tracheotomized, intubated and mechanically ventilated (Servo 900c; Siemens-Elema, Solna, Sweden) in a volume-controlled mode, with a tidal volume of 8mL·kg−1, PEEP of 0 cmH2O, inspiratory-to-expiratory ratio of 1:2, inspired oxygen fraction of 0.6, and a respiratory rate adjusted to maintain an end-tidal CO between 35–45mmHg. The anesthesia was maintained with a continuous intravenous infusion of ketamine (15–20mg·kg−1·h−1), midazolam (1–3mg·kg−1·h−1) and muscular blockade with rocuronium bromide (1mg·kg−1·h−1). Ringer's lactate solution (6mL·kg−1·h−1) was administered as maintenance fluid therapy.
Hemodynamic monitoringA pediatric esophageal Doppler probe (KDP72; CardioQ Combi, Deltex Medical, Chichester, UK) was introduced into the esophagus until the optimal outline and maximal peak velocity of aortic blood waveform was obtained. Consecutive transesophageal Doppler measurements for 60seconds at the beginning and after 1, 2, 3 and 4h (end of experiment) were completed and averaged to calculate variables as the heart rate (HR), stroke volume (SV) and cardiac index (CI). Systolic, diastolic and mean arterial pressure, were continuously measured by an indwelling femoral artery catheter connected to a pressure transducer (TruWave®, Edwards Lifesciences LLC, Irvine, CA, USA).
Respiratory monitoringContinuous non-invasive measurements of flow, pressure and volume in the animal's airway were obtained using a spirometry monitor (Datex-Ohmeda M-COV, Helsinki, Finland) with a neonatal flow sensor (Patient Spirometry Kit 8004382, GE Healthcare, Helsinki, Finland) connected directly to the animal's tracheal tube. Dynamic compliance of the respiratory system (Cdyn) and the peak pressure (Ppeak) were measured and averaged during a period of 60s at the beginning and after 2, 3 and 4h (end of experiment).
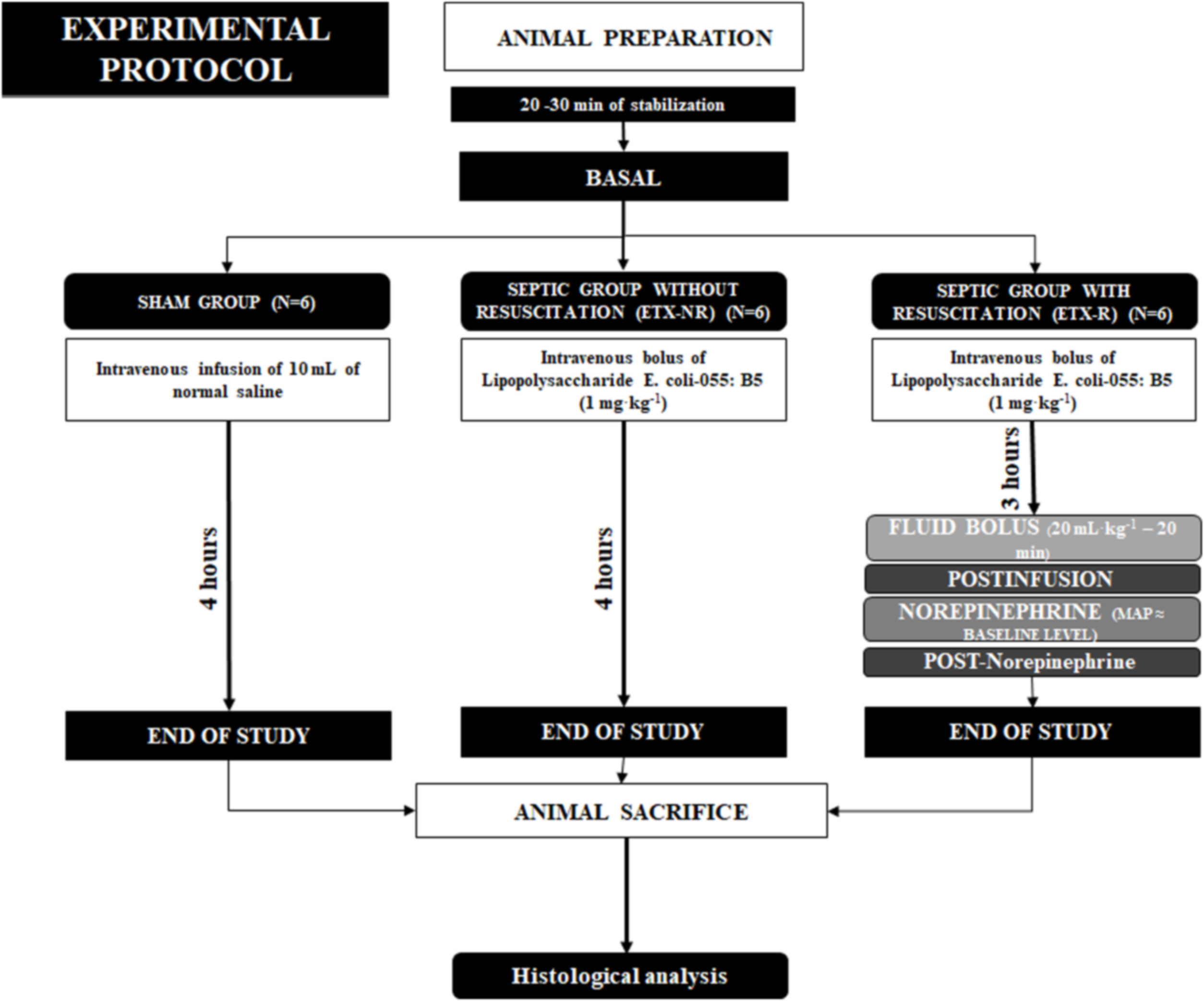
Experimental protocolAfter 5min of stabilization and baseline measurements of hemodynamic and lung mechanics, the animals were assigned using a computer-generated random sequence to three groups (6 animals each): a sham-operated group (SHAM), a non-resuscitated septic group (ETX-NR), and a septic group with hemodynamic resuscitation (ETX-R). In septic animals, a purified endotoxin-lipopolysaccharide (ETX) prepared from Escherichia coli 055:B5 (Sigma Chemical, St. Louis, MO) was intravenously infused over a period of 10min (1mg·kg−1). The dose and rate of the ETX was selected based on previous publications.24 However, to determine the best way for ETX administration, we required 8 animals. SHAM animals received an equivalent volume of normal saline. Three hours after ETX infusion, animals in the ETX-R group received a fluid bolus (Ringer's lactate) of 20mL·kg−1, and if the mean arterial pressure (MAP) was lower than the baseline measurement, NE infusion was started at 0.25mcg·kg−1·min−1. NE was increased 0.10mcg·kg−1·min−1 every 3min until reaching a MAP similar to the baseline level. A schematic representation of the experimental protocol is shown in Fig. 1. After completion of the study protocol, animals were euthanized by a lethal dose of chloride potassium.
Histological analysisAt the end of the experiment, the left lung was excised and immersed in 10% formaldehyde for at least 24h. Tissue samples were dehydrated with graded alcohol, embedded in paraffin, and cut in a series of 5μm-thick slices that were stained with hematoxylin and eosin. After the histological preparations were obtained, they were scanned to obtain digital preparations (3DHistech, Budapest, Hungary). An expert pathologist then evaluated these tissue sections in a blinded fashion using the following scoring system to determine the degree of lung injury: 0, no damage; 1, mild damage (present in 1–3 areas of 1mm2); 2, moderate damage (present in more than 3 areas of 1mm2 and less than 75% of the tissue section); 3, severe damage (present in more than 75% of the tissue section). These scores used the combined assessments of six parameters: accumulation of neutrophils in alveolar or interstitial space, reactive type II pneumocytes with atypical nuclei in the alveolus, alveolar congestion/collapse, alveolar wall thickening, alveolar hemorrhage and hyaline membrane formation, presenting a score from 0 to 18 ranging from normal histology to maximum damage.25,26,27
Statistical analysisData are expressed as the mean±standard deviation (SD), unless otherwise stated. Normality of data was checked by the Shapiro–Wilk test. We used a two-way analysis of variance (ANOVA) for repeated measures to determine the statistical significance of group differences in the respiratory and hemodynamic parameters at different time points. The Greenhouse–Geisser correction was used when violation of sphericity was detected by the Mauchly test. When statistical significance was indicated, it was further examined by a post hoc analysis (Bonferroni test). Baseline parameters and the differences within-subjects were evaluated using a repeated-measures ANOVA. The data obtained from the histopathological study were analyzed according to intensity and extension score using the non-parametric Kruskal Wallis test. The differences between each pair of 2 groups were assessed by Mann–Whitney U test. A p value <0.05 was considered statistically significant, unless otherwise indicated. Data were analyzed by using MedCalc Statistical Software version 16.8 (MedCalc Software bvba, Ostend, Belgium) and SPSS (SPSS 21, SPPS Inc, Chicago, IL).
ResultsIn the 18 rabbits randomly allocated to three different groups, we found that each group had similar baseline characteristics for any of the hemodynamic and respiratory variables measured, except for the Cdyn (Supplementary File, Table S1).
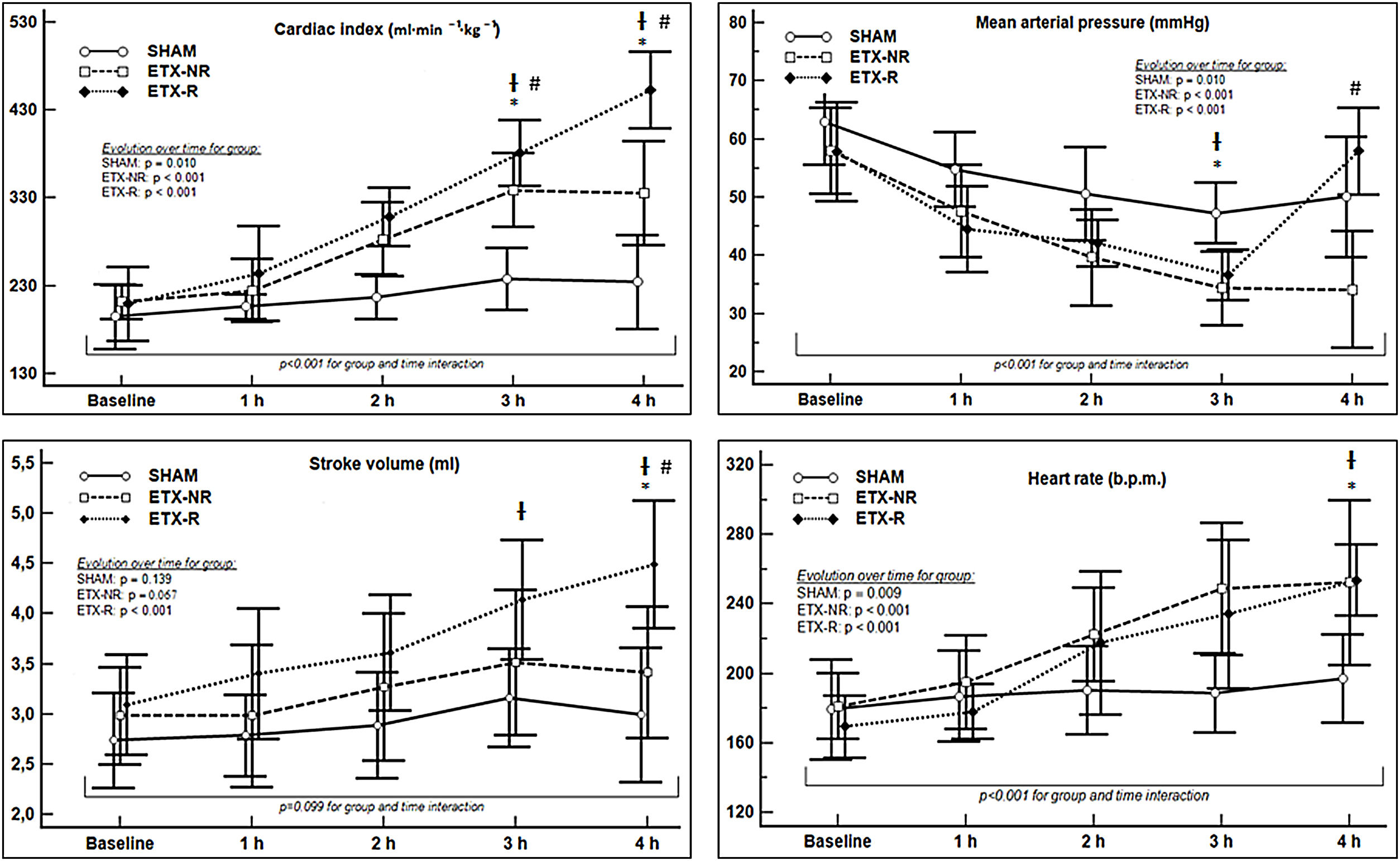
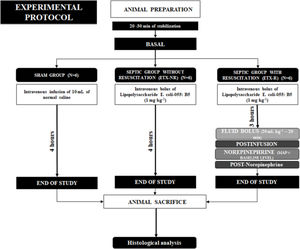
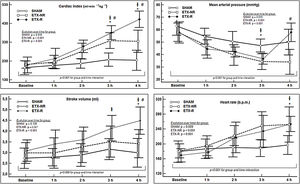
Hemodynamic changesInfusion of ETX resulted in a hyperdynamic hemodynamic profile with a progressive increase in CI (mostly secondary to a higher HR) and a reduction in blood pressure. In the ETX-R group, although the administration of fluids increased CI, stroke volume (SV) and MAP by 20%, 11% and 18% respectively, MAP's baseline levels were not reached, so NE was necessary in all cases (Fig. 2). Hemodynamic effects during different experimental stages are detailed in Supplementary File, Table S2.
Evolution over time of hemodynamic measurements of each group. Circles represent mean values and vertical lines are SD. SHAM: Sham-operated group, ETX-NR: Non-resuscitated septic group. ETX-R: Resuscitated septic group. Cardiac index (CI), mean arterial pressure (MAP), stroke volume (SV) and heart rate (HR). When ETX was administered there was an increase in the CI (secondary to an increase in HR), with a progressive reduction in MAP. The administration of fluids increased CI, SV and MAP; However MAP's baseline levels were not reached, so all animals required dose of norepinephrine. *p<0.005 ETX-R vs. basal.
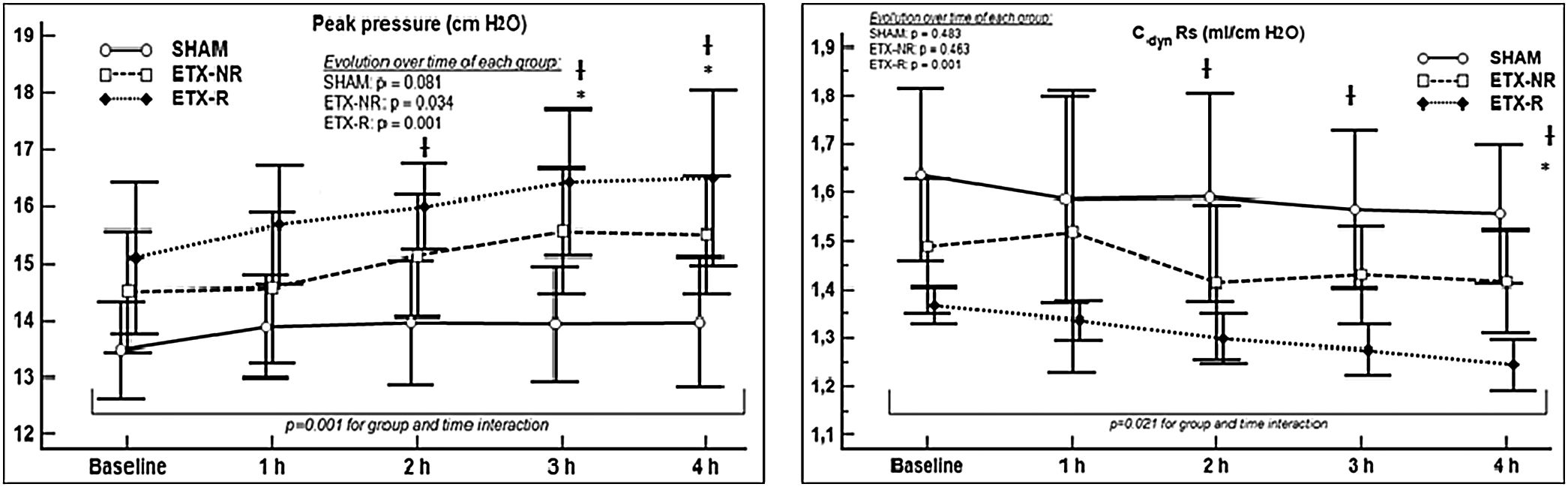
p<0.005 ETX-R vs. SHAM. #p<0.005 ETX-R vs. ETX-NR.As shown in Fig. 3, infusion of ETX caused a significance decrease and increase in the Cdyn and Ppeak respectively. However, although resuscitation worsened Cdyn by 20% and Ppeak by 16% in the ETX-R group compared to the ETX-NR group, differences were no statistically significant. Respiratory effects during the different experimental stages are summarized in Supplementary File, Table S3.
Evolution over time of respiratory measurements of each group. SHAM: sham-operated group; ETX: non-resuscitated septic group; ETX-R: resuscitated septic group. Circles represent mean values and vertical lines are SD. Dynamic compliance of respiratory system (Cdyn) and the peak pressure (Ppeak) during endotoxemia and resuscitation monitoring period. Although hemodynamic resuscitation worsened the lung mechanics, it was not significant. *p<0.005 ETX-R vs. basal.
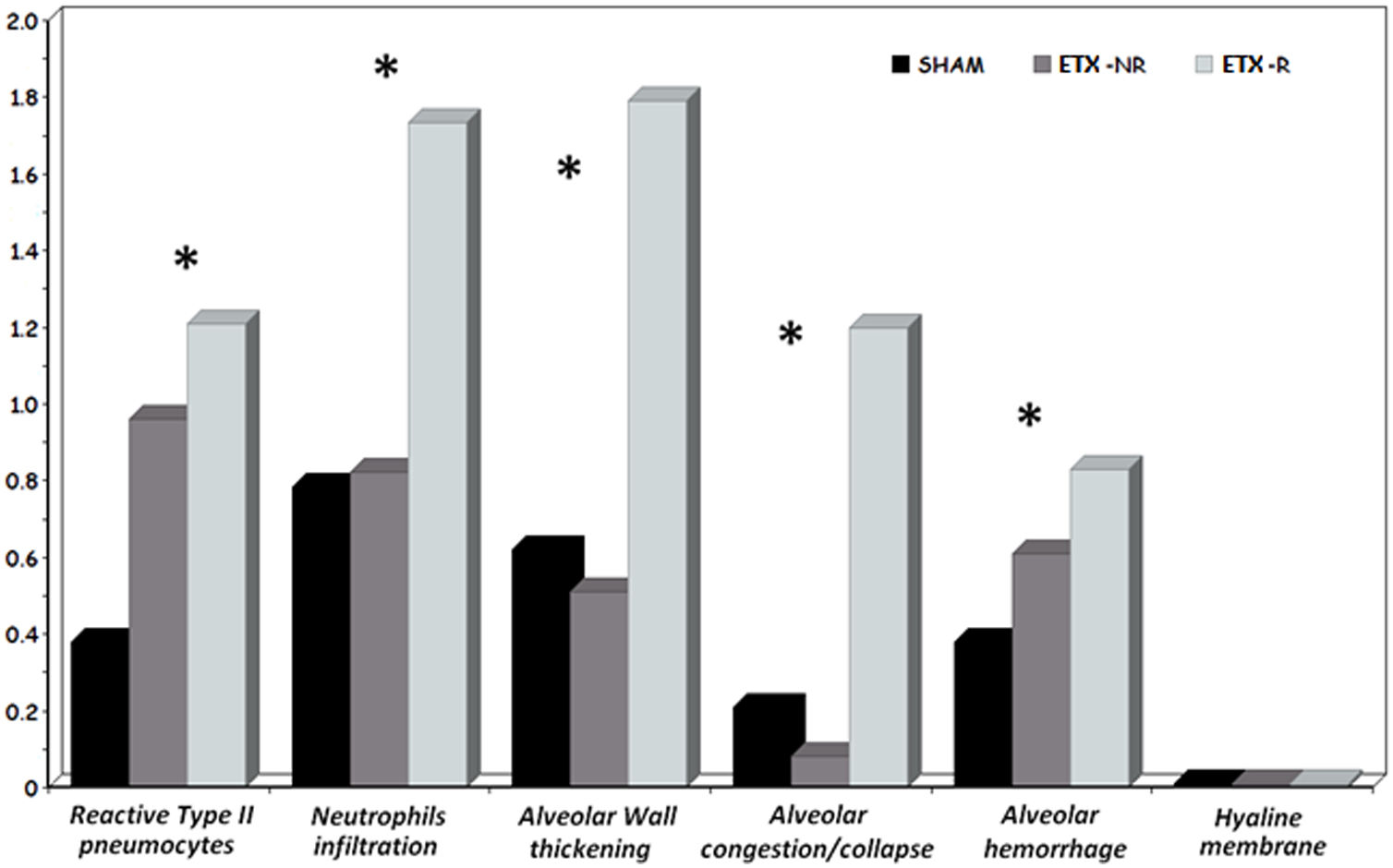
p<0.005 ETX-R vs. SHAM. #p<0.005 ETX-R vs. ETX-NR.As can be seen in Figs. 4 and 5, the main difference between both ETX groups and the SHAM group was the greatest presence of reactive type II pneumocytes in both septic groups (SHAM: 0.38; ETX-NR: 0.96; ETX-R: 1.21; p: <0.001). In the ETX-R group, a greater accumulation of neutrophils in the alveolar or the interstitial space (SHAM: 0.79; ETX-NR: 0.83; ETX-R: 1.72; p 0.003), thickening of the alveolar wall (SHAM: 0.62; ETX-NR: 0.51; ETX-R: 1.79; p <0.001), alveolar hemorrhage (SHAM: 0.38; ETX-NR: 0.61; ETX-R: 0.83; p <0.001) and alveolar congestion/collapse with non-aerated areas (SHAM: 0.21; ETX-NR: 0.08; ETX-R: 1.20; p <0.001) were observed. No hyaline membranes were observed in any of the animals. Lung injury scores were larger in the ETX-R group than for the SHAM group and the ETX-NR group (6.75, 2.71 and 2.96 respectively; p <0.001).
Graphical representation of histopathological variables (mean value of intensity and extension) analyzed in each group. Lung injury score 0–3. SHAM: sham-operated group; ETX: non-resuscitated septic group; ETX-R: resuscitated septic group. Hemodynamic resuscitation resulted in greater score of lung injury. *Indicate statistically significant difference from SHAM, ETX-NR and ETX-R by the non-parametric Kruskal Wallis test (p<0.01).
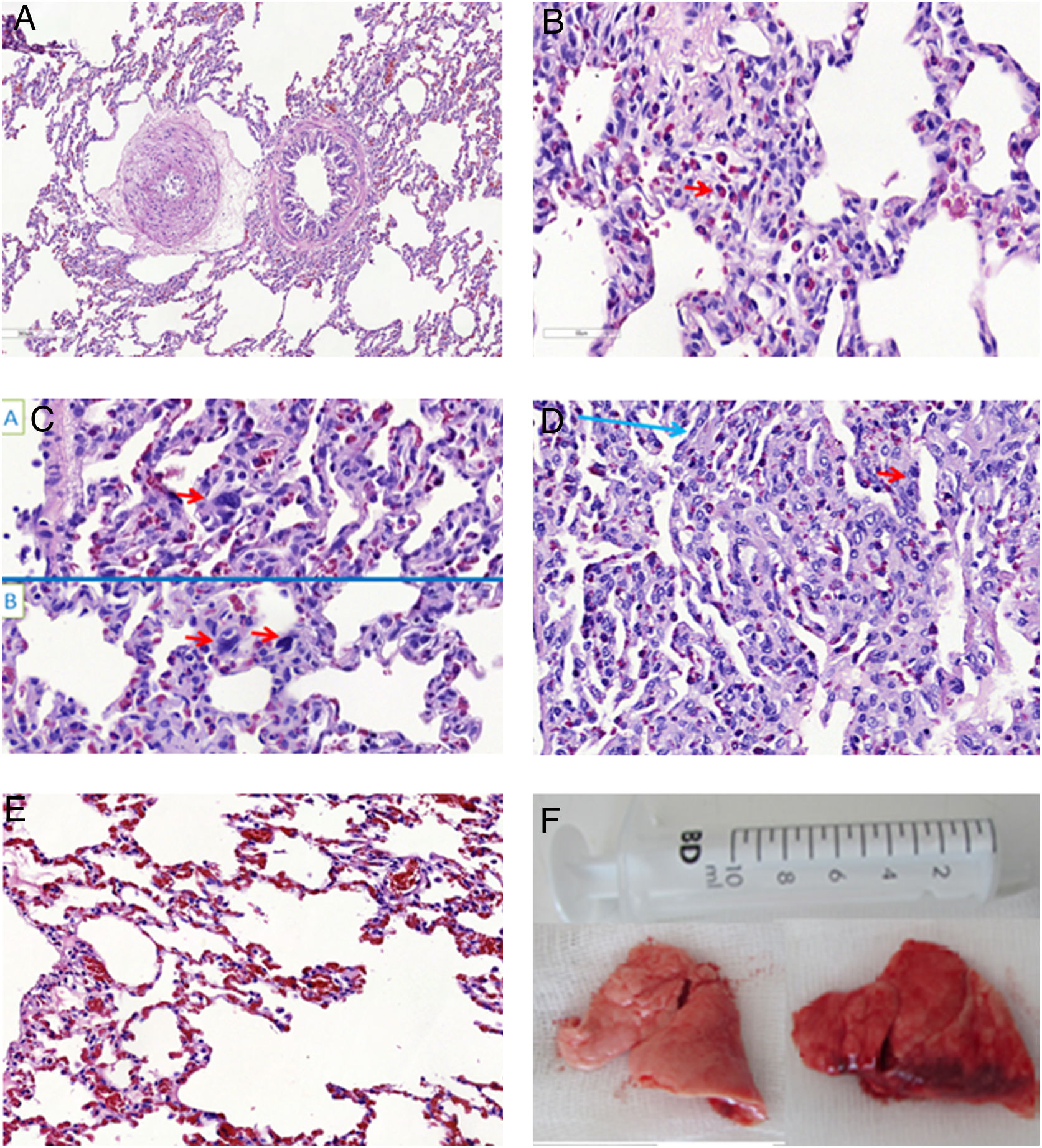
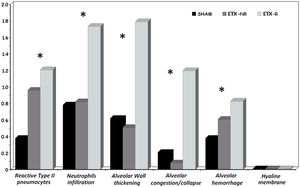
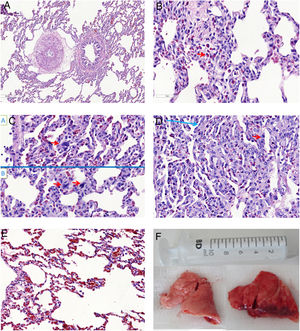
Microscopic aspects of the lungs from SHAM group (A), and the ETX-R (B, C, D, E). (A) normal lung. (B) Neutrophils in interstitium and alveolar wall. (C) Reactive Type II pneumocytes with hyperchromatic nuclei and nuclear membrane irregularity. (D) Thickened alveolar walls with intramural neutrophils, macrophages and fibroblasts. (E) Area with pulmonary emphysema. (F) Gross pathology surface of lung in the SHAM (left) and ETX-R (right) animals. Last one shows more intense damage, especially hemorrhagic areas and lung edema. SHAM: sham-operated group; ETX: non-resuscitated septic group; ETX-R: resuscitated septic group (HE ×40).
Hemodynamic resuscitation by means of fluids and NE is currently considered as the first-line resuscitation therapy by the international SSC.3 This guideline recommends an initial bolus of 30mL·kg−1 of fluid followed by infusion of vasopressors if the blood pressure goal is not achieved. The most important finding of our experimental study was that bolus fluid administration and late NE in the early hours of endotoxic septic shock has a negative influence on lung injury development. When ETX was administered to induce septic shock, an increase in reactive type II pneumocytes was found, which suggests epithelial damage.28 However, when fluids and NE were administered to restore blood pressure, they resulted in greater histopathological findings with an increase in neutrophils infiltration, reactive type II pneumocytes, alveolar congestion/collapse and alveolar wall thickening, and a larger lung injury score.
It can be assumed that increased pulmonary blood flow, due to administration of fluids (20mL·kg−1) and NE infusion, combined with a capillary permeability disorder, were the mechanisms responsible for the worsening of lung inflammatory damage initiated by endotoxemia. Although this is likely to be the price to pay for adequate hemodynamic optimization, our findings provide valuable information about the consequences and damaging effects of this strategy in septic shock.
ARDS is a catastrophic form of lung injury characterized by diffuse alveolar damage with severe inflammation and high permeability protein-rich edema.12 Recent studies suggest that the development of lung damage appears as a consequence of several impacts, which act as if it were a chain reaction.29 So, when an initial insult occurs, it primes an inflammatory response which damages and sensitizes the lungs without developing ARDS. However, if further impacts occur (second hits), even if they are less intense, an exaggerated inflammatory response arises, which can lead to gradual progression from initial lung injury to clinical ARDS. Our results suggest that this strategy of resuscitation might behave as a second hit influencing the progression of lung injury and favoring the development of ARDS.
Previous studies have shown how the amount of administered fluids increases the likelihood of developing ARDS among patients at risk.13,30 Jia et al. retrospectively demonstrated that net fluid balance during the first 48h of mechanically ventilated patients was associated with the development of ARDS.14 In addition, Hughes et al. found that among patients admitted after major surgery, the amount of fluid infused during surgery was independently associated with ARDS.18
Despite multiple studies showing the overall association between the amount of fluids administered and the development of ARDS, the consequences of the aggressive amount of fluid during hemodynamic resuscitation in the early phase of septic shock has not been well analyzed. Unfortunately, most of the studies have been retrospective in nature with small sample sizes. Our experimental study addresses this question and found an association between fluid administration and late NE used in the early phase of endotoxic septic shock and the development of lung injury. Seethala et al. highlighted the role of the amount of fluid administered to septic patients during the first 6h of care and the development of ARDS. However, this association was present in patients without shock.31 Similarly, Chang et al. retrospectively examined a cohort of 75 patients hospitalized with ARDS secondary to severe sepsis or septic shock and demonstrated that total volume of fluid infused during the first 6 or 24h of care did not increase the risk of ARDS after 72h of hospitalization.32 However, the novelty of our experimental study is that histopathological findings suggest that, although clinically it may not be manifested during the first days of hospitalization, the damaged lung can be silently initiated during resuscitation in the early phase of septic shock. Andrews et al. conducted a randomized controlled trial in Zambian patients with septic shock and found that the amount of fluid during the first 6h of care (4 vs. 2.5l; p<0.001) was associated with increased mortality (48% vs. 33%; p<0.03), but also with an increase of respiratory complications, such as hypoxemia and tachypnea (35.8% vs. 22.3%; p<0.03).33 A recent experimental study with an ovine septic shock model observed that fluid resuscitation led to increase in biomarker cardiac stress and endothelial glycocalyx shedding.34
NE could also contribute in a detrimental way to the development of lung injury. It is known that when NE is administered after fluid replacement, it is able to boost cardiac output through an improve in cardiac preload and cardiac contractility in septic shock patients. It increases the flow and perfusion in the pulmonary vascular system, which worsens the severity of lung damage.19–20 In addition NE, as it rises pulmonary vascular pressure, can also increase capillary hydrostatic pressure, which would extend transcapillary filtration, favoring pulmonary edema.35,36
An important application of this study is that, although the administration of fluids and NE in the early hours of septic shock is adequate at a cardiovascular point of view, we should assess whether the increase in pulmonary blood flow produced by this strategy, could have harmful effects on the lungs. We believe that a restrictive resuscitation strategy with less fluid in patients with septic shock could restore hemodynamics as well as reduce lung damage. In this context, several studies have investigated the consequences of restrictive strategies in patients with septic shock.37 For example, Permpikul et al. recently observed how with the early perfusion of NE, the amount of fluid administered in the first hours of admission was lower, without greater vasopressor requirement or a higher mortality rate.38 Ranjit et al., in addition to the decreased fluid administration, also observed a lower need for ventilator support in pediatric patients.39 However, the consequences of this strategy on ARDS have not yet been investigated. We suggest that this strategy would decrease the high incidence and severity of ARDS in septic patients.
Our study has several limitations. First, the short duration of our experiment does not allow us to evaluate the final evolution of lung damage. The follow-up of only four hours is limited to assess the consequences of the period of lung ischemia in the ETX-NR group. However, our aim was to determine the direct consequences of increased pulmonary blood flow during the first phase of hemodynamic resuscitation. Moreover, even in the early phase of this resuscitation, histopathological findings showed alterations suggestive of ARDS. Second, the evaluation the state of shock and guide resuscitation was not based on parameters of tissue hypoperfusion used in clinical practice, such as arterial pH, blood lactate, or P(v-a)CO. Furthermore, blood gases as a measure of lung function were not used, so lung damage was only determined by lung histology. Third, the amount of fluids administered in our study was less than recommended by the SSC. However, our intention was to evaluate if, even with the administration of less quantity of fluids than recommended, lung injury was already established. Also, the deleterious effects of aggressive fluid administration are well-known. Therefore, although the SSC recommends the administration of 30mL·kg−1 in the first hours of resuscitation, nowadays we tend to limit the volume contribution to the optimization of VO2/DO2 dependency. It's likely that a dose of 30mL·kg−1 would have been more appropriate from the hemodynamic point of view, however, the histopathological alterations would probably also have been greater. Fourth, the use of mechanical ventilation without PEEP could influence the development of lung injury behaving as an additional impact, although it was common in all groups. Experimental studies have shown that when lung injury is induced by mechanical ventilation without PEEP, there are other histopathological findings.26 Fifth, although our findings show that this strategy can be detrimental to lung injury, we did not analyze the effects of fluids nor NE alone. Nevertheless, Passmore et al. already analyzed this in an ovine model of septic shock, and did not find any histopathological lung differences between both treatments.40 Finally, our experimental study was carried out in young rabbits, so our results should be interpreted with caution when extrapolated to human lung injury.
ConclusionsOur experimental study shows that increasing pulmonary blood flow with fluids and NE until reaching a MAP endpoint in endotoxic septic shock has direct deleterious effects on lung damage. The relations between this strategy and the inflammatory lung injury were explained by histopathologic findings. These findings suggest that once lung damage has been initiated by endotexemia, the aggressive administration of fluids and NE act as second hits. More studies are required to assess whether the prevention of these second hits could result in a decrease in the incidence and severity of lung damage.
Contributions of the authorsP.G.G.: designed the study, participated in the experiments, acquired and interpreted the data, performed the statistical analysis, and draft the manuscript. M.I.M.G. and M.G.R.: participated in the experiments, acquired and interpreted the data, performed the statistical analysis and helped to draft the manuscript. A.G.C.: designed the study, acquired and interpreted the data, performed the statistical analysis, and helped to draft the manuscript. M.G.R.: Pathology study. M.C.: contributed in the conception and design of the study. All the authors approved the final manuscript, and have also ensured that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
FundingThis work was supported by the St George's University of London, UK, and performed at the Servicio Central de Experimentación y Producción Animal (SEPA) of the University of Cadiz, Spain.
Conflict of interestsM.I.M.G. is consultant to Edwards Lifescences and received honoraria and/or travel expenses from Deltex Medical. M.C. has received honoraria and/ot travel expenses from Edwards Lifescences, LiDCO, Cheetah, Bmeye, Masimo and Deltex Medical. P.G.G., M.G.R. and A.G.C. have no relevant financial relationships or conflicts of interest to disclose.
We thank Dr. Carlos Costela Villodres, from the Servicio Central de Experimentación y Producción Animal (SEPA) of the University of Cádiz, for his valuable technical assistance. The work was performed at the Servicio Central de Experimentación y Producción Animal (SEPA) of the University of Cadiz, Spain.