Higher blood nitrate and nitrite levels have been found in coronavirus disease 2019 (COVID-19) patients than in healthy subjects. The present study explores the potential association between serum nitrate levels and mortality in COVID-19 patients.
DesignA prospective observation study was carried out.
SettingEight Intensive Care Units (ICUs) from 6 hospitals in the Canary Islands (Spain).
PatientsCOVID-19 patients admitted to the ICU.
InterventionsDetermination of serum nitrate levels at ICU admission.
Main variable of interestMortality at 30 days.
ResultsNon-surviving (n=11) compared to surviving patients (n=42) showed higher APACHE-II (p<0.001) and SOFA scores (p=0.004), and higher serum nitrate levels (p=0.001). Logistic regression analyses showed serum nitrate levels to be associated to 30-day mortality after controlling for SOFA (OR=1.021; 95%CI=1.006–1.036; p=0.01) or APACHE-II (OR=1.023; 95%CI=1.006–1.041; p=0.01). There were no differences in the area under the curve (AUC) for mortality prediction by serum nitrate levels (AUC=83%; 95%CI=73–92%; p<0.001), APACHE II (AUC=85%; 95%CI=75–96%; p<0.001) and SOFA (AUC=78%; 95%CI=63–92%; p=0.005) based on the DeLong method. The Kaplan–Meier analysis found patients with serum nitrates levels>68.4μmol/l to have a higher mortality rate (hazard ratio=138.8; 95%CI=22.3–863.9; p<0.001).
ConclusionsThe main novel finding was the association between serum nitrate levels and mortality in COVID-19 patients controlling for the SOFA or APACHE-II scores, though larger studies are needed to confirm this observation.
Se han encontrado niveles más elevados de nitratos en la sangre de pacientes con enfermedad del coronavirus 2019 (COVID-19) que en sujetos sanos. Por lo tanto, el objetivo de estudio consistió en explorar la posible asociación entre los niveles séricos de nitratos y la mortalidad de pacientes por COVID-19.
DiseñoEstudio observacional y prospectivo.
ÁmbitoOcho unidades de cuidados intensivos (UCI) de 6 hospitales de las Islas Canarias (España).
PacientesPacientes COVID-19 ingresados en la UCI.
IntervencionesSe midieron los niveles séricos de nitratos al ingreso en la UCI.
Variable de interés principalMortalidad a los 30 días.
ResultadosLos pacientes fallecidos (n=11) comparados con los supervivientes (n=42) presentaron mayores APACHE-II (p<0,001), SOFA (p=0,004) y niveles séricos de nitratos (p=0,001). Los análisis de regresión logística mostraron una asociación entre los niveles séricos de nitratos al ingreso en la UCI y la mortalidad a los 30 días controlando por SOFA (OR:1.021; IC 95%:1.006-1.036; p=0,01) o APACHE-II (OR:1.023; IC 95%:1.006-1.041; p=0,01). No encontramos diferencias en el área bajo la curva (ABC) para la predicción de mortalidad entre los niveles séricos de nitratos (ABC:83%; IC 95%:73-92%; p<0,001), APACHE-II (ABC:85%; IC 95%:75-96%; p<0,001) y SOFA (ABC:78%; IC 95%:63-92%; p=0,005) con el método de DeLong. El análisis de Kaplan-Meier mostró que los pacientes que tenían niveles séricos de nitratos al ingreso en la UCI>68,4μmol/l presentaban mayor riesgo de fallecer (hazard ratio:138,8; IC 95%:22,3-863,9; p<0,001).
ConclusionesEl principal nuevo hallazgo fue la asociación entre los niveles séricos de nitratos y la mortalidad de pacientes COVID-19 controlando por SOFA o APACHE-II; pero estudios de mayor tamaño muestral son necesarios para confirmar este resultado.
Coronavirus disease 2019 (COVID-19), an emerging health threat in the world, is the disease produced by the novel coronavirus named severe acute respiratory syndrome coronavirus 2 (SARSCoV-2) that was detected for the first time in Wuhan (China) in December 2019. To September 15 of 2020 approximately had 29,459,649 confirmed cases and 932,997 deaths (3.2%) from COVID-19.1,2 Several factors have been associated with higher death as age, several comorbidities (cardiovascular diseases, arterial hypertension, chronic obstructive pulmonary disease, smoking, diabetes mellitus or cerebrovascular diseases), some blood biomarkers (of kidney dysfunction, inflammation, cardiac injury, muscle injury, liver dysfunction and coagulation alterations), and some clinical data as the appearance of acute respiratory distress syndrome (ARDS).3–8
In sepsis, nitric oxide synthesis is dysregulated with exaggerated production leading to cardiovascular dysfunction, bioenergetic failure and cellular toxicity.9–16 Nitric oxide is a very unstable and short half-life gas that breaks rapidly to the stable products as nitrate and nitrite.17,18 Higher blood nitrate and nitrite levels has been found in patients with higher sepsis severity,19–23 and in non-survivor than in survivor septic patients.23 Increased blood nitrate and nitrite levels has been found in COVID-19 patients than in healthy subjects24; however, we have not found data about serum nitrate levels and prognosis of COVID-19 patients. Thus, the objective of this study was to explore the potential association between serum nitrate levels and mortality of COVID-19 patients.
MethodsDesign and subjectsEigth Intensive Care Units from 6 hospitals of Canary Islands (Spain) participated in this prospective and observational study. The patients were recruited during March and April of 2020. The Ethics Committee (Protocol code CHUC-2020-26) from each hospital approved the protocol study. Due to the context of the rapid emergence of this infectious disease and the prohibition of patient visits by the Government of Spain due to the public health outbreak policy, and due to that data were prospectively collected, the requirement of written informed consent for participate in the study was waived.
Patients admitted to the ICU during April of 2020 with laboratory-confirmed COVID-19 by an assay of real time fluorescence reverse transcription-polymerase chain reaction (RT-PCR) from nasopharyngeal swab sample or a bronchial aspirate were included.
Determination of serum nitrate levelsWe collected blood samples at ICU admission in serum separator tubes and serum was stored immediately at −+80°C until to the end of recruitment. All determinations of serum nitrate concentrations were performed blindly to clinical data in the same Laboratory Department. Nitrate determination was performed following the procedure described by Navarro-Gonzálvez et al.25 with some modifications. The measurement was made on a Cobas C 501 analyzer (Roche Diagnostics). Each of the 5 batches into which the samples were divided for analysis was processed together with five calibrators (concentrations 0, 5, 20, 40, 80mM) processed analogously to the samples, as well as 2 controls (of concentrations 20.5mM and 71mM, from which a coefficient of variation of 9.5% and 5.1% respectively was obtained).
Variables recordedData patients were anonymized. In respect to demographic and clinical characteristics, we recorded the following data: sex, body max index, age, and history of chronic renal failure, diabetes mellitus, chronic obstructive pulmonary disease (COPD), chronic liver disease, smoking cessation, active smoking, ischemic heart disease, arterial hypertension, steroid agents, hematological tumor, human immunodeficiency virus (HIV) and solid tumor. Besides, we recorded temperature, chest radiograpy findings, Sepsis-related Organ Failure Assessment [SOFA] score,26 Acute Physiology and Chronic Health Evaluation (APACHE)-II score,27 and the development of septic shock28 and ARDS.29
Regarding to laboratory data at ICU admission, we recorded glucose, lactic acid, creatinine, sodium, protein, albumin, creatine kinase, bilirubin, alanine transaminase, aspartate transaminase, gamma-glutamyl transpeptidase, alkaline phosphatase, lactate dehydrogenase, procalcitonin, C-reactive protein, ferritin, N-terminal prohormone of brain natriuretic peptide (NTproBNP), interleukin-6, hematocrit, hemoglobin, white blood cell, lymphocytes, neutrophils, monocytes, basophils, eosinophils, platelets, activated partial thromboplastin time (aPTT), international normalized ratio (INR), d-dimer, fibrinogen, pressure of arterial oxygen (PaO2), fraction inspired of oxygen (FIO2).
In respect to ICU treatment, we recorded prone position, neuromuscular blockers, respiratory support, hydroxicloroquine, lopinavir/ritonavir, interferon, tocilizumab, steroid agents, vasopressors, and intermittent and continuous renal replacement therapy. Finally, we considered survival at 30 days as our endpoint study.
Statistical methodsMedians (percentile 25–75), frequencies (percentages), Mann–Whitney U test and chi-square test were used to describe and compare continuous and categorical variables between surviving and non-surviving patient groups. We tested the ability of serum nitrate levels, SOFA and APACHE-II to predict mortality using receiver operating characteristic (ROC) analysis, and areas under curves were compared with the method of DeLong et al.30 We constructed Kaplan–Meier 30-day survival curves using serum nitrates concentrations ≤68.4μmol/L and >68.4μmol/L (level point selected in basis to Youden J index), and curves were compared with log-rank test. We performed logistic regression analyses to determine the possible association between serum nitrate levels and 30-day mortality after to control by SOFA or APACHE-II. We constructed two models with only two predictor variables in each model due to the number of death patients was 11, and Odds Ratio and its 95% confidence intervals were reported. We used Spearman's rank correlation coefficient to test association between continuous variables. A point p<0.05 was used to consider significant differences. Statistical analyses were carried out with SPSS 17.0 (SPSS Inc., Chicago, IL, USA).
ResultsA total of 53 patients were included, all admitted in ICU with respiratory insufficiency, with SOFA of 5.7±2.5 and APACHE-II of 13.4±6.5. Of them, 42 (79.2%) received invasive mechanical ventilation, 40 (75.5%) vasopressors, and 8 (15.1%) renal replacement therapy. We found that non-surviving (n=11) compared to surviving patients (n=42) showed higher temperature (p=0.03), septic shock (p=0.048), APACHE-II (p<0.001) and SOFA (p=0.004) (Table 1). In addition, non-surviving showed higher serum nitrate levels (p=0.001) and NTproBNP (p=0.04), and lower platelet count (p=0.02) (Table 2). Besides, non-surviving patients received more frequently CRRT (p=0.048) and vasopressors (p=0.047) during its ICU stay (Table 3).
Demographic and clinical data of non-surviving and surviving patients.
| Survivors(n=42) | Non-survivors(n=11) | p value | |
|---|---|---|---|
| Gender female – n (%) | 27 (64.3) | 7 (63.6) | 0.99 |
| Age (years) – median (p 25–75) | 65 (51–70) | 70 (59–75) | 0.10 |
| Body max index (kg/m2) – median (p 25–75) | 28.1 (24.8–32.4) | 27.1 (23.0–30.2) | 0.29 |
| Diabetes mellitus – n (%) | 12 (28.6) | 3 (27.3) | 0.99 |
| COPD – n (%) | 5 (11.9) | 3 (27.3) | 0.34 |
| Ischemic heart disease – n (%) | 1 (2.4) | 1 (9.1) | 0.38 |
| Smoking – n (%) | 2 (4.8) | 2 (18.2) | 0.19 |
| Arterial hypertension – n (%) | 16 (38.1) | 6 (54.5) | 0.49 |
| Steroid agents – n (%) | 1 (2.4) | 2 (18.2) | 0.11 |
| Temperature (°C) – median (p 25–75) | 37.0 (36.0–37.5) | 35.2 (34.1–36.7) | 0.03 |
| Chest radiography findings – n (%) | 0.95 | ||
| Consolidation only | 5 (11.9) | 1 (9.1) | |
| Ground glass opacity plus consolidation | 21 (50.0) | 6 (54.5) | |
| Ground glass opacity only | 16 (38.1) | 4 (36.4) | |
| ARDS – n (%) | 36 (85.7) | 9 (81.8) | 0.67 |
| Septic shock – n (%) | 4 (9.5) | 4 (36.4) | 0.048 |
| APACHE-II score – median (p 25–75) | 12 (7–15) | 18 (16–20) | <0.001 |
| SOFA score – median (p 25–75) | 5 (3–7) | 8 (5–9) | 0.004 |
COPD=Chronic Obstructive Pulmonary Disease; APACHE=Acute Physiology and Chronic Health Evaluation; SOFA=Sepsis-related Organ Failure Assessment; ARDS=acute respiratory distress syndrome.
Laboratory data at ICU admission of non-surviving and surviving patients.
| Survivors(n=42) | Non-survivors(n=11) | p value | |
|---|---|---|---|
| Serum nitrates levels (μmol/L) – median (p 25–75) | 20 (15–43) | 86 (40–224) | 0.001 |
| Lactic acid (mmol/L) – median (p 25–75) | 1.33 (1.09–1.80) | 1.60 (1.30–2.20) | 0.11 |
| Sodium (mEq/L)- median (p 25–75) | 138 (134–141) | 140 (135–144) | 0.20 |
| Creatinine (mg/dl) – median (p 25–75) | 0.87 (0.68–1.03) | 1.07 (0.72–1.73) | 0.23 |
| Protein (g/L) – median (p 25–75) | 6.4 (5.8–7.1) | 6.0 (5.6–7.0) | 0.60 |
| C-reactive protein (mg/gl) – median (p 25–75) | 20 (10–76) | 24 (18–67) | 0.34 |
| Procalcitonin (ng/ml) – median (p 25–75) | 0.17 (0.08–0.48) | 0.58 (0.06–0.76) | 0.49 |
| Ferritin (ng/ml) – median (p 25–75) | 1039 (653–1817) | 1383 (859–2761) | 0.50 |
| NTproBNP (pg/ml) – median (p 25–75) | 451 (137–1379) | 5953 (5534–6371) | 0.04 |
| Interleukin-6 (pg/ml) – median (p 25–75) | 50 (6–179) | 61 (24–140) | 0.77 |
| Hemoglobin (g/dL) – median (p 25–75) | 12.8 (11.7–14.4) | 12.8 (11.0–15.0) | 0.95 |
| White blood cell – median*103/mm3 (p 25–75) | 7.7 (6.0–11.6) | 7.9 (5.3–13.1) | 0.95 |
| Platelets – median*103/mm3 (p 25–75) | 246 (173–383) | 158 (108–278) | 0.02 |
| INR – median (p 25–75) | 1.17 (1.06–1.36) | 1.18 (1.02–1.32) | 0.83 |
| aPTT (seconds) – median (p 25–75) | 27 (25–32) | 30 (23–36) | 0.52 |
| Fibrinogen (mg/dl) – median (p 25–75) | 711 (506–829) | 699 (600–910) | 0.49 |
| D-dimer (ng/mL) – median (p 25–75) | 1102 (744–2202) | 3516 (682–21480) | 0.21 |
| PaO2/FI02 ratio – median (p 25–75) | 133 (103–201) | 111 (100–140) | 0.30 |
NTproBNP=N-terminal prohormone of brain natriuretic peptide; INR=International normalized ratio; aPTT=Activated partial thromboplastin time; PaO2=pressure of arterial oxygen; FIO2=fraction inspired oxygen.
Treatment data in ICU of non-surviving and surviving patients.
| Survivors(n=42) | Non-survivors(n=11) | p value | |
|---|---|---|---|
| Respiratory support – n (%) | 0.30 | ||
| Conventional oxygen therapy | 4 (9.5) | 0 | |
| High-flow nasal cannula | 4 (9.5) | 0 | |
| Non-invasive mechanical ventilation | 3 (7.1) | 0 | |
| Invasive mechanical ventilation | 31 (73.8) | 11 (100) | |
| Neuromuscular blockers – n (%) | 26 (61.9) | 7 (63.6) | 0.99 |
| Prone position – n (%) | 13 (31.0) | 4 (36.4) | 0.73 |
| Lopinavir/Ritonavir – n (%) | 39 (92.9) | 10 (90.9) | 0.99 |
| Hydroxychloroquine – n (%) | 39 (92.9) | 11 (100) | 0.99 |
| Interferon Beta 1-B – n (%) | 26 (61.9) | 7 (63.6) | 0.99 |
| Tocilizumab – n (%) | 15 (35.7) | 6 (54.5) | 0.31 |
| Steroid agents – n (%) | 31 (73.8) | 9 (81.8) | 0.71 |
| Intermittent renal replacement therapy – n (%) | 0 | 0 | – |
| Continuous renal replacement therapy – n (%) | 4 (9.5) | 4 (36.4) | 0.048 |
| Vasopressors – n (%) | 29 (69.0) | 11 (100) | 0.047 |
Logistic regression analyses showed that serum nitrate levels were associated with 30-day mortality after controlling for SOFA (OR=1.021; 95% CI=1.006–1.036; p=0.01), APACHE-II (OR=1.023; 95% CI=1.006–1.041; p=0.01) or serum creatinine concentrations (OR=1.022; 95% CI=1.007–1.038; p=0.004) (Table 4).
Multiple logistic regression analyses to predict mortality at 30 days.
| Odds ratio | 95% confidence interval | p-Value | |
|---|---|---|---|
| Model 1: | |||
| Serum nitrates levels (μmol/L) | 1.021 | 1.006–1.036 | 0.01 |
| SOFA score (points) | 1.496 | 1.022–2.189 | 0.04 |
| Model 2: | |||
| Serum nitrates levels (μmol/L) | 1.023 | 1.006–1.041 | 0.01 |
| APACHE-II (points) | 1.336 | 1.076–1.657 | 0.01 |
| Model 3: | |||
| Serum nitrates levels (μmol/L) | 1.022 | 1.007–1.038 | 0.004 |
| Creatinine (mg/dl) | 1.093 | 0.948–1.260 | 0.22 |
SOFA=Sepsis-related Organ Failure Assessment; APACHE=Acute Physiology and Chronic Health Evaluation.
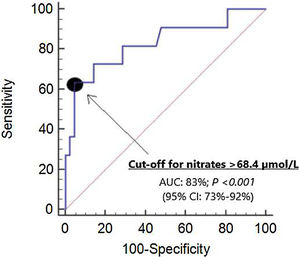
We did not find differences in the area under curve (AUC) for mortality prediction by serum nitrate levels (AUC=83%; 95% CI=73–92%; p<0.001) (Fig. 1), APACHE II (AUC=85%; 95% CI=75–96%; p<0.001) and SOFA (AUC=78%; 95% CI=63–92%; p=0.005).
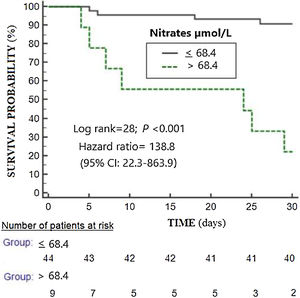
The selected point of serum nitrates levels>68.4μmol/L for mortality prediction had 64% of sensitivity (31–89%), 95% of specificity (84–99%), 13.4 of positive likelihood ratio (3.2–55.5), 0.4 of negative likelihood ratio (0.2–0.8), 78% of positive predictive value (46–94%) and 91% of negative predictive value (82–96%). In Kaplan–Meier analysis was found that patients with serum nitrates levels>68.4μmol/L had a higher mortality rate (Hazard ratio=138.8; 95% CI=22.3–863.9; p<0.001) (Fig. 2).
We did not find significant differences in serum nitrate levels between patients with and without septic shock (p=0.05). We did not find a significant association of serum nitrate levels with norepinephrine dosage at the time of patient inclusion (n=53; rho=0.24; p=0.09) and NTproBNP (n=20; rho=0.30; p=0.20).
DiscussionTo our knowledge, our series is the first one reporting serum nitrate levels in COVID-19 patients and the main new finding was the association between serum nitrate levels and mortality controlling for SOFA or APACHE-II.
Serum nitrite and nitrate are the stable end products of nitric oxide.17,18 Increased serum levels of these metabolites could be due to increased nitric oxide production and/or decreased elimination of serum nitrite and nitrate due to alterations in renal function.31 We have not found significant differences between survivor and non-survivor patients in renal function at ICU admission when were obtained blood samples for the determination of serum nitrate levels. In addition, we have found an association between serum nitrate levels and mortality after to control for serum creatinine concentrations. Thus, it is possible that those higher serum nitrate levels from non-survivor patients could be due mainly to a higher nitric oxide production.
Besides of serum nitrate levels, other variables with significant difference between non-surviving and surviving patients were septic shock, APACHE-II, SOFA, NTproBNP, and platelet count. Other factors that have been associated with higher death as age, several comorbidities, blood biomarkers of inflammation and coagulation alterations3–8; however, in our series no other significant differences were found possibly due to the small sample size of our study. In addition, the prevalence of symptomatic COVID-19 has been found to be higher in men than in women32; however, in our series had more woman possibly due to the small sample size of our study, although no had significant differences in mortality according the sex.
Increased nitric oxide production occurs in sepsis due to increased mRNA expression of the enzyme named inducible nitric oxide synthase (iNOS) in different cells as endothelial cells, platelets, macrophages and cardiac myocytes after exposure to bacterial lipopolysaccharide.33 This increase of nitric oxide contribute to cardiovascular dysfunction (vasodilation, hyporeactivity to vasopressors and negative inotropic effect), bioenergetic failure (inhibition of mitochondrial respiration due to that alter the function of the mitochondrial respiratory complex IV interrupting the normal transport of electrons leading to decreased adenosine triphosphate production) and cellular toxicity (activation of intrinsic apoptosis pathway trough the opening of mitochondrial transition pores allowing the transportation of cytochrome c from the mitochondria into the cytosol, which activate caspase-9 and that activate caspases 3 that leds to death cell by apoptosis).9–16 In our study, we found a trend to a higher serum nitrate levels in patients with septic shock, and a trend to positive associations of serum nitrate levels with norepinephrine dosage at the time of patient inclusion and with blood NTproBNP concentrations; although it is possible that the sample size contribute in the absence of significant differences. In addition, we have not studied mitochondrial respiratory complex and apoptosis, and those were some limitations of our study. We think that this association between high serum nitrate levels and high COVID-19 patients mortality found in our study could be due to a higher nitric oxide production which lead to cardiovascular dysfunction, bioenergetic failure and cellular toxicity and all this can contribute to organ dysfunction and finally the death of the patients. Our novel findings about higher serum nitrate levels in non-survivor than in survivor COVID-19 patients are in line with those found in septic patients.23
In a study of pulmonary autopsy specimens from patients who died from Covid-19 or from influenza A(H1N1) infection or from uninfected patients was found higher angiogenesis in lungs from patients with Covid-19.34 In addition, nitric oxide has been associated with angiogenesis in different diseases as cancer35 or chronic liver diseases.36,37 Thus, it is possible that if we had studied pulmonary autopsy specimens and serum nitrate levels during patient evolution, we would have observed in non-survivor patients persistently higher serum nitrate levels and a great angiogenesis in autopsies. However, those were other limitations of our study.
Other limitation of our study was that the limited number of deaths prevented that more variables were included in the same regression model. However, a strength of the study was that the association between serum nitrate levels and mortality remained after controlling for SOFA or APACHE-II. Another interesting finding of our study was that the capacity for mortality prediction provided by serum nitrate levels was not different that those of SOFA and APACHE-II. We think that the practical usefulness of our study lies in the fact that serum nitrate levels, with an easy and cheap determination, could help to clinicians in the estimation of prognosis in COVID-19 patients.
In recent years, different approaches to modulate nitric oxide activity in septic patients (agents that reducing nitric oxide production inhibiting iNOS or increasing nitric oxide elimination acting as scavengers) have improved systemic vascular resistance and blood pressure in patients with septic shock without obtain a reduction in mortality rate.13,14 The development of other pharmacological strategies to reduce iNOS to decrease nitric oxide production could improve the prognosis of those patients. Recently, the inhibition of iNOS by agmatine has attenuated vascular dysfunction and mortality rate in rat endotoxemic model.33 In addition, in a small study were administered three antioxidants (methylene blue, vitamin C and N-acetyl cysteine) to five COVID-19 patients and serum nitrate levels decreased after treatment.24 Other therapeutic aspect in COVID-19 patients is the potential use of inhaled nitric oxide due to its vasodilation effect to reverse pulmonary hypertension and due to its potential antiviral activity against SARS-CoV-2 infection.38,39
ConclusionsThe main new finding of our study was the association between serum nitrate levels and mortality in COVID-19 patients controlling for SOFA or APACHE-II, but larger studies are needed to confirm this result.
Authors’ contributionsLLo conceived, designed and coordinated the study, participated in acquisition and interpretation of data, and drafted the manuscript.
MMM, MA, AP, LRG, JSV, JAMR and NO participated in acquisition of data.
FGB and JANG carried out the determinations of serum nitrates levels.
AJ participated in the interpretation of data.
All authors revised the manuscript critically for important intellectual content and made the final approval of the version to be published.
FundingThis study was supported by a grant from Instituto de Salud Carlos III (PI-18-00500) (Madrid, Spain) and co-financed with Fondo Europeo de Desarrollo Regional (FEDER).
Conflicts of interestThe authors declare that they have no competing interests.
Investigators of Working Group of COVID-19 Canary ICU: Leonardo Lorente, Fuensanta Gómez-Bernal, José Antonio Navarro-Gonzálvez, Alina Perez, Alejandro Jiménez, Alejandra Pérez-Llombet, Luis Uribe, Lourdes González, Rocío Alvarez, (Hospital Universitario de Canarias. La Laguna. Tenerife. Spain); María M. Martín, Julia Alcoba-Flórez, Miguel Angel Rodríguez, Albano Estupiñan (Hospital Universitario Nuestra Señora de Candelaria. Santa Cruz de Tenerife. Spain); Mónica Argueso, Juan J. Cáceres, Marta Riaño-Ruiz, Juan Guillermo O Campo, Paula Vega, Lucía Gonzalez (Hospital Insular. Las Palmas de Gran Canaria. Spain); Jordi Solé-Violán, Nazario Ojeda, Sergio López, Aurelio Rodríguez-Pérez, Casimira Domínguez (Hospital Universitario Dr. Negrín. Las Palmas de Gran Canaria. Spain); José Alberto Marcos y Ramos, María F. Zapata, Tamara Cantera (Hospital Doctor José Molina Orosa. Lanzarote. Spain); Luis Ramos-Gómez, Raquel Ortiz-López, Leopoldo Martín Martín (Hospital General La Palma. La Palma. Spain).