Different genetic polymorphisms of human leukocyte antigen (HLA) have been associated with the risk and prognosis of autoimmune and infectious diseases. The objectives of this study were to determine whether there is an association between HLA genetic polymorphisms and the susceptibility to and mortality of coronavirus disease 2019 (COVID-19) patients.
DesignObservational and prospective study.
SettingEight Intensive Care Units (ICU) from 6 hospitals of Canary Islands (Spain).
PatientsCOVID-19 patients admitted in ICU and healthy subjects.
InterventionsDetermination of HLA genetic polymorphisms.
Main variable of interestMortality at 30 days.
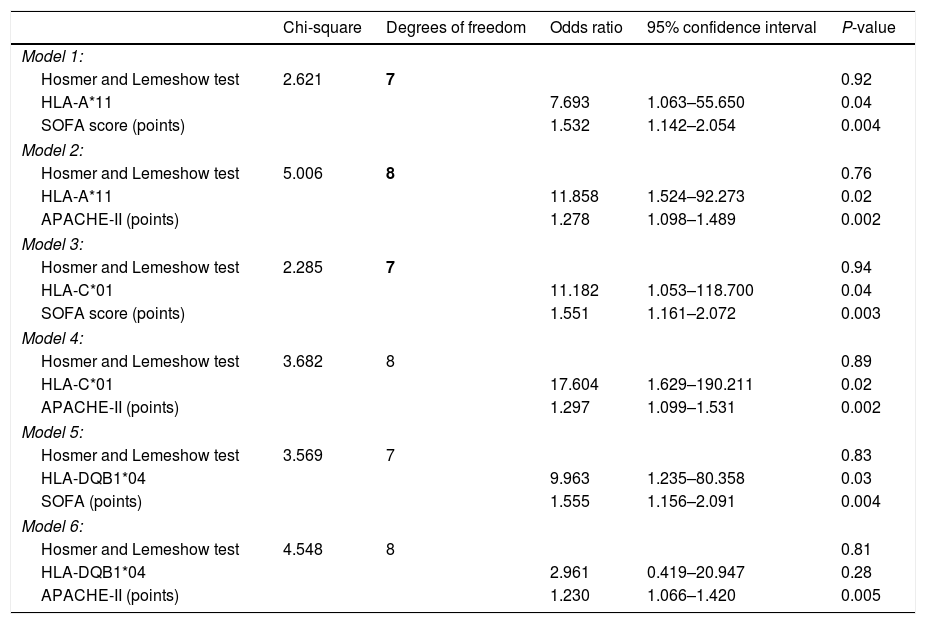
ResultsA total of 3886 healthy controls and 72 COVID-19 patients (10 non-survivors and 62 survivor patients at 30 days) were included. We found a trend to a higher rate of the alleles HLA-A*32 (p=0.004) in healthy controls than in COVID-19 patients, and of the alleles HLA-B*39 (p=0.02) and HLA-C*16 (p=0.02) in COVID-19 patients than in healthy controls; however, all these p-values were not significant after correction for multiple comparisons. Logistic regression analysis showed that the presence of certain alleles was associated with higher mortality, such as the allele HLA-A*11 after controlling for SOFA (OR=7.693; 95% CI=1.063–55.650; p=0.04) or APACHE-II (OR=11.858; 95% CI=1.524–92.273; p=0.02), the allele HLA-C*01 after controlling for SOFA (OR=11.182; 95% CI=1.053–118.700; p=0.04) or APACHE-II (OR=17.604; 95% CI=1.629–190.211; p=0.02), and the allele HLA-DQB1*04 after controlling for SOFA (OR=9.963; 95% CI=1.235–80.358; p=0.03).
ConclusionsThe new finding from our preliminary study of small sample size was that HLA genetic polymorphisms could be associated with COVID-19 mortality; however, studies with a larger sample size before definitive conclusions can be drawn.
Diferentes polimorfismos genéticos de los antígenos leucocitarios humanos (HLA) están asociados con el riesgo y el pronóstico de enfermedades autoinmunes e infecciosas. Los objetivos de estudio fueron determinar si existe una asociación entre polimorfismos genéticos de HLA y la susceptibilidad y mortalidad de pacientes con la enfermedad del coronavirus 2019 (COVID-19).
DiseñoEstudio observacional y prospectivo.
ÁmbitoOcho unidades de cuidados intensivos (UCI) de 6 hospitales de las Islas Canarias (España).
PacientesPacientes COVID-19 ingresados en la UCI y sujetos sanos.
IntervencionesSe determinaron los polimorfismos genéticos de los HLA.
Variable de interés principalMortalidad a los 30 días.
ResultadosSe incluyeron 3.886 sujetos sanos y 72 pacientes COVID-19 (10 fallecidos y 62 supervivientes a 30 días). Encontramos una tendencia a una mayor frecuencia de los alelos HLA-A*32 (p=0,004) en sujetos sanos que en pacientes COVID-19, y de los alelos HLA-B*39 (p=0,02) y HLA-C*16 (p=0,02) en pacientes COVID-19 que en sujetos sanos; sin embargo, no fueron significativos al corregir por comparaciones múltiples. En la regresión logística encontramos que la presencia de ciertos alelos estuvo asociada con mayor mortalidad, como el alelo HLA-A*11 controlando por SOFA (OR=7.693; IC del 95%=1.063-55.650; p=0,04) o APACHE-II (OR=11.858; IC del 95%=1.524-92.273; p=0,02), el alelo HLA-C*01 controlando por SOFA (OR=11.182; IC del 95%=1.053-118.700; p=0,04) o APACHE-II (OR=17.604; IC del 95%=1.629-190.211; p=0,02) y el alelo HLA-DQB1*04 controlando por SOFA (OR=9.963; IC del 95%=1.235-80.358; p=0,03).
ConclusionesLos nuevos hallazgos de nuestro preliminar estudio de pequeño tamaño muestral fueron que determinados polimorfismos genéticos de los HLA podrían estar asociados con la mortalidad de pacientes COVID-19; sin embargo, son necesarios estudios de mayor tamaño muestral para concluirlo definitivamente.
The novel coronavirus called as severe acute respiratory syndrome coronavirus 2 (SARSCoV-2) was detected for the first time in December 2019 in Wuhan (China) and the disease it causes is named as coronavirus disease 2019 (COVID-19). COVID-19 is an emerging health threat in the world. To June 8, 2020 there were 7,106,010 confirmed cases and 406,395 deaths (5.7%) from COVID-19.1,2 Several factors have been associated with higher death as age, some comorbidities (arterial hypertension, diabetes mellitus, smoking, chronic obstructive pulmonary disease, cerebrovascular or cardiovascular diseases), blood biomarkers (of inflammation, muscle injury, cardiac injury, liver dysfunction, coagulation alterations and kidney dysfunction), and clinical data as the development of acute respiratory distress syndrome (ARDS).3–8
Human Leukocyte Antigen (HLA), located in the short arm of human chromosome 6 (6p21.3), represents one of the most highly polymorphic systems in the human genome and plays a central role in the regulation of immune response. The HLA system include near to 27,000 alleles in three distinct classes of genes (Class I, II and III). Of the three classes of genes, HLA class I-A, B, C and class II-DR, DP, DQ play a crucial role in various immunological functions in human including antigen presentation to T lymphocytes and recognition of self and non-self proteins. HLA class I and II gene polymorphisms provide the strongest and most consistent alleles for autoimmune diseases susceptibility.9,10
HLA plays a central role in antigen presentation and therefore different allelic polymorphism could be involved in the susceptibility to infectious diseases. Different genetic polymorphisms of HLA have been associated with predisposition and/or outcome of different infectious diseases such as hepatitis B virus (HBV), hepatitis C virus (HCV), Chikungunya, Chagas, dengue, influenza A(H1N1) and tuberculosis.11–30 In addition, a recently published study found a higher rate of some HLA alleles in 82 COVID-19 non-critically ill patients than in control subjects.31 However, we have not found data about HLA and prognosis of COVID-19 patients. Thus, the objectives of this study were to determine whether there is an association between HLA genetic polymorphisms and susceptibility to and mortality of COVID-19 patients.
MethodsDesign and subjectsIn this prospective and observational study participated 8 Intensive Care Units from 6 hospitals of Canary Islands (Spain). The study was conducted with the approval in all hospitals of the Ethics Committee (Protocol code CHUC-2020-26). The requirement for written informed consent of each patient was waived given that data were prospectively collected, the context of the rapid emergence of this infectious disease and the public health outbreak policy of forbid patient visits by the Government of Spain.
We included patients admitted to the ICU with laboratory-confirmed COVID-19 by means of a positive result for COVID-19 nucleic acids by a real-time fluorescence reverse transcription-polymerase chain reaction (RT-PCR) assay of a nasopharyngeal swab sample or a bronchial aspirate.
Determination of genetic polymorphisms of HLAGenomic DNA was extracted from peripheral blood samples by using standard protocols in a Maxwell® Rapid Sample Concentrator (RSC) Instrument (Promega Corp, USA). HLA typing was performed by polymerase chain reaction sequence-specific oligonucleotide (PCR-SSO) technique developed by LIFECODES® HLA-SSO Typing (Immucor Inc, USA). We genotyped HLA-A, HLA-B, HLA-C, HLA-DRB1 and HLA-DQB1 loci in COVID-19 patients. We used as controls a group of healthy subject representative of the allelic frequency of our population (Canary Islands) registered as voluntary donors in the Spanish Register of Bone Marrow Donors (REDMO is the acronym from Spain).
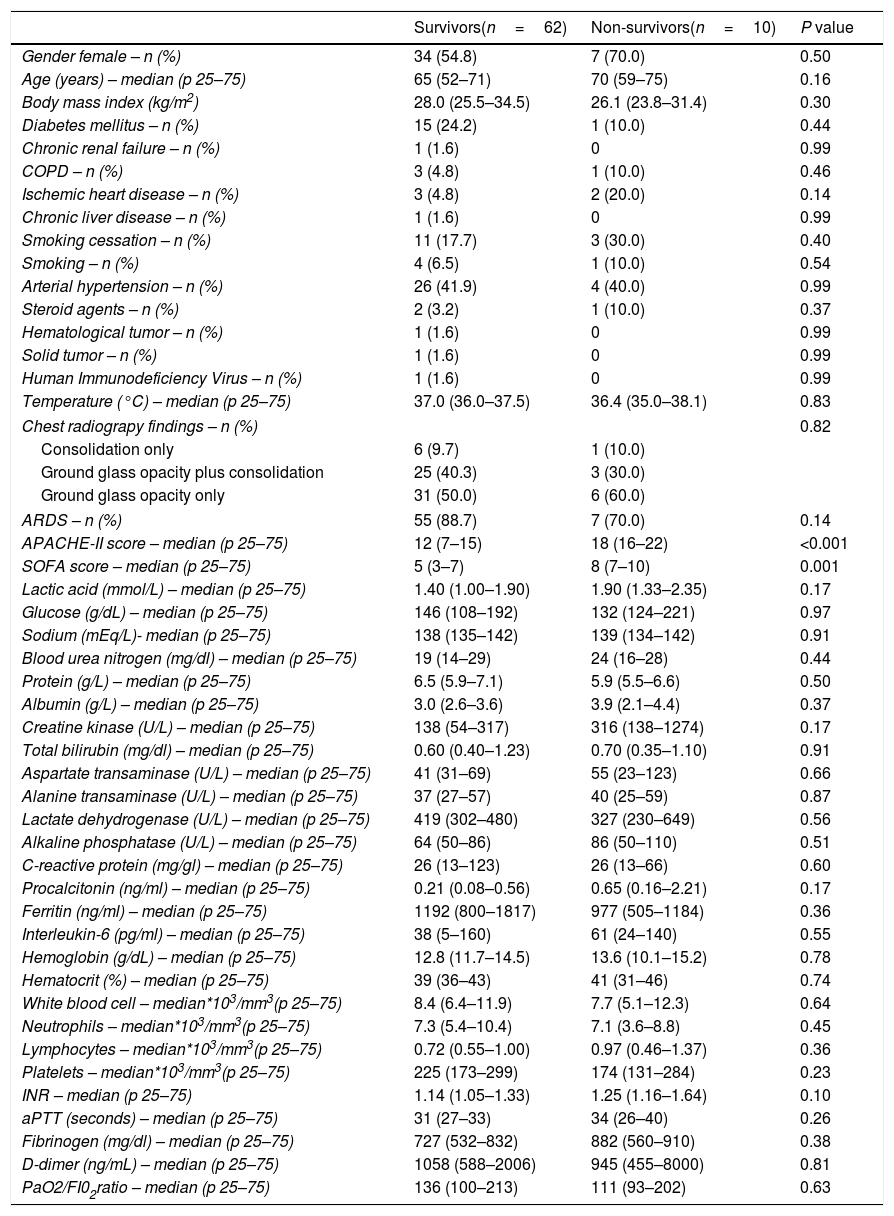
Variables recordedWe recorded the following variables regarding to demographic and clinical data: age, sex, body mass index, and history of chronic renal failure, diabetes mellitus, chronic obstructive pulmonary disease (COPD), active smoking, smoking cessation, chronic liver disease, ischemic heart disease, arterial hypertension, steroid agents, human immunodeficiency virus (HIV), solid tumor and hematological tumor. We also recorded body temperature, chest radiography findings, Acute Physiology and Chronic Health Evaluation (APACHE)-II score,32 Sepsis-related Organ Failure Assessment [SOFA] score33 and the development of ARDS.34
Besides, we registered the following laboratory data at ICU admission: lactate, sodium, glucose, blood urea nitrogen, protein, albumin, creatine kinase, bilirubin, aspartate transaminase, alanine transaminase, lactate dehydrogenase, alkaline phosphatase, procalcitonin, C-reactive protein, interleukin-6, ferritin, hemoglobin, hematocrit, white blood cell, neutrophils, lymphocytes, platelets, activated partial thromboplastin time (aPTT), international normalized ratio (INR), d-dimer, fibrinogen, pressure of arterial oxygen (PaO2) and fraction of inspired oxygen (FiO2).
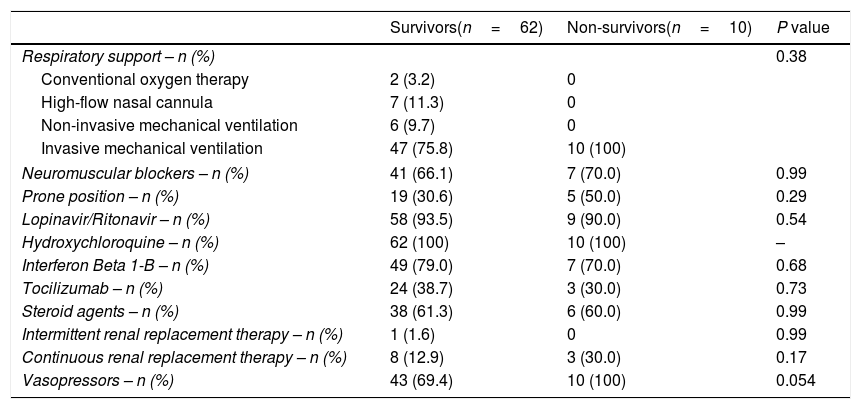
In respect to ICU treatment, respiratory support, prone position, neuromuscular blockers, lopinavir/ritonavir, interferon, hydroxicloroquine, tocilizumab, steroid agents, continuous and intermittent renal replacement therapy, and vasopressors were recorded. Survival at 30 days was our endpoint study.
Statistical methodsWe used frequencies (percentages) and medians (percentile 25–75) to describe categorical and continuous variables. We used chi-square test and Mann–Whitney U test to compare categorical and continuous variables between patient groups (surviving and non-surviving). We tested the possible association between some HLA allele and 30-day mortality using logistic regression analysis, and Odds Ratio and its 95% confidence intervals were calculated as measurement of the clinical impact of the predictor variables. As 10 was the number of non-surviving patients at 30 days, we constructed several logistic regression models with only two predictor variables in each model to avoid over fitting effect.35 In each model was included one HLA allele and one clinical variable with p-value lower than 0.10 in the comparison between non-surviving and surviving patients. Thus, we performed six logistic regression models including HLA-A*11, HLA-C*01 and HLA-DQB1*04 as HLA alleles, and SOFA and APACHE-II as clinical variables. Hosmer–Lemeshow test was reported for each regression model. We used the point p<0.05 for the establishment of significant differences, and the programs NCSS 2000 (Kaysville, Utah) and SPSS 17.0 (SPSS Inc., Chicago, IL, USA) for the analyses.
ResultsGenotyping of HLA-A, HLA-B, HLA-C, HLA-DRB1 and HLA-DQB1 loci was performed in 3886 healthy controls and 72 COVID-19 patients (Supplementary Tables 1–5). We found a trend to a higher rate of the alleles HLA-A*32 (p=0.004) in healthy controls than in COVID-19 patients, and of the alleles HLA-A*03 (p=0.047), HLA-B*39 (p=0.02) and HLA-C*16 (p=0.02) in COVID-19 patients than in healthy controls; however, all these p-values no were significant after correction for multiple comparisons.
We found that non-surviving (n=10) compared to surviving patients (n=62) showed higher APACHE-II (p<0.001) and SOFA (p<0.001) (Tables 1 and 2). We found a trend to a higher rate of the alleles HLA-A*11 (p=0.051), HLA-C*01 (p=0.09) and HLA-DQB1*04 (p=0.051) in non-surviving than in surviving patients (Supplementary Tables 1–5). Logistic regression analysis showed that the presence of allele HLA-A*11 was associated with higher mortality after controlling for SOFA (OR=7.693; 95% CI=1.063–55.650; p=0.04) or APACHE-II (OR=11.858; 95% CI=1.524–92.273; p=0.02). In addition, the allele HLA-C*01 was associated with higher mortality after controlling for SOFA (OR=11.182; 95% CI=1.053–118.700; p=0.04) or APACHE-II (OR=17.604; 95% CI=1.629–190.211; p=0.02). Besides, the presence of allele HLA-DQB1*04 was associated with higher mortality after controlling for SOFA (OR=9.963; 95% CI=1.235–80.358; p=0.03), but no controlling for APACHE-II (Table 3).
Demographic and clinical data of non-surviving and surviving patients.
| Survivors(n=62) | Non-survivors(n=10) | P value | |
|---|---|---|---|
| Gender female – n (%) | 34 (54.8) | 7 (70.0) | 0.50 |
| Age (years) – median (p 25–75) | 65 (52–71) | 70 (59–75) | 0.16 |
| Body mass index (kg/m2) | 28.0 (25.5–34.5) | 26.1 (23.8–31.4) | 0.30 |
| Diabetes mellitus – n (%) | 15 (24.2) | 1 (10.0) | 0.44 |
| Chronic renal failure – n (%) | 1 (1.6) | 0 | 0.99 |
| COPD – n (%) | 3 (4.8) | 1 (10.0) | 0.46 |
| Ischemic heart disease – n (%) | 3 (4.8) | 2 (20.0) | 0.14 |
| Chronic liver disease – n (%) | 1 (1.6) | 0 | 0.99 |
| Smoking cessation – n (%) | 11 (17.7) | 3 (30.0) | 0.40 |
| Smoking – n (%) | 4 (6.5) | 1 (10.0) | 0.54 |
| Arterial hypertension – n (%) | 26 (41.9) | 4 (40.0) | 0.99 |
| Steroid agents – n (%) | 2 (3.2) | 1 (10.0) | 0.37 |
| Hematological tumor – n (%) | 1 (1.6) | 0 | 0.99 |
| Solid tumor – n (%) | 1 (1.6) | 0 | 0.99 |
| Human Immunodeficiency Virus – n (%) | 1 (1.6) | 0 | 0.99 |
| Temperature (°C) – median (p 25–75) | 37.0 (36.0–37.5) | 36.4 (35.0–38.1) | 0.83 |
| Chest radiograpy findings – n (%) | 0.82 | ||
| Consolidation only | 6 (9.7) | 1 (10.0) | |
| Ground glass opacity plus consolidation | 25 (40.3) | 3 (30.0) | |
| Ground glass opacity only | 31 (50.0) | 6 (60.0) | |
| ARDS – n (%) | 55 (88.7) | 7 (70.0) | 0.14 |
| APACHE-II score – median (p 25–75) | 12 (7–15) | 18 (16–22) | <0.001 |
| SOFA score – median (p 25–75) | 5 (3–7) | 8 (7–10) | 0.001 |
| Lactic acid (mmol/L) – median (p 25–75) | 1.40 (1.00–1.90) | 1.90 (1.33–2.35) | 0.17 |
| Glucose (g/dL) – median (p 25–75) | 146 (108–192) | 132 (124–221) | 0.97 |
| Sodium (mEq/L)- median (p 25–75) | 138 (135–142) | 139 (134–142) | 0.91 |
| Blood urea nitrogen (mg/dl) – median (p 25–75) | 19 (14–29) | 24 (16–28) | 0.44 |
| Protein (g/L) – median (p 25–75) | 6.5 (5.9–7.1) | 5.9 (5.5–6.6) | 0.50 |
| Albumin (g/L) – median (p 25–75) | 3.0 (2.6–3.6) | 3.9 (2.1–4.4) | 0.37 |
| Creatine kinase (U/L) – median (p 25–75) | 138 (54–317) | 316 (138–1274) | 0.17 |
| Total bilirubin (mg/dl) – median (p 25–75) | 0.60 (0.40–1.23) | 0.70 (0.35–1.10) | 0.91 |
| Aspartate transaminase (U/L) – median (p 25–75) | 41 (31–69) | 55 (23–123) | 0.66 |
| Alanine transaminase (U/L) – median (p 25–75) | 37 (27–57) | 40 (25–59) | 0.87 |
| Lactate dehydrogenase (U/L) – median (p 25–75) | 419 (302–480) | 327 (230–649) | 0.56 |
| Alkaline phosphatase (U/L) – median (p 25–75) | 64 (50–86) | 86 (50–110) | 0.51 |
| C-reactive protein (mg/gl) – median (p 25–75) | 26 (13–123) | 26 (13–66) | 0.60 |
| Procalcitonin (ng/ml) – median (p 25–75) | 0.21 (0.08–0.56) | 0.65 (0.16–2.21) | 0.17 |
| Ferritin (ng/ml) – median (p 25–75) | 1192 (800–1817) | 977 (505–1184) | 0.36 |
| Interleukin-6 (pg/ml) – median (p 25–75) | 38 (5–160) | 61 (24–140) | 0.55 |
| Hemoglobin (g/dL) – median (p 25–75) | 12.8 (11.7–14.5) | 13.6 (10.1–15.2) | 0.78 |
| Hematocrit (%) – median (p 25–75) | 39 (36–43) | 41 (31–46) | 0.74 |
| White blood cell – median*103/mm3(p 25–75) | 8.4 (6.4–11.9) | 7.7 (5.1–12.3) | 0.64 |
| Neutrophils – median*103/mm3(p 25–75) | 7.3 (5.4–10.4) | 7.1 (3.6–8.8) | 0.45 |
| Lymphocytes – median*103/mm3(p 25–75) | 0.72 (0.55–1.00) | 0.97 (0.46–1.37) | 0.36 |
| Platelets – median*103/mm3(p 25–75) | 225 (173–299) | 174 (131–284) | 0.23 |
| INR – median (p 25–75) | 1.14 (1.05–1.33) | 1.25 (1.16–1.64) | 0.10 |
| aPTT (seconds) – median (p 25–75) | 31 (27–33) | 34 (26–40) | 0.26 |
| Fibrinogen (mg/dl) – median (p 25–75) | 727 (532–832) | 882 (560–910) | 0.38 |
| D-dimer (ng/mL) – median (p 25–75) | 1058 (588–2006) | 945 (455–8000) | 0.81 |
| PaO2/FI02ratio – median (p 25–75) | 136 (100–213) | 111 (93–202) | 0.63 |
COPD=Chronic Obstructive Pulmonary Disease; APACHE=Acute Physiology and Chronic Health Evaluation; SOFA=Sepsis-related Organ Failure Assessment; ARDS=acute respiratory distress syndrome; INR=International normalized ratio; aPTT=Activated partial thromboplastin time; PaO2=pressure of arterial oxygen; FIO2=fraction inspired oxygen.
Treatment of non-surviving and surviving patients.
| Survivors(n=62) | Non-survivors(n=10) | P value | |
|---|---|---|---|
| Respiratory support – n (%) | 0.38 | ||
| Conventional oxygen therapy | 2 (3.2) | 0 | |
| High-flow nasal cannula | 7 (11.3) | 0 | |
| Non-invasive mechanical ventilation | 6 (9.7) | 0 | |
| Invasive mechanical ventilation | 47 (75.8) | 10 (100) | |
| Neuromuscular blockers – n (%) | 41 (66.1) | 7 (70.0) | 0.99 |
| Prone position – n (%) | 19 (30.6) | 5 (50.0) | 0.29 |
| Lopinavir/Ritonavir – n (%) | 58 (93.5) | 9 (90.0) | 0.54 |
| Hydroxychloroquine – n (%) | 62 (100) | 10 (100) | – |
| Interferon Beta 1-B – n (%) | 49 (79.0) | 7 (70.0) | 0.68 |
| Tocilizumab – n (%) | 24 (38.7) | 3 (30.0) | 0.73 |
| Steroid agents – n (%) | 38 (61.3) | 6 (60.0) | 0.99 |
| Intermittent renal replacement therapy – n (%) | 1 (1.6) | 0 | 0.99 |
| Continuous renal replacement therapy – n (%) | 8 (12.9) | 3 (30.0) | 0.17 |
| Vasopressors – n (%) | 43 (69.4) | 10 (100) | 0.054 |
Multiple logistic regression analyses to predict mortality at 30 days.
| Chi-square | Degrees of freedom | Odds ratio | 95% confidence interval | P-value | |
|---|---|---|---|---|---|
| Model 1: | |||||
| Hosmer and Lemeshow test | 2.621 | 7 | 0.92 | ||
| HLA-A*11 | 7.693 | 1.063–55.650 | 0.04 | ||
| SOFA score (points) | 1.532 | 1.142–2.054 | 0.004 | ||
| Model 2: | |||||
| Hosmer and Lemeshow test | 5.006 | 8 | 0.76 | ||
| HLA-A*11 | 11.858 | 1.524–92.273 | 0.02 | ||
| APACHE-II (points) | 1.278 | 1.098–1.489 | 0.002 | ||
| Model 3: | |||||
| Hosmer and Lemeshow test | 2.285 | 7 | 0.94 | ||
| HLA-C*01 | 11.182 | 1.053–118.700 | 0.04 | ||
| SOFA score (points) | 1.551 | 1.161–2.072 | 0.003 | ||
| Model 4: | |||||
| Hosmer and Lemeshow test | 3.682 | 8 | 0.89 | ||
| HLA-C*01 | 17.604 | 1.629–190.211 | 0.02 | ||
| APACHE-II (points) | 1.297 | 1.099–1.531 | 0.002 | ||
| Model 5: | |||||
| Hosmer and Lemeshow test | 3.569 | 7 | 0.83 | ||
| HLA-DQB1*04 | 9.963 | 1.235–80.358 | 0.03 | ||
| SOFA (points) | 1.555 | 1.156–2.091 | 0.004 | ||
| Model 6: | |||||
| Hosmer and Lemeshow test | 4.548 | 8 | 0.81 | ||
| HLA-DQB1*04 | 2.961 | 0.419–20.947 | 0.28 | ||
| APACHE-II (points) | 1.230 | 1.066–1.420 | 0.005 | ||
SOFA=Sepsis-related Organ Failure Assessment; APACHE=Acute Physiology and Chronic Health Evaluation.
To our knowledge, this is the first study reporting data on HLA genetic polymorphisms and susceptibility to and prognosis in COVID-19 patients. We found a trend to a higher rate of alleles HLA-A*32 in healthy controls than in COVID-19 patients, and of alleles HLA-A*03, HLA-B*39 and HLA-C*16 in COVID-19 patients than in healthy controls. The small sample size of our COVID-19 population could explain the absence of significant differences after Bonferroni correction in those and other HLA genetic polymorphisms.
We found that non-surviving compared to surviving patients showed higher APACHE-II and SOFA. We also found a trend to higher rate of the alleles HLA-A*11, HLA-C*01 and HLA-DQB1*04 in non-surviving than in surviving patients. As previously mentioned, the small sample size of our study could have explained these results. Therefore, we decided performed regression analyses to test the potential association of those HLA alleles and mortality. As 10 was the number of non-surviving patients at 30 days, we constructed several multiple binomial logistic regression models with only two predictor variables in each model to avoid over fitting effect. We included HLA-A*11 and SOFA in the first model, HLA-A*11 and APACHE-II in the second model, HLA-C*01 and SOFA in the third model, HLA-C*01 and APACHE-II in the fourth model, HLA-DQB1*04 and SOFA in the fifth model, and HLA-DQB1*04 and APACHE-II in the sixth model. An important finding is that all 3 alleles were associated with the mortality after controlling for SOFA, and the same was observed for alleles HLA-A*11 and HLA-C*01when controlled for APACHE-II.
The results of our study are in line with those of other studies in patients with other infectious diseases. Regarding to susceptibility, higher rate of HLA-A*32 has been found in patients with chronic HCV infection than in control subjects.11 A restricted response of cytotoxic T lymphocytes for HCV in presence of HLA-A*03 has been reported.12 Higher rate of HLA-B*39 was also found in patients with tuberculosis,13 Chagas disease14 and osteoarticular complications due to brucellosis15 in comparison with controls. There has been found higher rate of HLA-A*11 in patients with panbronchiolitis,17 dengue disease,18 HBV infection,19 influenza A(H1N1)pdm09 virus infection20 or invasive meningococcal disease than in control subjects.21 Higher frequency of HLA-C*01 has been found in patients with acute viral encephalitis than in control subjects.24 There has been found higher rate of HLA-DQB1*04 in patients with HBV,25 human papillomavirus (HPV) infection,26 post-histoplasmosis fibrosing mediastinitis27 or chikungunya viral infection encephalitis than in control subjects.28 In a recently published study has been found a higher rate of HLA-C*07:29 and HLA-B*15:27 in 82 COVID-19 non-critically ill patients than in control subjects.31 However, our study was underpowered to find an association between HLA genetic polymorphism and susceptibility to COVID-19 patients.
Interestingly, we found certain HLA genetic polymorphisms that could increase the risk of death in patients COVID-19. Previously, regarding to prognosis, the presence of HLA-C*16 increase the risk of rapid progression in patients with Acquired Immune Deficiency Syndrome.16 Also, the presence of HLA-A*11 has been associated with poor evolution of patients with HBV22 or active pulmonary tuberculosis.23 Besides, the presence of HLA-DQB1*04 has been associated with poor evolution of patients with HBV.29,30 However, to the best of our knowledge, no study has evaluated this aspect in patients with COVID-19.
We must recognize some limitations of our study. First, we only included two variables in each regression analysis model due to that the small number of death patients in our study, which precludes the inclusion of several variables,35 as previously has been reported in other researchs.36,37 That small number of death patients in our study was due to the small sample size and to the low mortality rate in our series (14%), which was in the lower limit of the range published by other series from ICU of our country (15–34%).38–40 Second, we studied mortality at 30 days and mortality at other moment of time have been not studied. Third, the rate of SDRA was higher, not statistically significant, in survivor than in non-survivor patients, possibly due to the small sample size. However, we think that the strengths of the study were that two HLA alleles in our study (HLA-A*11 and HLA-C*01) were associated with the mortality after controlling for SOFA or APACHE-II. In addition, the three HLA alleles associated with the mortality in our study (HLA-A*11, HLA-DQB1*04 and HLA-C*01), previously were associated with poor evolution in other infectious diseases (HLA-A*1122,23 and HLA-DQB1*0429,30) or with the risk of other infectious diseases (HLA-C*0124).
ConclusionsThe new finding from our preliminary study of small sample size was that HLA genetic polymorphisms could be associated with COVID-19 mortality; however, studies with a larger sample size before definitive conclusions can be drawn.
Authors’ contributionsLLo conceived, designed and coordinated the study, participated in acquisition and interpretation of data, and drafted the manuscript.
MMM, JJC, JSV, AP, JAMR, LRG, SL, APC, APL, LU, JAF, AE, PV, LuG, LoG, RA, MFZ, ROL, NO, ARP and CD participated in acquisition of data.
AF and YB carried out the determinations of HLA genetic polymorphisms.
AJ participated in the interpretation of data.
All authors revised the manuscript critically for important intellectual content and made the final approval of the version to be published.
FundingThis study was supported by a grant from Instituto de Salud Carlos III (PI-18-00500) (Madrid, Spain) and co-financed with Fondo Europeo de Desarrollo Regional (FEDER).
Conflicts of interestThe authors declare that they have no competing interests.
Leonardo Lorente, Andrés Franco, Yvelise Barrios, Alina Perez, Alejandro Jiménez, Antonia Pérez-Cejas, Alejandra Pérez-Llombet, Luis Uribe, Lourdes González, Rocío Alvarez (Hospital Universitario de Canarias. La Laguna. Tenerife. Spain); María M. Martín, Julia Alcoba-Flórez, Albano Estupiñan (Hospital Universitario Nuestra Señora de Candelaria. Santa Cruz de Tenerife. Spain); Juan J. Cáceres, Paula Vega, Lucía Gonzalez (Hospital Insular. Las Palmas de Gran Canaria. Spain); Jordi Solé-Violán, Nazario Ojeda, Sergio López, Aurelio Rodríguez-Pérez, Casimira Domínguez (Hospital Universitario Dr. Negrín. Las Palmas de Gran Canaria. Spain); José Alberto Marcos y Ramos, María F. Zapata (Hospital Doctor José Molina Orosa. Lanzarote. Spain); Luis Ramos-Gómez, Raquel Ortiz-López (Hospital General La Palma. La Palma. Spain).