Mechanical ventilation is a procedure that is used to save the life of patients with severe respiratory failure. Although mechanical ventilation per se does not cure this process, its application can be detrimental to whom may receive it. This ventilator induced lung injury (VILI) should be minimized by individualizing mechanical ventilation.1 Although it is always difficult to make estimates, it is recommended to apply a tidal volume (TV) of 4–6 mL/kg of ideal weight; delta pressure (ΔP) <15 cmH2O; plateau pressure (Pplat) <27 cmH2O; mechanical power (MP) <17 J/min, and apply PEEP defined by optimal static compliance.2 The targets would be to find proper oxygenation to guarantee enough oxygen supply to tissues and remove CO2 to prevent severe hypercapnia, which is a known factor associated with morbidity and mortality.3
But what can happen if we cannot keep all these premises? We could adjust the ventilator to perform potentially damaging mechanical ventilation or use an extracorporeal respiratory support system? The latter seems like the best option. However, to this date, there is not enough evidence on this issue to draw definitive conclusions yet. The main study conducted among patients with acute respiratory distress syndrome (ARDS) to assess the efficacy of veno-venous extracorporeal membrane oxygenation (VV-ECMO) used 3 different criteria for its application. Two of them were oxygenation criteria and the third one, respiratory acidosis: pH < 7.25 with partial pressure of CO2 >60 mmHg for over 6 h.4 The overall results showed a reduction of the brute overall mortality rate at 60 days in 11% of the cases. However, this difference was not statistically significant. We should mention that the group that benefited the most from the technique was clearly the one with respiratory acidosis as the inclusion criteria with a lower mortality rate of 31%.4 This benefit has been associated with less VILI since it was demonstrated that in this type of patients there is a reduction of TV, respiratory rate (RR), Pplat, ΔP, and MP.5
CO2 removal using extracorporeal devices (ECCO2R) is more efficient than ventilating the patient since, at the physiological level, the content of CO2 for a certain volume of venous blood is much higher compared to the content of oxygen. Also, CO2 is more soluble than oxygen and spreads better across the membrane of the circuit. To understand the performance of CO2 removal in these devices, it is of paramount importance to know that this removal will rise parallel to the increase of blood CO2 content, partial pressure of venous CO2, the membrane surface area, sweep gas flow, and blood flowing across the membrane with “ceiling effects” for both.6 The physiological and technical bases, as well as the different devices used for the extracorporeal removal of CO2 are discussed in a different issue of our journal.7
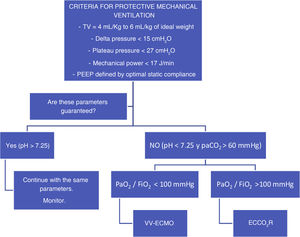
Prospective studies have been conducted in patients with hypoxemic respiratory failure treated with ultraprotective mechanical ventilation and it has been demonstrated that with an ECCO2R device it is possible to lower TV from 6 mL/kg down to 3 mL/kg or 4 mL/kg of ideal weight. Overall, we should say that in none of the 3 landmark studies conducted significant differences in both groups were seen regarding the days on mechanical ventilation, the ICU or hospital stay or mortality.8–10 (Fig. 1)
Proposal for the application of different extracorporeal devices in patients treated with mechanical ventilation. TV is the tidal volume; PaCO2 stands for arterial partial pressure of carbon dioxide; PaO2/FiO2 stands for the ratio between the oxygen arterial pressure and the fraction of inspired oxygen; VV-ECMO stands for venovenous extracorporeal membrane oxygenation; ECCO2R stands for extracorporeal carbon dioxide removal.
Therefore, after careful analysis of these studies we should say that the use of ECCO2R devices does not seem an option for all patients with moderate ARDS. However, if we look closely at what patients were included in these studies, we’ll see that most of them did not seem to have respiratory issues to solve. In the REST trial the ΔP of half of the patients from the ECCO2R group was <15 cmH2O. Another indication that is worth exploring here is its use in patients in whom protective ventilation cannot be kept and VV-ECMO has already proven effective. We used low-flow ECCO2R devices not to remove the entire production of CO2 out of the patient’s body but enough CO2 to keep pH > 7.25 with ΔP < 15 cmH2O, and RR < 30 rpm. If patients also have severe ARDS, therapy with VV-ECMO should be administered too. However, if the PaO2/FiO2 ratio is >100 mmHg, an ECCO2R device should be implanted into the patient through a 14-Fr cannulation similar to the one used in continuous venovenous hemofiltration. With these criteria in mind, at the present time, we have recruited 34 patients with a median pH of 7.17, pCO2 levels of 90 mmHg, and ΔP of 19 cmH2O. ECCO2R therapy achieved its targets in 50% of the patients in just 24 h, and in 67% of the patients after 72 h. The main complications were circuit coagulation in 82% of the cases, bleeding in 14% including almost 6% of cerebral hemorrhages, and mixed alkalosis in 20%. The group’s overall survival rate was 60%.
In conclusion, we can say that ECCO2R therapy is a viable alternative to VV-ECMO to treat hypercapnic acidosis since it guarantees ultraprotective mechanical ventilation in those patients who may need it. However, it would not be indicated for all patients with ARDS. Also, indications can be expanded to patients with COPD exacerbations, bronchial asthma, bronchopleural fistula or in patients weaning from ECMO. It could well be the case that the use of ECCO2R devices with pumps that reach intermediate flows of 400 mL/m–700 mL/m with an oxygenation membrane with large surface areas of, at least, 1.8 m2 is the ideal solution to demonstrate the net benefit of its use in patients with hypercapnic acidosis of any etiology guaranteeing mechanical ventilation capable of minimizing the development of VILI.