To evaluate the impact of early empirical anti-Methicillin-resistant Staphylococcus aureus (MRSA) therapy on the survival outcomes of patients with severe acute pancreatitis (SAP) in the intensive care units (ICUs).
DesignSecondary Analysis of the the Medical Information Mart for Intensive Care-IV (MIMIC-IV v3.1) on MRSA therapy in Intensive Care Units (ICUs).
Setting94,458 ICU hospitalization records from 65,366 unique patients between 2008 and 2019.
Patients494 patients diagnosed with acute pancreatitis first admitted to the ICU.
InterventionsAnti-MRSA (vancomycin or linezolid) agents.
Main variables of interest28-day and 90-day mortality rates, incidence of renal impairment, and total hospitalization costs.
ResultsA total of 494 patients were included, 302 (61.1%) patients received anti-MRSA therapy. After PSM, no significant differences were observed in ICU mortality (adjusted relative risk [aRR], 0.39; 95% CI, 0.11–1.38, p = 0.14) or hospital mortality (aRR, 0.53; 95% CI, 0.21–1.33, p = 0.18) between the two groups. Similarly, 28-day mortality and 90-day mortality did not significantly differ (p > 0.05). Empirical anti-MRSA therapy was associated with significantly longer ICU and hospital LOS (p < 0.001). Subgroup analysis revealed that anti-MRSA therapy significantly increased acute kidney injury incidence (p = 0.002), particularly in patients without pre-existing kidney disease (p < 0.001).
ConclusionsEmpirical anti-MRSA therapy was not associated with improved short- or long-term survival in SAP patients, and may lead to prolonged ICU and hospital stays. These findings highlight the importance of integrating local MRSA epidemiology into antimicrobial stewardship decisions.
Evaluar el impacto de la terapia empírica temprana contra Staphylococcus aureus resistente a meticilina (MRSA) sobre los desenlaces de supervivencia de pacientes con pancreatitis aguda grave (PAG) en unidades de cuidados intensivos (UCI).
DiseñoAnálisis secundario de la base de datos Medical Information Mart for Intensive Care-IV (MIMIC-IV v3.1) sobre la terapia contra MRSA en UCI.
Ámbito94.458 registros de hospitalización en UCI correspondientes a 65.366 pacientes únicos entre 2008 y 2019.
Pacientes494 pacientes diagnosticados de pancreatitis aguda e ingresados por primera vez en UCI.
IntervencionesAgentes anti-MRSA (vancomicina o linezolid).
Variables de interés principalesTasas de mortalidad a 28 y 90 días, incidencia de insuficiencia renal y costos totales de hospitalización.
ResultadosSe incluyeron 494 pacientes, de los cuales 302 (61,1%) recibieron terapia anti-MRSA. Tras el emparejamiento por puntaje de propensión (PSM), no se observaron diferencias significativas en la mortalidad en UCI (riesgo relativo ajustado [aRR], 0,39; IC del 95%, 0,11–1,38; p = 0,14) ni en la mortalidad hospitalaria (aRR, 0,53; IC del 95%, 0,21–1,33; p = 0,18) entre los grupos. De forma similar, la mortalidad a 28 y 90 días no mostró diferencias significativas (p > 0,05). La terapia empírica contra MRSA se asoció con una estancia significativamente más prolongada en UCI y hospital (p < 0,001). El análisis de subgrupos reveló que la terapia anti-MRSA incrementó de forma significativa la incidencia de lesión renal aguda (p = 0,002), especialmente en pacientes sin enfermedad renal previa (p < 0,001).
ConclusionesLa terapia empírica contra MRSA no se asoció con una mejora en la supervivencia a corto o largo plazo en pacientes con PAG y podría conllevar una prolongación de la estancia en UCI y hospital. Estos hallazgos subrayan la importancia de integrar la epidemiología local de MRSA en las decisiones de optimización del uso de antimicrobianos.
Acute pancreatitis (AP) is a leading cause of acute abdominal pain worldwide, with annual incidence rising by 2–5%.1 Approximately 20–40% of AP patients develop severe acute pancreatitis (SAP), a life-threatening condition characterized by high intensive care unit (ICU) admission rates and a mortality rate exceeding 30%.2,3 Infectious complications further aggravate the prognosis of SAP by triggering uncontrolled systemic inflammation and multi-organ failure.4
SAP patients are particularly susceptible to hospital-acquired infections, especially multidrug-resistant pathogens, due to persistent systemic inflammation, immune dysregulation, and prolonged invasive treatments, etc.5 Among these, Methicillin-resistant Staphylococcus aureus (MRSA) has emerged as a critical pathogen, accounting for 30.2–36.0% of ICU-associated device-related infections, including ventilator-associated pneumonia and catheter-related bloodstream infections.6–9 MRSA-related mortality ranks second among drug-resistant pathogens in ICU, following third-generation cephalosporin-resistant Gram-negative bacteria as reported by the European burden of disease study on drug-resistant bacterial infections.10 Notably, SAP patients with MRSA infection experience significantly worse outcomes compared to those infected with non-resistant bacteria or uninfected patients.11,12
Early identification of infections in critically ill SAP patients remains challenging. While next-generation sequencing facilitates faster pathogen detection, its clinical utility is substantially limited in SAP due to the low microbial load in necrotic tissues.13–16 This leaves clinicians with the difficult decision of whether to initiate empirical anti-MRSA therapy in rapidly deteriorating SAP patients. Current guidelines do not provide clear recommendations on whether early MRSA treatment is necessary for high-risk SAP patients.17 This study aimed to evaluate the impact of adding empirical anti-MRSA therapy to standard treatment (covering Gram-negative and sensitive Gram-positive bacteria) on in-hospital mortality, as well as 28-day and 90-day survival outcomes in ICU-admitted SAP patients.
MethodologyData sourceThis was a retrospective cohort study using the Medical Information Mart for Intensive Care-IV (MIMIC-IV v3.1), a publicly available critical care database containing 94,458 ICU hospitalization records from 65,366 unique patients. Database access was authorized following completion of the Collaborative Institutional Training Initiative (CITI) program (certification ID: 12259631) by the investigator. As all patient identifiers have been anonymized, institutional ethics committee approval and informed consent were waived.18
Study population and definitionsPatients diagnosed with acute pancreatitis admitted to the ICU were included. SAP was defined by the following criteria: (1) primary discharge diagnosis of AP (ICD-9: 577.0; ICD-10: K85.x); (2) Sequential Organ Failure Assessment (SOFA) score ≥2, persisting for >48 h, with organ failure involving at least one system (respiratory, circulatory, or renal), meeting the revised Atlanta classification criteria.19 Exclusion criteria included: (1) ICU stay <48 h; (2) age <18 years; (3) initiation of antibiotic treatment for a confirmed infection ≥24 h prior to ICU admission; (4) recurrent pancreatitis with prior SAP-related ICU admission; (5) major active infection at ICU admission (e.g., severe pneumonia, urinary tract infection, or sepsis); (6) initiation of anti-MRSA therapy beyond 72 h; and (7) incomplete baseline data (<80%).
Variable extractionThe primary exposure was defined as intravenous administration of anti-MRSA agents initiated within 72 h of ICU admission, prior to obtaining any microbial culture results. The patients in the Standard therapy group received guideline-directed empirical antimicrobial therapy without MRSA-active coverage. Baseline characteristics were extracted within 24 h of ICU admission using SQL queries, including demographics (age, sex, race), disease severity scores, and vital signs, etc. Additionally, laboratory markers and comorbidities were recorded. The primary outcomes were 28-day and 90-day all-cause mortality, while secondary outcomes included ICU length of stay (LOS) and total hospital LOS.
Statistical analysisTo evaluate independent effect of treatment on survival outcomes, Propensity score matching (PSM) was performed using a multivariable logistic regression model incorporating all baseline covariates listed in Table 1 (excluding outcomes).20 Covariate balance was assessed using standardized mean differences (SMDs), with values <0.1 indicating acceptable balance. The Cox proportional hazards model was adopted to estimate the adjusted hazard ratios (aHRs) and 95% confidence intervals (CIs) for mortality outcomes. Kaplan–Meier curves were generated for survival comparisons, and the proportional hazards assumption was evaluated with time-dependent covariates. PSM was also applied to assess differences in ICU LOS and hospital LOS. Robustness was tested via inverse probability of treatment weighting (IPTW).
Characteristics of 494 patients admitted to the intensive care unit with severe acute pancreatitis.
| Characteristic | All cohort (n = 494) | Anti-MRSA-used (n = 302) | Non-anti-MRSA used (n = 192) | p-value |
|---|---|---|---|---|
| Demographic | ||||
| Male, (n%) | 279 (56.5) | 174 (57.6) | 105 (54.7) | 0.052 |
| Age, median [IQR] | 61.0 [46.0, 74.0] | 59.0 [45.0, 71.0] | 64.5 [47.0, 78.0] | 0.035 |
| Vital signs (median [IQR]) | ||||
| Heart rate | 95.0 [80.7, 107.4] | 96.5 [82.1, 109.8] | 91.3 [77.9, 103.7] | 0.006 |
| Systolic blood pressure | 115.8 [106.5, 129.7] | 112.1 [105.3, 123.9] | 123.4 [108.7, 137.1] | < 0.001 |
| Diastolic blood pressure | 64.1 [57.9, 72.9] | 63.3 [56.5, 70.9] | 66.4 [60.2, 76.2] | 0.004 |
| Respiratory rate | 20.6 [17.7, 24.2] | 21.5 [18.5, 24.9] | 19.3 [17.1, 22.3] | < 0.001 |
| Temperature | 36.9 [36.6, 37.3] | 36.9 [36.6, 37.4] | 36.9 [36.6, 37.2] | 0.375 |
| SpO2 | 96.3 [94.9, 97.7] | 96.4 [94.9, 97.7 | 96.1 [94.9, 97.6] | 0.676 |
| Laboratory variables on ICU day 1 (median [IQR]) | ||||
| WBC_min, cell/mm3 | 10.5 [7.1, 15.4] | 10.3 [6.9, 15.8] | 10.9 [7.5, 14.7] | 0.585 |
| WBC_max, cell/mm3 | 14.7 [10.4, 20.2] | 14.9 [10.3, 21.6] | 14.0 [10.5, 18.6] | 0.09 |
| Hemoglobin_min, mg/dL | 10.3 [8.6, 12.0] | 10.0 [8.4, 11.8] | 10.7 [9.1, 12.2] | 0.005 |
| Hemoglobin_max, mg/dL | 11.8 [10.10, 13.8] | 11.8 [9.9, 13.8] | 11.8 [10.6, 13.5] | 0.275 |
| Platelets_min, cell/mm3 | 151.0 [105.5, 218.0] | 149.0 [99.8, 214.5] | 155.0 [113.0, 226.8] | 0.132 |
| Platelets_max, cell/mm3 | 196.5 [142.0, 272.0] | 196.0 [139.8, 274.3] | 196.5 [146.3, 271.0] | 0.742 |
| BUN_max, mg/dL | 26.0 [15.0, 43.0] | 28.0 [17.8, 49.3] | 21.0 [12.3, 36.0] | < 0.001 |
| Aniongap_min, mEq/L | 13.0 [11.0, 16.0] | 14.0 [11.0, 17.0] | 13.0 [11.0, 14.8] | 0.002 |
| Aniongap_max mEq/L | 17.0 [14.0, 21.0] | 18.0 [15.0, 23.0] | 16.0 [14.0, 19.8] | < 0.001 |
| Bicarbonate_min, mEq/L | 19.0 [16.0, 23.0] | 18.0 [14.0, 21.0] | 22.0 [18.0, 24.0] | < 0.001 |
| Calcium_min, mEq/L | 7.6 [6.9, 8.2] | 7.4 [6.7, 8.0] | 7.9 [7.3, 8.3] | < 0.001 |
| Calcium_max, mEq/L | 8.3 [7.8, 8.8] | 8.2 [7.7, 8.7] | 8.3 [7.9, 8.9] | 0.058 |
| Chloride_min, mEq/L | 101.0 [97.0, 106.0] | 101.0 [97.0, 106.0] | 102.0 [98.0, 106.0] | 0.344 |
| Chloride_max, mEq/L | 106.0 [102.0, 111.0] | 107.0 [102.0, 111.0] | 106.0 [102.0, 110.0] | 0.147 |
| Creatinine_max, mEq/L | 1.4 [0.9, 2.6] | 1.7 [1.0, 3.3] | 1.1 [0.8, 1.8] | < 0.001 |
| Glucose_min, mg/dL | 108.0 [90.0, 129.0] | 109.0 [90.0, 133.3] | 106.0 [91.0, 123.0] | 0.418 |
| Glucose_max, mg/dL | 152.0 [118.0, 213.0] | 158.5 [124.8, 223.3] | 140.5 [113.0, 195.5] | 0.001 |
| Sodium_min, mEq/L | 137.0 [133.0, 140.0] | 136.0 [132.0, 140.0] | 137.0 [134.0, 139.8] | 0.163 |
| Sodium_max, mEq/L | 140.0 [137.0, 143.0] | 140.0 [136.0, 143.0] | 140.0 [138.0, 142.0] | 0.843 |
| Potassium_min, mEq/L | 3.7 [3.4, 4.1] | 3.7 [3.4, 4.2] | 3.7 [3.4, 4.0] | 0.601 |
| Potassium_max, mEq/L | 4.4 [4.0, 5.0] | 4.5 [4.1, 5.1] | 4.3 [3.9, 4.8] | < 0.001 |
| INR_max | 1.4 [1.2, 1.8] | 1.5 [1.2, 2.1] | 1.4 [1.2, 1.6] | < 0.001 |
| PT_max, sec | 15.6 [13.7, 19.9] | 16.4 [13.8, 22.5] | 15.2 [13.5, 17.8] | 0.002 |
| PTT_max, sec | 33.5 [28.7, 44.0] | 34.8 [29.5, 48.2] | 31.8 [27.9, 40.7] | 0.001 |
| ALT_max, U/L | 92.0 [33.5, 256.0] | 91.5 [33.2, 277.3] | 92.0 [33.5, 246.0] | 0.642 |
| ALP_max, U/L | 119.0 [77.5, 221.0] | 119.0 [77.0, 214.5] | 121.0 [80.0, 237.5] | 0.451 |
| AST_max, U/L | 124.5 [55.8, 329.8] | 135.0 [56.5, 367.5] | 112.0 [53.0, 264.0] | 0.025 |
| Urine output | 1375.0 [769.5, 2202.8] | 1227.5 [547.5, 1908.8] | 1566.6 [1007.5, 2446.3] | < 0.001 |
| Aetiology, (n%) | ||||
| Multi-infection (n%) | 128 (25.9) | 114 (37.7) | 14 (7.3) | < 0.001 |
| No aetiology, (n%) | 225 (45.5) | 92 (30.5) | 133 (69.3) | < 0.001 |
| Bacteria, (n%) | 225 (45.5) | 174 (57.6) | 51 (26.6) | < 0.001 |
| Fungi, (n%) | 137 (27.7) | 120 (39.7) | 17 (8.9) | < 0.001 |
| Virus, (n%) | 1 (0.2) | 1 (0.3) | 0 (0) | 1 |
| MRSA screen | 329 (66.7) | 222 (73.5) | 107 (55.7) | < 0.001 |
| MRSA infection | 13 (2.6) | 6 (2.0) | 7 (3.6) | 0.266 |
| Severity (median [IQR]) | ||||
| APSIII | 53.0 [40.0, 73.0] | 63.0 [47.0, 83.0] | 43.5 [33.0, 53.0] | < 0.001 |
| SOFA | 7.0 [4.0, 10.0] | 8.0 [5.0, 11.0] | 5.0 [3.0, 7.0] | < 0.001 |
| SIRS | 3.0 [3.0, 4.0] | 3.0 [3.0, 4.0] | 3.0 [2.0, 3.0] | < 0.001 |
| LODS | 6.0 [3.0, 8.0] | 7.0 [5.0, 9.0] | 4.0 [2.0, 6.0] | < 0.001 |
| Comobidity | ||||
| Charlson comorbidity index, mean, median [IQR] | 4.0 [2.0, 6.0] | 4.0 [2.0, 6.0] | 4.0 [2.0, 6.0] | 0.313 |
| Renal disease | 89 (18.0) | 60 (19.9) | 29 (15.1) | 0.189 |
| Diabetes | 162 (32.8) | 105 (34.8) | 57 (29.7) | 0.28 |
| Severe liver disease | 63 (12.8) | 38 (12.6) | 15 (7.8) | 0.103 |
| Malignant cancer | 38 (7.7) | 26 (8.6) | 12 (6.3) | 0.389 |
| Congestive heart failure | 92 (18.6) | 58 (19.2) | 34 (17.7) | 0.723 |
| Chronic pulmonary disease | 78 (15.8) | 44 (14.6) | 34 (17.7) | 0.377 |
| Outcomes | ||||
| LOS ICU, median [IQR] | 3.8 [2.0, 8.9] | 5.9 [2.6, 14.7] | 2.5 [2.1, 4.7] | < 0.001 |
| LOS hospital, median [IQR] | 12.9 [7.5, 22.5] | 17.7 [9.7, 27.0] | 9.4 [5.7, 13.9] | < 0.001 |
| Reinfection, (n%) | 82 (16.6%) | 48 (15.9) | 34 (17.7) | 0.6 |
Categorical variables were summarized as counts (%) and compared using chi-square or Fisher’s exact tests. Continuous variables were reported as mean ± standard deviation or median [interquartile range (IQR)], and compared using Student’s t-test or Mann–Whitney U test, as appropriate. Analyses were conducted in R (v4.3.0) and SPSS (v25.0), with two-sided p < 0.05 considered statistically significant.
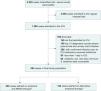
ResultsBaseline demographic and clinical characteristicsA total of 494 ICU-admitted patients with severe acute pancreatitis were included (Fig. 1). The median age was 61.0 [46.0–74.0] years, and the majority were male (56.5%, 279/494). The median Charlson comorbidity index was 4.0 [2.0, 6.0]. The most prevalent comorbidities were diabetes (32.8%), followed by congestive heart failure (18.6%, 92/494), renal disease (18.0%, 89/494), and chronic pulmonary disease (15.8 %, 78/494) (Table 1).
Study Population. a: empirical anti-MRSA therpy were defined as the first antibiotic used that has inhibitory effects on methicillin-resistant Staphylococcus aureus (298 cases started on vancomycin, 4 cases started on linezolid); b: Alternative empirical therapy is defined as antibiotics with the ability to suppress/eliminate pathogens, including β-lactams (penicillin, ampicillin, amoxicillin, ampicillin-sulbactam, piperacillin-tazobactam, piperacillin-tazobactam sodium); cephalosporins (cefotaxime, ceftriaxone), carbapenems (meropenem), macrolides (azithromycin, clarithromycin, erythromycin), fluoroquinolones (ciprofloxacin hydrochloride, intravenous ciprofloxacin, levofloxacin), aminoglycosides (amikacin, gentamicin, gentamicin sulfate, tobramycin), etc.
Pathogens were identified in 269 (54.5%) patients, with Escherichia coli (20.9%, 103/494) and coagulase-positive Staphylococcus aureus (9.7%, 48/494) being the most frequently isolated organisms (Table S1). MRSA screening was performed in 329 (66.7%) patients, with a positive rate of 2.6% (13/494) (Table 1). Regarding antibiotic therapy, vancomycin was the most commonly administered agent (44.3%, 219/494), followed by piperacillin–tazobactam (32.5%, 160/494) and cefepime (24.2%, 119/494) (Table S2).
Patient Characteristics Stratified by Empirical MRSA TherapyOf the 494 ICU-admitted SAP patients, 61.1% (302/494) received empirical anti-MRSA therapy, primarily vancomycin (298 cases) and, less frequently, linezolid (4 cases). 66.7% of patients in the anti-MRSA group initiated treatment within 24 h of ICU admission. 38.9% (192/494) received standard empirical treatment without anti-MRSA coverage. There were no significant differences between the groups in sex distribution (male: 57.6% vs. 54.7%, p = 0.052) or Charlson Comorbidity Index (median 4.0 [IQR 2.0, 6.0] vs. 4.0 [2.0, 6.0], p = 0.31). However, patients receiving anti-MRSA therapy exhibited significantly higher baseline severity, as indicated by elevated APS III, SOFA, SIRS, and LODS scores (all p < 0.001) (Table 1).
On ICU day 1, the anti-MRSA group had higher levels of heart rate, BUN_max, aniongap_max, creatinine_max, glucose_max, potassium_max, INR_max, PT_max, PTT_max, and AST_max compared with the standard therapy group, and lower values of systolic blood pressure, hemoglobin_min, bicarbonate_min, and urine output (all p < 0.05), reflecting more severe physiological derangement (Table 1).
Additionally, the anti-MRSA group had higher rates of identified infection burden with increased rates of bacterial infection (57.6% vs. 26.6%), fungal infection (39.7% vs. 8.9%), and polymicrobial infection (37.7% vs. 7.3%) (all p < 0.001). Among the total cohort, 329 patients underwent MRSA screening, of whom 13 (2.6%) tested positive, and 6 received empirical anti-MRSA therapy (Table 1).
In unadjusted comparisons, patients receiving anti-MRSA group had significantly longer ICU stays (median 5.9 [IQR 2.6, 14.7] vs. 2.1 [1.4, 3.7] days, p < 0.001) and total hospital stays (median 17.7 [IQR 9.7, 27.0] vs. 9.4 [5.7, 13.9] days, p < 0.001). ICU readmission due to pancreatitis recurrence occurred in 16.6% (82/494) of the overall cohort, with no significant difference between groups (p = 0.60) (Table 1).
Adjusted outcomes after propensity score matchingAfter propensity score matching, baseline characteristics between the empirical anti-MRSA group and the standard therapy group were well balanced, with adequate overlap in propensity score distribution (AUC = 92.5%) (Fig. 2, Table S3). Covariate balance was achieved across all matched subgroups after weighting (Fig. S1, Table S4).
Relative Distribution of Propensity Scores for Treatment With Anti - Methicillin-Resistant Staphylococcus aureus (MRSA) therapy. Conditional density curves demonstrating relative distributions of propensity scores for treatment with anti-MRSA therapy. The standardized mean difference (SMD) between the groups was 0.04.
After adjustment, there was no significant difference between groups in ICU mortality (aRR 0.39; 95% CI, 0.11–1.38; p = 0.14), hospital mortality (aRR 0.53; 95% CI, 0.21–1.33; p = 0.18), 28-day mortality (aRR 1.28; 95% CI, 0.64-2.56; p = 0.48), or 90-day mortality (aRR 0.63; 95% CI, 0.37–1.07; p = 0.09) (Table 2). Kaplan–Meier analysis showed no significant difference in 28-day survival between the empirical anti-MRSA and standard therapy groups (P = 0.50), nor in 90-day survival (P = 0.09) (Fig. 3). However, the anti-MRSA group had significantly longer ICU stay (median 3.8 (IQR 3.0, 8.1] vs. 2.7 [2.0, 4.1], p < 0.001), and total hospital stay (median 13.0 [IQR 8.1, 19.8] vs. 9.7 [5.7, 14.5], p < 0.001) compared to the standard treatment group (Table S4).
Adjusted risk ratios for mortality among inverse probability-weighted analyses.
| Outcome | RRa/aRRb; 95% CI (unadjusted/adjusted) | p (unadjusted/adjusted) |
|---|---|---|
| ICU mortality | 11.61 (3.56, 37.97)/0.39 (0.11, 1.38) | < 0.001/0.14 |
| Hospital mortality | 10.24 (4.36, 24.10)/0.53 (0.21, 1.33) | < 0.001/0.18 |
| 28-day mortality | 0.87 (0.54, 1.39)/1.28 (0.64, 2.56) | 0.55/0.48 |
| 90-day mortality | 0.70 (0.47, 1.03)/0.63 (0.37, 1.07) | 0.77/0.09 |
a: RR: Relative Risk; b: aRR: adjusted Relative Risk.
To validate the consistency of the findings, three sensitivity analyses were conducted using distinct statistical approaches. The estimated hazard ratios (HRs) for mortality were consistent across methods: 1.25 (95% CI: 0.66–2.36) in the PSM model, 1.21 (95% CI: 0.55–2.66) in the inverse probability of treatment weighting (IPTW) model (effective sample size [ESS] = 115), and 1.11 (95% CI: 0.53–2.31) in the doubly robust model (Table 3).
Comparison of treatment effect estimates using different sensitivity analysis.
| Method | HRa | 95% CIb | p value | ESSc |
|---|---|---|---|---|
| Original PSMd | 1.25 | (0.66, 2.36) | 0.51 | 256 |
| IPTWe Basic Model | 1.21 | (0.55, 2.66) | 0.63 | 115 |
| IPTW Doubly Robust | 1.11 | (0.53, 2.31) | 0.78 | 115 |
a. HR: hazard ratio, b. CI: confidence interval, c. ESS: Effective Sample Size, d. PSM: Propensity Score Matching, e. IPTW: Inverse Probability of Treatment Weighting.
Across all models, empirical anti-MRSA therapy was not associated with a statistically significant reduction in mortality (all p > 0.05), confirming the consistency of the results. Although the IPTW model experienced a 55% effective sample size reduction due to extreme weighting, the direction and magnitude of the effect remained stable.
Subgroup analysisAfter propensity score matching, the incidence of acute kidney injury (AKI) was significantly higher in the anti-MRSA therapy group compared to the standard therapy group (33.6% vs. 17.2%, p = 0.002). This increased risk was most evident in patients without pre-existing kidney disease (24.2% vs. 8.6%, p < 0.001), whereas no significant difference was observed in those with baseline kidney disease (9.4% vs. 8.6%, p = 0.50) (Fig. 4, Table 4).
Risk of Acute Kidney Injury (AKI) associated with empirical anti-MRSA therapy in the propensity-matched Cohort. This forest plot compares AKI incidence between patients receiving empirical anti-MRSA therapy (n = 128) versus standard therapy (n = 128). Comparison of AKI severity and recovery rate were restricted to patients who developed AKI. Estimates are presented as risk ratios (RR) with 95% confidence intervals, with the standard therapy group as the reference (RR = 1.0).
Acute kidney injury in propensity-matched Cohort.
| Characteristic | Adjusted anti-MRSA therapy (n = 128) | Adjusted standard therapy (n = 128) | p-value |
|---|---|---|---|
| AKI Incidence, n (%) | |||
| Overall | 43 (33.6) | 22 (17.2) | 0.002 |
| With baseline kidney disease | 12 (9.4) | 11 (8.6) | 0.5 |
| Without baseline kidney disease | 31 (24.2) | 11 (8.6) | < 0.001 |
| AKI severity among affected, n (%) | |||
| AKI stage 1 | 32 (74.4) | 18 (81.8) | 0.37 |
| AKI stage 3 | 11 (25.6) | 4 (18.2) | 0.37 |
| Recovery among AKI patients, n (%) | |||
| AKI recovery | 27 (62.8) | 16 (72.7) | 0.3 |
Regarding AKI severity, stage 1 AKI was the most common in both groups. The proportion of stage 3 AKI was higher (25.6% vs. 18.2%, p = 0.37), and the recovery rate of AKI was lower in the anti-MRSA group (62.8% vs. 72.7%, p = 0.30), although neither difference reached statistical significance (Table 4).
DiscussionThis large-scale retrospective analysis of ICU-admitted SAP patients in the MIMIC-IV database reveals a critical therapeutic paradox: despite early initiation - within 24 h of ICU admission in over 60% of cases, empirical anti-MRSA therapy demonstrated no mortality benefit at either 28 days (aRR 1.28, 95% CI 0.64–2.56; p = 0.48) or 90 days (aRR 0.63, 95% CI 0.37–1.07; p = 0.09), while significantly prolonging ICU stays and total hospital stay (all p < 0.001). These findings remained consistent after adjustment for baseline severity using propensity score matching. These findings align with current guidelines that recommend reserving antibiotics for cases of suspected or confirmed infected pancreatic necrosis.21–23 Previous randomized controlled trials and meta-analyses have similarly shown no mortality benefit from prophylactic antibiotic use in SAP.24–26
Several factors may underlie this lack of benefit. First, the prevalence of MRSA was extremely low in this cohort (2.6%). Second, the timing of therapy may not align with disease pathophysiology. Infected pancreatic necrosis rarely develops during the first week of illness. Furthermore, early empirical anti-MRSA therapy - most commonly vancomycin (accounting for 94% of cases) - is intended to introduce microbiome disruption. A notably higher rate of fungal and polymicrobial infections was observed in this group, consistent with previously reported pathogen profiles in SAP.27
Beyond microbiome disruption, empirical anti-MRSA therapy was associated with a significantly increased risk of acute kidney injury, particularly among those without underlying renal disease (RR = 3.40, 95% CI:1.75−6.61, p < 0.001). Although no statistically significant differences were observed in AKI severity stages or recovery rates, the anti-MRSA treatment group had a higher incidence of stage 3 AKI (25.6% vs 18.2%) and a lower recovery rate (62.8% vs 72.7%). These findings are consistent with the established evidence of vancomycin-induced nephrotoxicity, particularly when co-administered with agents like piperacillin-tazobactam.28,29 Recent studies have proposed that alternative agents such as daptomycin, which targets bacterial protein synthesis, when combined with β-lactam antibiotics, may offer improved outcomes in bacteremia management.30–32
Despite technological advances, significant diagnostic blind spots remain, as highlighted by our findings. Only 13 of 329 ICU patients screened for MRSA tested positive (2.6%), yet their in-hospital mortality reached 30.8%, suggesting that current diagnostic tools may miss high-risk infections.33,34 This uncertainty likely fuels the prevalent use of empiric broad-spectrum and anti-MRSA agents, which - when not guided by microbiological evidence - may fail to improve outcomes and instead introduce harm.35–38 Such overuse may increase the risk of nephrotoxicity and fungal overgrowth, as demonstrated in our cohort. To improve precision, future strategies should enhance the sensitivity of pathogen detection in low-burden samples like necrotic pancreatic tissue and integrate clinical, laboratory, and radiologic data to develop dynamic, individualized risk prediction models.
LimitationsAlthough multiple causal inference methods (PSM, IPTW, and double robust models) were employed to adjust for confounding, several limitations remain. As 94% of patients in the anti-MRSA group received vancomycin, the findings predominantly reflect its impact and may not be generalizable to newer agents (e.g., tigecycline, oritavancin). The limited use of linezolid also precluded evaluation of its independent effects. Second, the applicability of these results to settings with high MRSA prevalence in ICU SAP patients may be limited, and region-specific strategies could be warranted. Third, despite rigorous adjustment, residual confounding cannot be excluded, as patients receiving anti-MRSA therapy had more comorbidities and higher baseline severity, which may have influenced outcomes through unmeasured factors. Selective decontamination or other forms of topical/enteral administration of vancomycin (aimed at suppressing MRSA colonization) are not captured as discrete variables in the MIMIC-IV dataset, and therefore we were unable to identify or adjust for such practices. In our cohort the vast majority of anti-MRSA exposure was systemic intravenous therapy (298 of 302 anti-MRSA cases received IV vancomycin), which is the exposure analyzed. Unmeasured use of topical or enteral decontamination could theoretically affect local microbial ecology and individual infection risk.
Given the inherent limitations of this retrospective and nonrandomized design, well-designed prospective, randomized controlled trials are warranted to elucidate the true clinical value of empirical anti-MRSA therapy in patients at high risk for severe acute pancreatitis. In parallel, large-scale, multicenter observational studies are needed to identify robust predictors of MRSA infection through comprehensive multivariable modeling, thereby facilitating risk stratification and optimizing patient selection for empirical therapy. Moreover, procalcitonin-guided antibiotic stewardship has been shown to reduce unnecessary antibiotic exposure in SAP without increasing the risk of infection or organ dysfunction underscoring its potential as a valuable tool in individualized treatment.39 Future prospective investigations should validate such biomarker-based approaches and determine whether tailored antibiotic strategies informed by predictive markers can translate into improved clinical outcomes.
ConclusionsOur study discovered that empiric anti-MRSA therapy was not associated with reduced short- or long-term mortality in ICU patients with severe acute pancreatitis, and may be associated with prolonged hospitalization.
CRediT authorship contribution statementConceptualization: Yinghui Hong, Mingliang Ye. Data curation: Yinghui Hong, Mingliang Ye. Formal analysis: Yinghui Hong, Mingliang Ye. Methodology: Junshi Wang, Yuhang Chen. Software: Yinghui Hong, Mingliang Ye. Supervision: Bin Huang, Xi Li and Lei Huang. Writing-original draft: Yinghui Hong, Mingliang Ye. Writing-review and editing: Yinghui Hong, Mingliang Ye. Yinghui Hong and Mingliang Ye contributed equally and are co-first authors. All authors read and approved the final manuscript.
FundingThis work was supported by grants from Peking University Shenzhen Hospital Scientific Research Fund (No. KYQD2024428).
Consent for publicationNot applicable.
Ethics approval and consent to participateEthical approval for the MIMIC-IV project was granted by the Institutional Review Boards of the Massachusetts Institute of Technology and Beth Israel Deaconess Medical Center. Due to comprehensive de-identification of patient data, this study qualifies for exemption from informed consent requirements.
Declaration of Generative AI and AI-assisted technologies in the writing processNo artificial intelligence tools have been used in the generation of figures or in the creation or refinement of the text.
Availability of data and materialsStudy data were presented in the manuscript and supplemental materials. More information in current study are available from the corresponding author on reasonable request.
The authors declare that they have no competing interests.