The purpose of this scoping review was to evaluate literature involving opioid-sparing medications in critically ill patients with a focus on clinically meaningful outcomes.
DesignScoping review using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews.
SettingIntensive care unit.
Patients or participantsAdult patients in an intensive care unit setting.
InterventionsNone.
Main variables of interestPubMed and Cochrane Library were searched from October 1, 2019 to June 1, 2023. Inclusion criteria consisted of randomized controlled trials evaluating adjunctive analgesic use in adult patients in an intensive care unit setting.
ResultsThere were 343 citations and titles identified in the initial search, with 328 remaining after removal of duplicates, 294 excluded at title and abstract screening, 34 available for full text review, and six included in the scoping review. Most studies reported modest reductions in opioid use as a secondary endpoint. Improvement in clinical outcomes such as reduction in duration of mechanical ventilation or delirium were reported in two trials with dexmedetomidine.
ConclusionsIn recently published trials of adjunctive agents in critically ill patients, opioid-sparing effects were small. Data to support improvements in clinical outcomes remains limited.
El propósito de esta revisión de alcance fue evaluar la literatura sobre medicamentos ahorradores de opioides en pacientes críticos con un enfoque en resultados clínicamente significativos.
DiseñoRevisión de alcance utilizando los elementos de informes preferidos para revisiones sistemáticas y la extensión de metanálisis para revisiones de alcance.
ÁmbitoUnidad de Cuidados Intensivos.
Pacientes o participantesPacientes adultos en una unidad de cuidados intensivos.
IntervencionesNinguno.
Variables de interés principalesSe realizaron búsquedas en PubMed y Cochrane Library desde el 1 de octubre de 2019 hasta el 1 de junio de 2023. Los criterios de inclusión consistieron en ensayos controlados aleatorios que evaluaron el uso de analgésicos complementarios en pacientes adultos en una unidad de cuidados intensivos.
ResultadosSe identificaron 343 citas y títulos en la búsqueda inicial, 328 restantes después de la eliminación de duplicados, 294 excluidas en la selección de títulos y resúmenes, 34 disponibles para revisión del texto completo y seis incluidas en la revisión de alcance. La mayoría de los estudios informaron reducciones modestas en el uso de opioides como criterio de valoración secundario. En dos ensayos con dexmedetomidina se informaron mejoras en los resultados clínicos, como la reducción de la duración de la ventilación mecánica o el delirio.
ConclusionesEn ensayos publicados recientemente sobre agentes complementarios en pacientes críticamente enfermos, los efectos ahorradores de opioides fueron pequeños. Los datos que respaldan las mejoras en los resultados clínicos siguen siendo limitados.
The Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU (PADIS Guidelines) suggest a protocol-based, stepwise approach for pain and sedation management that promotes treating pain over initiating a sedative agent (conditional recommendation, moderate quality of evidence).1 Opioid analgesics remain the most utilized agents to achieve this endpoint despite well-established adverse effects including but not limited to respiratory depression, constipation, delirium, and immunosuppression. Although there is substantial variation in the method of administration and agent selected, available data indicates that opioids are utilized in nearly 85% of mechanically ventilated patient-days.2
In 2016, guidelines published by the Centers for Disease Control (CDC) noted that long-term opioid use often begins when opioids are prescribed for acute pain.3 Since this time, the majority of every clinical practice guideline with recommendations related to pain management advocates for the use of opioid-sparing interventions in conjunction with non-pharmacologic approaches when available.4–9 In critically ill patient populations, the PADIS Guidelines suggest use of acetaminophen as an adjunct to opioids in critically ill patients and conditionally suggest use of ketamine for post-surgical pain and gabapentinoids for neuropathic pain to reduce opioid requirements and improve pain control.1 Nefopam is also recommended as an adjunct or alternatives to opioids but it is not commercially available in the United States or Canada.
The efficacy and safety of nonopioid analgesics for patients in an intensive care unit (ICU) setting was evaluated in a review of studies published up to October 1, 2019 by Wheeler et al. 10 The investigators found that the use of any adjunct agent compared to opioids alone was associated with decreased opioid use. However, the decrease in oral morphine equivalents over 24 hours was small (mean difference, 25.89 mg less; 95% CI, 19.97–31.81 mg) and of low certainty.10 Debate exists regarding what defines a substantial opioid-sparing effect, whether a reduction in opioids translates to improved patient outcomes, and what defines an acceptable benefit-to-risk ratio in critically ill patient populations at risk for adverse drug events.
In recent years, various groups and organizations including the Food and Drug Administration (FDA) have drafted guidance documents that address future research design for studies evaluating opioid-sparing medications and outcomes that would be considered clinically meaningful.11,12 The purpose of this scoping review is to provide an update to the review by Wheeler et al. and discuss if medications commonly recommended as opioid-sparing agents have evidence of an acceptable benefit-to-risk profile as elucidated in guidance documents to justify their use in heterogeneous populations of critically ill patients in ICU settings.
Patients and methodsProtocol and registrationThe Preferred Reporting Items for Systematic Reviews and Meta-Analysis extension for Scoping Reviews (PRISMA-ScR) was utilized for drafting and revision of the protocol by the research team.13
Eligibility criteriaInclusion criteria consisted of randomized controlled trials evaluating adjunctive analgesic use in adult patients (aged 18 years or older) in an ICU setting from October 1, 2019, to June 1, 2023. Studies were excluded if interventions were initiated outside of the ICU (e.g., emergency department, operative room, post-anesthesia care units), if interventions were peri-procedural such as line insertions or dressing changes, if they were regional in nature (e.g., peripheral nerve blocks, epidural or intrathecal catheters), or if single-doses were studied. Other exclusion criteria included studies without an English-language translation and studies done in mixed populations of ICU and floor patients. The criteria were selected to mirror that of the previous systematic review by Wheeler et al.10
Human subjectsThis review did not involve human subjects and thus approval from the institutional review board was not necessary.
Information resourcesTwo bibliographic databases were searched to identify relevant literature from October 1, 2019, to June 1, 2023, which included PubMed (National Library of Medicine, 2019–2023) and Cochrane Library (Wiley, 2019–2023). To limit the possibility of missed studies, the search was performed with the assistance of a medical librarian using different databases and reviewing reference lists of included trials to help identify any missing articles. An initial search was completed in December of 2022. An updated search was performed in February and May of 2023. The final search, done prior to completion of the manuscript to capture any studies recently published, was completed utilizing PubMed on June 1st, 2023. Keyword concepts for the search included the following terms: “intensive care unit,” “opioid,” and “analgesia.” The full search strategy can be found in Appendix A. Duplicate articles were identified and removed, and the database reviews were supplemented by reviewing references of key reviewed articles.
Selection of sources of evidenceTo provide consistency in data extraction, a single reviewer screened articles for the review. Two other reviewers then reviewed the included articles to form a final consensus.
Screenings were conducted in two sequential stages with the first comprising an initial assessment of the title/abstract, if no exclusion criteria were noted the next stage was a full review of the article. Reasons for exclusion were recorded at each stage. To reduce clinical heterogeneity, studies evaluating regional anesthesia interventions and interventions initiated in non-ICU settings were excluded.
Data charting processThe data charting process was completed in accordance with previous scoping review protocols.14 Key study characteristics and detailed information on all metrics to determine opioid sparing effects were made jointly by the reviewers. One reviewer independently charted the data. Discussions, with all reviewers, were held to resolve disagreements on criteria. The variables of interest included year of publication, country, study design and setting, number of subjects, patient population, types of interventions provided and their relationship to opioid usage, and clinically important endpoints such as ICU length of stay, duration of mechanical ventilation, and incidence/duration of delirium. A data extraction table was developed prior to data extraction to provide a format for documenting key study characteristics. The article PMID and titles were charted in excel and duplicates were removed throughout the data extraction process.
Critical appraisal of individual sources of evidenceScoping reviews, unlike systematic reviews, examine literature where it is uncertain what questions may be posed. Thus, critical appraisals are not pertinent for this scoping review.
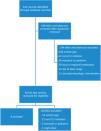
Data analysis and synthesis of resultsFull text articles were assessed for eligibility after the initial screening process. For a depiction of the process used in synthesizing the data for this scoping review see the PRISMA flow diagram (Fig. 1).
Risk of bias assessmentRisk of bias assessment was not necessary for this scoping review.
ResultsThe PRISMA diagram for the scoping review is outlined in Fig. 1. A total of six randomized controlled trials were included for analysis.15–20 The patient populations evaluated in the trials included mixed medical/surgical ICU patients (n = 2), cardiothoracic ICU patients undergoing elective coronary artery bypass graft (CABG) surgery (n = 2), oral and maxillofacial surgical patients (n = 1), and neuroscience ICU patients (n = 1). One-half of the studies were completed in patients admitted to the ICU post-operatively. Two of the studies compared a non-opioid agent (ketamine in one study, gabapentin in the other study) to a control group, one study compared gabapentin to placebo, two studies compared dexmedetomidine to other agents (clonidine in one study, midazolam in the other study), and one study compared ketorolac to paracetamol. Full characteristics of the included trials are described in Table 1.
Characteristics of Included Studies.
| Study | Standard Care and Opioids Utilized | Comparison Groups | Duration of intervention | Patient subgroup and number of patients | Mean age (yr) | % Female patients | Outcome |
|---|---|---|---|---|---|---|---|
| Wang et al.15 | Continuous infusion hydromorphone (4–8 mcg/kg/hour) | IV Midazolam (MID) (0.04–0.2 mcg/kg/hour)orIV Dexmedetomidine (DEX) (1 mcg/kg over 10 minutes and then continuous infusion 0.2–0.7 mcg/kg/hour; maximum 1 mcg/kg/hour) | 12 hours | Oral and maxillofacial surgical patients, mechanically ventilatedDEX n = 20; MID n = 20 | MID = 60.5DEX = 60.05 | 27.5 | Lower mean hydromorphone requirements in DEX group vs MID (3.87 ± 1.13 mg vs 5.69 ± 0.77 mg; p = 0.001) |
| Shokri et al.16 | Morphine 1–2 mg rescue doses allowed in both groups.Midazolam 15 mcg/kg permitted in DEX group when maximum dose inadequate | IV DEX (0.7–1.2 mcg/kg/hour; maximum 1.4 mcg/kg/hour)orClonidine (0.5 mcg/kg over 10–15 minutes followed by continuous infusion at 1–2 mcg/kg/hour; maximum 2 mcg/kg/hour) | 24 hours or until ICU discharge up to 72 hours | Adults between 60–70 years of age undergoing coronary artery bypass graft (CABG) surgery, mechanically ventilatedDEX n = 144; Clonidine n = 142 | DEX = 63.75 Clonidine = 64.38 | 51.4 | Lower mean post-operative morphine consumption over 3 days in DEX group vs clonidine (18.24 ± 8.62 mg vs 22.37 ± 10.73 mg; p < 0.001) |
| Javaherforooshzadeh et al.17 | Standard protocol for sedation after surgery consisting of propofol 0.5 mg/kg/hour and morphine 0.1 mg/kg/hourPCA morphine after extubation | IV Ketorolac 0.5 mg/kg every 6 hours orIV Paracetamol 10 mg/kg every 6 hours | Study drug was continued for 24 hoursMorphine consumption was collected for 48 hours | Adults between 30–70 years of age, elective on-pump CABG; ejection fraction ≥ 30%; CTICUKetorolac n = 30; Paracetamol = 30 | Ketorolac = 61.83Paracetamol = 58.41 | 38.3 | Lower mean morphine consumption at 24 hours in the paracetamol group vs the ketorolac group (0.29 ± 0.41 mg vs 1.71 ± 0.53 mg; p = 0.027) and at 48 hours (0.22 ± 0.15 mg vs 2.18 ± 0.52 mg; p = 0.007) |
| Dhakal et al.18 | Protocolized opioid regimen consisting of fentanyl 25–50 mcg every hour as needed with conversion to oxycodone 5–10 mg every 4 hours as needed if pain inadequately relieved with fentanyl Opioid dose converted to morphine equivalents (unclear if IV or oral morphine equivalents) | Gabapentin (100 mg three times daily; titrated every 24 hours up to a maximum dose of 900 mg three times daily)orPlacebo | 7 days | Patients 18 years of age and older with aneurysmal SAH in neuro ICUGabapentin n = 8; Placebo n = 8 | Gabapentin = 52.7Placebo = 49.8 | 75 | No significant difference in mean morphine equivalent requirements at day 7 in the gabapentin group vs placebo group (19.4 ± 14.8 mg vs 20.2 ± 13.5 mg; p = 0.79) |
| Amer et al19 | Standard ICU analgesia/sedation protocol used at study institution. Opioid regimen consisted of fentanyl | Ketamine (1–2 mcg/kg/min)orControl group receiving standard of care | Treatment duration up to 48 hours | Admission to medical, surgical, or transplant ICU, mechanical ventilation anticipated for at least 24 hours after enrollment(N = 83, ketamine n = 40) | Median ketamine = 59Median control group = 61 | 38.6 | No significant difference in median cumulative weight-based dose of fentanyl (69.6 mcg/kg in ketamine group vs 63.5 mcg/kg in control group; p = 0.69) or non-weight-based dose of fentanyl (4400 mcg in ketamine group vs cg in control group, p = 0.67)at 48 hours |
| Salarian et al.20 | Fentanyl (1–2 mcg/kg/hour)andmidazolam (0.06–0.2 mg/kg/hour) | Gabapentin (300 mg every 8 hours)OrControl group | Not reported | Mixed medical and surgical/trauma patients between the ages of 18–70 receiving mechanical ventilation for more than 3 daysGabapentin n = 23Control n = 20 | Gabapentin = 57.7Control group = 52.4 | 53.5 | Significant reductions in mean fentanyl dose in the gabapentin group vs control (1.8 ± 0.1 mcg/kg/hour vs 4.1 ± 1.5 mcg/kg/hour; p < 0.01) |
Of the trials published since 2019, the opioid-sparing effects of the intervention groups were commonly secondary endpoints with the majority demonstrating modest reductions in opioid use.15,16,19,20 Primary endpoints evaluated were commonly those that might be considered clinically important in critically ill patient populations (e.g., incidence and duration of delirium, duration of mechanical ventilation), but with no intent to relate them to opioid-sparing effects. Additionally, benefits with respect to the incidence of delirium and duration of mechanical ventilation are potentially confounded in the two studies that utilized benzodiazepines in the control group.15,20
The trials included in this scoping review demonstrated variable results with respect to opioid-sparing effects, with four trials15–17,20 revealing statistically significant reductions in opioid requirements and two trials18,19 reporting no differences in opioid requirements. Wang et al. reported a mean reduction in intravenous hydromorphone of 1.82 mg in mechanically ventilated patients undergoing maxillofacial surgery using a sedation regimen consisting of dexmedetomidine compared to midazolam.15 Another study by Shokri et al. reported a mean intravenous morphine reduction of 4.13 mg over 72 hours in mechanically ventilated patients undergoing CABG surgery that received dexmedetomidine compared to clonidine for sedation.16 Javaherforooshzadeh et al. evaluated morphine requirements with adjunctive paracetamol compared to ketorolac in postoperative CABG patients and reported a mean reduction 1.42 mg at 24 hours with paracetamol.17 Finally, Salarian et al. reported a mean difference in fentanyl infusion rate of 2.3 mcg/kg/hour in a mixed population of critically ill patients receiving mechanical ventilation with gabapentin added to standard care.20
The incidence and duration of delirium were evaluated in four out of six trials included in this scoping review. Two studies reported reductions in the incidence and duration of delirium with dexmedetomidine compared to midazolam or clonidine infusions for sedation.15,16 In one trial by Salarian et al. that compared the addition of gabapentin to a sedation regimen consisting of fentanyl and midazolam to a control group that received the same sedation regimen without gabapentin, the incidence of delirium was evaluated as a secondary endpoint.20 Patients that received gabapentin had lower rates of delirium compared to control (43% vs 17.3%, p = 0.03). Finally, a study using adjunctive ketamine reported no differences in the incidence of delirium compared to a protocolized, nurse-driven sedation regimen with daily spontaneous awakening trials and spontaneous breathing trials.19
Four out of the six randomized controlled trials compared duration of mechanical ventilation as either a primary or secondary outcome. Two of the trials reported reductions in duration of mechanical ventilation, both of which included dexmedetomidine in the intervention group.15,16 However, the absolute reductions in the duration of mechanical ventilation were small (6.3 minutes and 1.8 hours). The other two studies evaluating adjunctive ketamine and gabapentin reported no differences in the duration of mechanical ventilation.19,20
Of the trials included in this scoping review, none evaluated the impact of the intervention on opioid prescriptions at ICU transitions of care, hospital discharge, or long-term opioid utilization.
DiscussionThe results of this scoping review indicate that there have been a limited number of randomized controlled trials evaluating opioid-sparing agents in critically ill patient populations since a review by Wheeler et al. involving studies up to 2019.10 Additionally, there remains no consistent definition with respect to what absolute or percentage reduction in opioid requirements defines a clinically meaningful opioid-sparing agent.11,12 The review by Wheeler et al. concluded that opioid adjunctive medications in addition to opioids as compared to an opioids alone led to reductions in patient-reported pain scores at 24 hours (standard mean difference, –0.88; 95% CI, –1.29 to –0.47) and decreased opioid consumption (in oral morphine equivalents over 24 hour; mean difference, 25.89 mg less; 95% CI, 19.97–31.81 mg less), but both findings were of low certainty.
A recently published consensus document formulated by participants from universities, government agencies, industry, and patient advocacy organizations suggested that an opioid-sparing intervention must include the following elements without causing an unacceptable increase in pain: (1) prevents opioids from being started, (2) decreases opioid treatment duration, (3) decreases the cumulative opioid dose, or (4) reduces opioid-related adverse outcomes.11 In addition, the Food and Drug Administration (FDA) published a draft guidance document to address challenges of developing non-opioid medications to manage pain in light of the opioid crisis which questioned whether the term “opioid-sparing” was sufficient to justify labeling regarding reduced opioid requirements.12 While these guidance documents do not specifically address critically ill patient populations, the basic tenets generally remain applicable. Of the six included studies in this scoping review, none prevented an opioid from being started, none decreased opioid treatment duration, four trials showed a modest reduction in cumulative opioid dose, and four studies addressed delirium and mechanical ventilation as described below. None of the trials addressed all elements recommended in guidance documents.
Preventing opioid initiation in postoperative/trauma-related pain or in mechanically ventilated patients using the protocol-based, stepwise approach for pain and sedation management suggested in the PADIS guidelines might be desirable but is challenging with currently available non-opioid therapeutic options in patients with more severe pain states.1 However, minimizing duration in critically ill patients, particularly with respect to discontinuation of therapy at transitions of care out of the ICU or prior to discharge would likely represent a clinically important outcome. Several studies involving critically ill patient populations opioid-naïve prior to ICU admission and that received opioids as part of an analgosedation or analgesia-first regimen were ultimately discharged from the hospital with a prescription rates for an opioid varying between 20% to 47.1%.21–24 In addition, persistent opioid use among opioid-naïve ICU survivors measured at various time points between 3 and 12 months after ICU discharge ranged from 2.6% to 23%.22,24–26 Given concerns related to adverse effects of opioids in general and long-term opioid use disorder in ICU survivors, opioid-sparing agents that reduce duration of opioid use are warranted. A useful discussion of opioid-sparing options for managing analgesia, sedation, and delirium in critically ill adult patients is provided in clinical practice guidelines compiled by the Pan-American and Iberian Federation of Societies of Critical Medicine and Intensive Therapy.27 These guidelines recommend decreasing sedation levels with the use of multimodal pain management strategies while recognizing the need for more research on alternatives to opioids. Agents such as dexmedetomidine, remifentanil, and ketamine are discussed in the guidelines. Of note, the terminology regarding sedation has implications for patient management. For example, analgesia-first sedation in which an analgesic is used before a sedative is different from analgesia-based sedation in which an analgesic is used instead of a sedative to manage agitation or facilitate mechanical ventilation.28
Potential risks of opioid sparing strategies include inappropriate pain control which may lead to prolonged duration of mechanical ventilation, ventilator dyssynchrony, increased ICU length of stay, and post-traumatic stress disorder. Additionally, interpretation of any opioid-sparing effects and reductions in duration of mechanical ventilation or delirium is often complicated by confounding variables (e.g., use of benzodiazepines) in published studies. Regardless, there is appeal to the use of agents such as dexmedetomidine that may reduce the duration of mechanical ventilation and delirium while providing sedation to critically ill patients.29 A useful paper for clinicians wishing to minimize the problems associated with oversedation was compiled by the Zero Oversedation Project of the Sedation, Analgesia and Delirium Working Group of the Spanish Society of Intensive and Critical Care Medicine and Coronary Units.30 The paper contains practical information with tools to help ensure optimal patient management including minimizing the risk of excessive sedation.
The study by Salarian et al.,20 which suggested that the addition of gabapentin to a sedation regimen led to a reduced rate of delirium, is not corroborated by the results of a recent study that demonstrated that perioperative gabapentin use was associated with increased risk of delirium among older patients after major surgery.31 Furthermore, prescription use of gabapentin has increased more than five-fold between 2006 and 2018 with a coexisting increase in gabapentin-related overdose death exacerbated by concomitant opioid use.32,33 Given the widespread use of opioids in critically ill patients and concerns about safety, adoption of gabapentin use as an opioid-sparing agent in this patient population requires additional study.
Reduced duration of mechanical ventilation represents another potential clinically important outcome of opioid-sparing agents assuming pain assessments are routinely employed. As noted by García Sánchez and Alcántara Carmona, 70% of patients receiving mechanical ventilation in Spanish ICUs receive opioids but only 52% of ICUs use routine pain assessment scales.34 The studies in our scoping review demonstrating that dexmedetomidine but not ketamine reduced the duration of mechanical ventilation are consistent with a previously published meta-analysis, which reported a reduction in the duration of mechanical ventilation with dexmedetomidine (low certainty of evidence) and no reduction in duration of mechanical ventilation with ketamine (moderate certainty of evidence).10 The cause and effect of the potential opioid-sparing effects of dexmedetomidine on duration of mechanical ventilation requires additional study.
Future directions for research should include a focus on the effectiveness and safety of non-opioid medications in critically ill patients and not rely on extrapolations from studies conducted in other patient populations. Critically ill patients are at increased risk for a number of organ dysfunctions that might preclude the administration of medications commonly administered in other patient settings. For example, critically ill patients are at risk for acute kidney injury and gastrointestinal bleeding, conditions that would necessitate the avoidance of nonsteroidal anti-inflammatory drugs (NSAIDs).
Future investigations also need to evaluate outcomes beyond opioid sparing effects. A medication’s benefit in terms of an opioid-sparing effect must outweigh safety-related concerns (i.e., benefit exceeding risks), which becomes complex in critically ill patients at substantial risk for complications such as bleeding, acute kidney injury (AKI), and respiratory failure. Potential clinically important endpoints that could be evaluated with opioid-sparing regimens include duration of mechanical ventilation (or ventilator-free days), incidence and duration of delirium, reduction in post-operative ileus, and incidence of respiratory failure leading to mechanical ventilation. Ideally, any improvements in these clinical outcomes would not be outweighed by adverse outcomes such as alterations in hemodynamics, AKI, bleeding complications, among others. Clinically meaningful outcomes related specifically to opioid sparing that should be investigated might include avoidance of opioid use, decreased duration or cumulative doses of opioid administration, or reductions in opioid-related adverse effects. Finally, there is a need for more research to identify novel analgesic agents that can either serve to eliminate the use of opioids or serve as adjunctive therapies.
ConclusionUtilization of adjunct agents to limit opioid exposure is a widespread practice amongst clinicians in the ICU setting, though it comes with difficulties and barriers. However, few studies have shown benefits (beyond decreased opioid consumption) of utilizing non-opioid medications in patients who are critically ill and requiring analgesics. Further studies are needed to determine the implications of opioid sparing amongst critically ill patients admitted to the ICU.
Author contributionsAll authors contributed to conception and design, acquisition, analysis, or interpretation of data, drafted, and critically revised the manuscript.
FundingNo funding was used for this manuscript.
Conflict of interestThe authors have no conflict of interest to declare.
Data availability statementThere is no shared data.
None.
Please cite this article as: Gambadoro G, Kopp BJ, Erstad BL. Implicaciones de los medicamentos ahorradores de opioides en pacientes críticos: una revisión exploratoria. Med Intensiva. 2024. https://doi.org/10.1016/j.medin.2024.06.001.