The primary objective of this study was to evaluate the impact of high-flow nasal cannula oxygen therapy [HFNC] on the diaphragm thickening fraction.
DesignProspective, descriptive, cohort study
SettingThe study was conducted in the Physiology and Respiratory Care Laboratory, Intensive Care Unit, Hospital Británico de Buenos Aires.
ParticipantsThirteen healthy subjects >18 years old
InterventionsHigh-flow nasal cannula oxygen therapy
Main variables of interestDemographic data (age and gender), anthropometric data (weight, height, and body mass index), and clinical and respiratory variables (Diaphragm thickening fraction [DTf], esophageal pressure swing, respiratory rate [RR], esophageal pressure-time product per minute [PTPes/min]).
ResultsMedian DTf decreased significantly as flow increased (p < 0.05). The baseline DTf measurement was 21.4 %, 18.3 % with 20 L/m, and 16.4 % with 40 L/m. We also observed a significant decrease in RR as flow increased in HFNC (p < 0.05). In the 8 subjects with recordings, the PTPes/min was 81.3 (±30.8) cmH2O/sec/min and 64.4 (±25.3) cmH2O/sec/min at baseline and 40 L/m respectively (p = 0.044).
ConclusionsThe use of high-flow oxygen therapy through nasal cannula of HFNC in healthy subjects decreases the DTf and RR in association with increased flow. In addition, the use of 40 L/m flow may reduce the muscular work associated with respiration.
El objetivo primario de este estudio fue evaluar el impacto de la oxigenoterapia con cánula nasal de alto flujo (HFNC) sobre la fracción de engrosamiento del diafragma.
DiseñoEstudio de cohorte, prospectivo, descriptivo.
ÁmbitoEl estudio se llevó a cabo en el Laboratorio de Fisiología y Cuidados Respiratorios, Unidad de Terapia Intensiva, Hospital Británico de Buenos Aires.
ParticipantesTrece sujetos sanos >18 años.
IntervencionesOxigenoterapia con cánula nasal de alto flujo.
Variables de interés principalesDatos demográficos (edad y sexo), datos antropométricos (peso, talla e índice de masa corporal), variables clínicas y respiratorias (fracción de engrosamiento del diafragma [DTf], presión esofágica, frecuencia respiratoria [FR], producto presión-tiempo esofágico por minuto [PTPes/min]).
ResultadosLa mediana de DTf disminuyó significativamente a medida que aumentaba el flujo programado (p < 0,05). La medición basal de la DTf fue del 21,4 %, del 18,3 % con 20 L/m y del 16,4 % con 40 L/m. También observamos una disminución significativa de la FR a medida que aumentaba el flujo en HFNC (p < 0,05). En los 8 sujetos con registros, la PTPes/min fue de 81,3 (±30,8) cmH2O/seg/min y 64,4 (±25,3) cmH2O/seg/min al inicio y 40 L/m respectivamente (p = 0,044).
ConclusionesEl uso de oxigenoterapia a alto flujo a través de cánula nasal en sujetos sanos disminuye la DTf y la FR conforme aumenta el flujo programado. Además, el uso de un flujo de 40 L/m puede reducir el trabajo muscular asociado a la respiración.
High flow oxygen therapy via nasal cannula (HFNC) involves the administration of humidified and heated gas at a high flow rate, with a variable fraction of inspired oxygen (FiO2).1 The use of HFNC at the intensive care unit (ICU) setting has increased due to the advantages it offers in treating certain diseases,1–3 as well as the benefits it provides vs conventional oxygen therapy.4–6 HFNC provides respiratory support by clearing dead space in the upper airways between breaths, allowing for optimization of the inspired gas composition for the patient. High velocity nasal insufflation (HVNI) is a variant of HFNC that uses a smaller diameter cannula, enabling the delivery of high flow at a higher velocity.7
Work of breathing (WOB) refers to the mechanical effort associated with breathing and is quantified as the pressure gradient required to achieve a change in lung volume, mainly determined by the resistance and elasticity offered by the respiratory system.8
Changes in esophageal pressure swing (Pes) are representative of pleural pressure and help establish the pressure gradient primarily generated by the diaphragm.9 Diaphragm ultrasound is a non-invasive imaging modality to complement Pes measurement, allow for the assessment of diaphragm function and reflect the extent of diaphragm fiber recruitment through the muscle thickening fraction.10
We hypothesized that the implementation of HFNC would have a significant impact on the diaphragm thickening fraction in healthy subjects. Specifically, we considered that HFNC would lead to a decrease in diaphragm thickening fraction, indicative of lower diaphragm fiber recruitment. Additionally, we expected that respiratory rate would show a favorable response to HFNC use, with a reduction in its values, suggesting an improvement in respiratory efficiency. Therefore, the primary endpoint of this study was to evaluate the impact of high-flow nasal cannula oxygen therapy on the diaphragm thickening fraction in healthy subjects. Secondarily, this study aimed to evaluate the behavior of respiratory rate and WOB in these subjects.
Patients and methodWe conducted a descriptive and prospective cohort study at the Physiology and Respiratory Care Laboratory, Intensive Care Unit of Hospital Británico de Buenos Aires, Argentina from March 1st through June 30th, 2022. A total of 13 healthy subjects older than 18 years were included. All subjects gave their prior written informed consent. Demographic data (age and gender), anthropometric data (weight, height, and body mass index [BMI]), and clinical and respiratory variables were recorded.
Clinical and respiratory dataThe following variables were recorded for each study participant:
- -
- -
Esophageal pressure swing. Difference between baseline Pes and maximum inspiratory Pes deflection.
- -
Respiratory rate (RR). Number of breaths per minute recorded over 60 s.
- -
Esophageal pressure-time product per minute (PTPes/min). Calculated as the area determined by the maximum inspiratory Pes deflection multiplied by the RR.12 A custom software developed in Matlab R2018b (The MathWorks, Inc., Natick, MA, United States) was used for this purpose.
For the study, we used: a FluxMed® pulmonary mechanics monitor with a latex balloon catheter of 7 cm in length (MBMed, Buenos Aires, Argentina) for measuring esophageal pressure; an Esaote MyLab® 40 ultrasound machine (Genoa, Italy) for diaphragm measurements, and a Precision Flow® high-flow oxygen therapy device with HVNI technology (Vapotherm Inc., Exeter, NH) and its disposable products.
Procedures performedAll partiipants had an esophageal balloon inserted through the nose (after topical anesthesia), to the middle third of the esophagus, approximately 30 cm from the nose. Balloon was inflated with 1 mL of air and connected to the pulmonary mechanics monitor. An occlusion test was performed to assess proper placement of the esophageal balloon. A tele-expiratory pause was performed, and airway pressure was recorded during an inspiratory effort as detailed in the Baydur test. An acceptable catheter position was defined when the ratio of changes in esophageal pressure to airway pressure (ΔPes/ΔPaw) was close to unity (between 0.8 and 1.2).13,14 Pes was digitized and recorded on a laptop after a stabilization period of 5 min. Data were recorded with each subject lying in a semi-seated position at 45 °, breathing at rest with a closed mouth under 3 different conditions: without HFNC, with HFNC at 20 L/min, and with HFNC at 40 L/min. In all cases, the fraction of inspired oxygen was 21% after a random flow sequence generated online at (https://www.randomizer.org/).
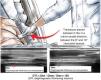
Each test condition was maintained for 5 min, during which Pes was continuously recorded. During the last minute of each of these 5-minute periods, RR was recorded, and the corresponding ultrasound measurements were taken by an expert operator (the same in all cases). The ultrasound measurement was taken using a 7−13 MHz high-frequency linear transducer in real-time B-mode. The transducer was placed between 2 ribs in a cranio-caudal direction, searching for the area with the best image resolution, between the 9th and 10th right intercostal spaces, medial to the anterior axillary line, locating the diaphragm between 2 parallel hyperechoic tissue layers (pleura and peritoneum) with a hypoechoic tissue layer (diaphragm) between them. Once the support area was located, diaphragm thickness at end-expiration was measured over 3 respiratory cycles using the equipment's electronic caliper.
In this position, diaphragm thickness at end-expiration and end-inspiration of the same respiratory cycle was measured (Fig. 1), and the mean value over 3 respiratory cycles was recorded. A 2-minute washout period was allowed, with the subject breathing at rest as comfortably as possible, between each test condition to avoid cumulative effects. DTf11 and PTPes/min12 were calculated for each subject under each available condition.
Statistical analysisDescriptive statistics used mean and standard deviation or median and interquartile range for quantitative variables, and absolute and relative frequencies for qualitative variables. The Shapiro–Wilk test and quantile-quantile plots of the differences were used to validate data normality. For analyzing variations in respiratory rate and DTf at different flows, a regression model belonging to the generalized linear model (generalized estimating equation) was used. This choice was based on the lack of data independence and the inability to ensure homoscedasticity among them. Sample size was determined by the maximum number of volunteers we could incorporate into the study and based on former studies.15 Statistical significance was considered for p < 0.05. Stata® 13 software was used for data analysis (StataCorp. 2011. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP).
This study was conducted in full compliance with the Declaration of Helsinki, and was approved by Hospital Británico deBuenos Aires Research Ethics Committee; approval: CRIHB #1220 PRIISABA No. 6256 and registered at https://clinicaltrials.gov/ #NCT06086769. All participants provided their prior written informed consent to participate. Data supporting the findings of this study are available through the corresponding author upon reasonable request. The Precision Flow® equipment and disposables were provided by JAEJ S.A. (Buenos Aires, Argentina). This study did not receive any funding.
ResultsData from 13 healthy subjects were analyzed, 31% of whom were women, with a mean age of 29.7 years (±3.4) (Table 1).
Demographic and anthropometric characteristics of the 13 study participants.
| Participant No. | Age (years) | Gender (F/M) | Height (cm) | Weight (kg) | BMI (kg/m2) |
|---|---|---|---|---|---|
| 1 | 26 | M | 174 | 72 | 23.7 |
| 2 | 27 | F | 167 | 69 | 24.7 |
| 3 | 28 | M | 165 | 72 | 26.4 |
| 4 | 33 | M | 178 | 70 | 22 |
| 5 | 32 | M | 165 | 80 | 29.4 |
| 6 | 35 | M | 184 | 110 | 32.5 |
| 7 | 30 | M | 172 | 72 | 24.3 |
| 8 | 28 | M | 170 | 75 | 26 |
| 9 | 25 | M | 185 | 88 | 25.7 |
| 10 | 26 | F | 150 | 50 | 22.2 |
| 11 | 31 | F | 146 | 45 | 21.1 |
| 12 | 35 | F | 170 | 58 | 20.1 |
| 13 | 30 | M | 178 | 75 | 23.7 |
| Total | **29.7 (±3.4)**a | **4 (30%)**b | **169.5 (±11.6)**a | **72 (±16.3)**a | **24.8 (±3.4)**a |
F, female; M, male; BMI, body mass index.
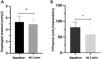
The median DTf dropped significantly as flow increased (p < 0.05). Baseline DTf was 21.4% (IQR, 17.4–30), 18.3% (IQR, 13–23.1) at 20 L/min, and 16.4% (IQR, 11.7–27.5) at 40 L/min (for each liter of flow applied, DTf dropped by 0.16% ± 0.06; 95%CI, 0.28−0.036; p = 0.011) (Fig. 2A). We also observed a significant decrease in RR as HFNC flow increased (p < 0.05). The baseline median RR was 16 breaths per minute (IQR, 14–18), 10 breaths per minute (IQR, 8–12) at 20 L/min, and 6 breaths per minute (IQR, 6–7) at 40 L/min; a reduction of 0.25 breaths per minute ± 0.02 (95%CI, 0.3−0.22; p < 0.0001) per liter of flow used (Fig. 2B).
Behavior of diaphragm thickening fraction (A) and respiratory rate (B): baseline, with HFNC at 20 L/min, and with HFNC at 40 L/min. (A) Generalized linear model considering data dependency (generalized estimating equation; GEE) (p < 0.011). DTf (%), diaphragm thickening fraction. (B) Generalized linear model considering data dependency (generalized estimating equation; GEE) (p < 0.0001). Respiratory rate expressed in breaths/minute.
Because of technical problems, not all signals could be processed (5 records were involuntarily deleted); however, in the 8 subjects with preserved records, a mean Pes of 5.31 cmH2O (±1.39) and 4.92 cmH2O (±1.30) was obtained; baseline and at 40 L/min, respectively (p = 0.75) (Fig. 3A). Additionally, baseline PTPes/min and the result of HFNC application at 40 L/min showed a significant decrease in WOB of 81.3 cmH2O/s/min (±30.8) and 64.4 cmH2O/s/min (±25.3) baseline and at 40 L/min, respectively (p = 0.044) (Fig. 3B).
DiscussionThis study describes the effect of HFNC on DTf, RR, and WOB in healthy participants. Key findings include: (1) application of HFNC at 40 L/min reduced DTf by 23%; (2) HFNC use impacted RR, significantly decreasing it with increased programmed flow; (3) a 20% reduction in WOB was evidenced through decreased PTPes/min with HFNC at 40 L/min.
Lung volume gain is mainly determined by the variation in pleural pressure generated by the action of respiratory muscles. As the main inspiratory muscle, the diaphragm exerts its effect by increasing the 3 thoracic diameters.16 Greater diaphragm thickening indicates greater muscle recruitment. Our findings reveal a reduction in DTf, possibly indicating decreased muscle recruitment. Interestingly, almost 70% of this reduction is achieved with a programmed flow of 20 L/min, while the remaining 30% is reached at 40 L/min. This difference could be attributed to the flow acceleration effect generated by HVNI technology and its impact on pressure, especially in CO2 washout. Based on fluid tests conducted with different cannula diameters (2.7 mm vs. 5.4 mm), and clinical experience, high-velocity nasal insufflation has been shown to require a lower flow in adults to completely purge CO2 from the extrathoracic anatomical reservoir between breaths.7,17 Consequently, significant effects on DTf can be achieved with a relatively low programmed flow. This could be considered for patients with poor adherence to HFNC use due to intolerance to high flow programming.17,18
In our study, a marked decrease in RR was also evident, which could be determined by the combined physiological advantages of HFNC with HVNI technology as it uses small-caliber nasal cannulas (usually 2.7 mm internal diameter in adult patients) that produce greater flow acceleration than the larger-caliber cannulas used in previous studies.17 One of the advantages of delivering high flow in the nasopharynx is the CO2 washout effect, reducing rebreathing.6 With a lower RR, HFNC can enhance dead space washout and further reduce rebreathing, thus decreasing WOB by optimizing minute ventilation and alveolar ventilation. As breathing slows down, lower flows achieve more effective washout, especially with higher flow delivery.19 This suggests that RR could be an important indicator not only of respiratory function but also of therapy efficacy at the flow used. Some studies do not report changes in RR with HFNC implementation, or if they do, the change is not clinically relevant.3,20 However, in studies evaluating response in patients with respiratory failure, the cause of increased minute ventilation is multifactorial.21
Of note that a lower RR alone does not justify a reduction in WOB per se. However, a reduced DTf undoubtedly reflects reduced muscle recruitment associated with a lower pressure gradient for inspired volume.20 Ultimately, possibly a lower workload to generate that volume change in a system with more homogeneous lung aeration, with a greater end-expiratory lung volume,5 and thus lower elastance. Therefore, the combination of these physiological effects evidenced with HFNC use in healthy subjects could determine a lower ventilatory load translatable to ICU patients. In this context, evidence suggests that when using non-invasive mechanical ventilation, both DTf and RR behavior and its relationship with DTf emerge as useful tools to anticipate prognosis in patients with hypoxemic respiratory failure.22 Similarly, although more studies are needed in this regard, these variables could serve as valid, feasible, and non-invasive tools to predict HFNC therapy outcomes in ICU patients.
For the same muscle pressure gradient, 2 different total respiratory system elastance ratios cause different changes in Pes.23 The variability of this ratio could explain the dissociation between DTf and Pes results found in our study, being statistically significant in the first case and not in the second. Additionally, expiratory resistance generated by high flow in healthy subjects could, like PEEP, trigger activation of expiratory muscles, impacting initial volume changes and thus affecting Pes values.4,24 Lastly, our healthy subjects were predominantly men (70%), which could lead to lower pressure generated by HFNC and influence Pes-related results.25
Vivier et al.26 previously documented a notable correlation between diaphragmatic PTP and DTf in non-invasively ventilated patients (ρ = 0.74; p < 0.001). Our study provides partial validation of these findings, observing a reduction in PTPes/min along with a reduction in DTf in the same direction. Notably, despite these trends, we did not identify any significant differences in the pleural pressure surrogate (Pes) across evaluated scenarios. Although our results do not allow us to draw definitive conclusions on the correlation between these variables, the behavior of both PTPes/min and DTf clearly suggests the possibility of such a correlation. The observed decrease in WOB in our participants under different scenarios, with no variation in Pes, could be explained by the reduction in RR. It is essential to recognize that PTPes/min depends on time, emphasizing that the relationship with DTf may not solely depend on pressure changes, but also be influenced by changes in RR with HFNC use and their impact on inspiratory time.
Finally, of note that as our study was conducted in healthy volunteers, it was carried out with an FiO2 of 21%. The use of higher O2 concentrations could influence RR response through chemoreceptor-mediated reduction in minute ventilation. However, this mechanism in normoxemic humans might be less relevant.27
Our study has some limitations. First, it was conducted in healthy participants, so data may not be generalizable to patients with different pathologies. However, the physiological basis of HFNC suggests that the benefits explained above could be reproduced in other populations. Second, this study was not blinded, which could introduce bias. However, due to the study design, it was impossible to avoid this. Lastly, the availability of variables related to Pes affected the number of records that could be evaluated. Despite this involuntary loss of records, a statistically significant difference was found with the evaluated records.
ConclusionsThe use of high-flow nasal cannula oxygen therapy in healthy subjects decreases diaphragm thickening fraction and respiratory rate in relation to increased flow. Additionally, the use of 40 L/min flow could significantly reduce the muscle work associated with breathing.
FundingNone declared.
Authors’ contributions- 1
Gustavo A. Plotnikow: Study design, data collection and analysis, literature search, manuscript preparation.
- 2
Facundo JF Bianchini: Data collection, literature search, manuscript review.
- 3
Roque S. Moracci: Data analysis, literature search, manuscript review.
- 4
Malena P. Loustau and Valeria S. Acevedo: Literature search, manuscript preparation.
- 5
Jaime A. Mackinlay, Emanuel Di Salvo, and Federico Melgarejo: Data collection, literature search.
- 6
Facundo J. Gutierrez, Javier Mariani, and Matias Madorno: Study design, data analysis.
GAP has received funding for educational programs from Medtronic LATAM and Vapotherm Inc., United States. The remaining authors declared no conflicts of interest whatsoever.
The authors wish to thank Dr. Gaston Murias, head of the Intensive Care Unit, and all members of the Physical Therapy and Respiratory Care staff at the Intensive Care Unit Rehabilitation Service of Hospital Británico de Buenos Aires, Argentina, for their collaboration and support. We also wish to thank Juan Ignacio Mithieux of JAEJ S.A. and Amy Bergeski, Senior Clinical Research Manager at Vapotherm Inc., for their generous support during the study.
Please cite this article as: G.A. Plotnikow, F.J.F. Bianchini, R. Moracci, J.A. Santana Mackinlay, F. Melgarejo, M. Paula Loustau, et al., Impacto de la oxigenoterapia a alto flujo a través de la insuflación de gas a alta velocidad sobre la fracción de engrosamiento diafragmático en sujetos sanos, Med Intensiva. 2024. https://doi.org/10.1016/j.medin.2024.05.010
This study was presented at the 32nd Argentine and International Congress of Intensive Care held from November 9 to 11, 2022 in the city of Mar del Plata, Buenos Aires, Argentina.