Cough is a fundamental defense mechanism for keeping the airway free of foreign elements. Life-threatening situations may arise when cough proves ineffective as a result of muscle weakness or altered mucociliary function.
When a patient is unable to cough effectively, techniques are required to either reinforce or replace cough capacity. The use of mechanical systems that facilitate or substitute cough function is increasingly common in Intensive Care Units, where it is relatively frequent to find situations of ineffective cough due to different clinical causes.
This review examines the current clinical practice recommendations referred to the indication and use of mechanical cough assist and intrapulmonary percussive ventilation systems.
La tos es un mecanismo de defensa fundamental para mantener la vía respiratoria libre de elementos extraños. Cuando la tos es ineficaz, por debilidad muscular o por alteración del normal funcionamiento del sistema mucociliar, se puede dar lugar a situaciones que pongan en riesgo la vida.
Cuando un paciente no es capaz de producir tos eficaz es cuando está indicada la aplicación de técnicas que, o bien potencien la tos del paciente, o bien la sustituyan. Actualmente el uso de sistemas mecánicos facilitadores o sustitutivos de la tos es creciente en las unidades de cuidados intensivos, donde con relativa frecuencia encontramos pacientes en esta situación por diversas causas clínicas.
Esta revisión se centra en las recomendaciones de práctica clínica actuales con respecto a la indicación y aplicación de la tos asistida mecánica y de la ventilación percutora intrapulmonar.
“Cough” (in Latin: tussis): the voluntary or reflex sudden, sharp and noisy expulsion of air from the lungs.
Cough is a defense mechanism with two basic functions: to keep the airway free of foreign elements, and to expel secretions that are produced in excess or under pathological conditions. Ineffective cough is defined as cough unable to adequately perform these functions. Cough can also manifest in acute or chronic form as a symptom of many disease conditions, drug side effects, etc.1,2
Many diseases can severely affect the cough reflex and result in ineffective cough, particularly disorders that cause muscle weakness, alterations in bronchial secretion characteristics and clearance, and a decrease and/or abolition of cough stimulation. The disorders that produce ineffective cough result in a tendency to retain bronchial secretions, with alteration of the normal ventilation/perfusion (V/Q) ratio, and an increased risk of respiratory infectious problems. On the other hand, in the presence of a significant amount of secretions in the upper airway that are not expelled correctly, patients experience excessive muscle labor, with a risk of muscle fatigue.1–4
When a patient is unable to cough effectively, techniques that either reinforce cough or replace it are indicated with the aim of improving inspiratory capacity or mobilizing the secretions to where they can be cleared by the patient or by physical means.5–7
Ineffective cough has been widely studied in patients with disorders causing muscle weakness, such as neuromuscular disease. In critical patients, the deficient management of secretions is a determining factor of respiratory failure, failed weaning from invasive mechanical ventilation, and failure of noninvasive mechanical ventilation.3,8–10
Although there is currently no solid scientific evidence warranting the systematic use of mechanical mucociliary clearance systems in the Intensive Care Unit (ICU) (the studies involving critical patients being few and heterogeneous), and the recommendations are practically based only on expert opinion, such systems are increasingly used in critical care – often in patients with spinal cord injuries or neuromuscular diseases, but also in situations of muscle weakness or difficult weaning from mechanical ventilation. Special mention must be made of the recent study published by Gonçalves et al., which demonstrates the benefits of these techniques when included in the mechanical ventilation weaning protocols in critical patients, affording lower reintubation rates and shorter ICU stays.10–13
The present review offers a brief description of the physiopathology of cough and comments on the current clinical practice recommendations regarding mechanical mucociliary clearance techniques and their application, including: intrapulmonary percussive ventilation (IPV) and mechanical insufflation–exsufflation therapy (MI–E) or mechanical cough assist (MC).
Assessment of cough efficacyClinical assessmentAssessment always should include the patient antecedents and updated clinical history. Special attention should focus on the duration of mechanical ventilation and on whether the patient has an artificial airway or not; the presence of neuromuscular disease; high spinal cord injury; diaphragmatic dysfunction (Table 1); chronic bronchial disease; or disease conditions altering the characteristics of the bronchial secretions, such as cystic fibrosis.
Main causes of diaphragmatic insufficiency.
| Neurological causes |
| Spinal cord injury |
| Multiple sclerosis |
| Amyotrophic lateral sclerosis |
| Cervical spondylitis |
| Poliomyelitis |
| Guillain-Barré syndrome |
| Phrenic involvement: |
| – Tumor disease |
| – Heart surgery “cold lesion” |
| – Trauma |
| – Viral disease |
| – Idiopathic |
| – Radiotherapy |
| – Chest surgery |
| Myasthenic crisis |
| Eaton-Lambert syndrome |
| Myopathic causes |
| Limb-girdle dystrophy |
| Hyper-/hypothyroidism |
| Malnutrition |
| Lysosomal alpha-glucosidase deficiency |
| Connective tissue diseases: |
| – Systemic lupus erythematosus |
| – Dermatomyositis |
| – Mixed connective tissue disease |
| Amyloidosis |
| Idiopathic myopathy |
| Others |
| Critical patient polyneuromyopathy |
| Chronic mechanical ventilation |
| Diaphragmatic dysfunction secondary to mechanical ventilation |
| Anti-Hu syndrome |
| Botulism |
| Lyme disease |
| Pharmacological-aminoglycosides |
| COPD-pulmonary hyperinsufflation |
In addition to the usual physical examination, quantification and evaluation of the characteristics of the bronchial secretions are required, along with assessment of the patient capacity to mobilize and expectorate the secretions, and the need for specific care measures such as cough incentivizing, tracheal aspiration (through the natural or artificial airway), auscultation and respiratory inspection.2,3,6–8
Consideration is also required of phonation and swallowing alterations, as well as of the capacity to perform an effective Valsalva maneuver – this being of great importance for spontaneous cough and for non-mechanical cough assist maneuvers.
Complementary tests are to be added to the physical examination.
Functional assessmentA number of determinations have been used for the functional assessment of cough. Such measurements can be made both in patients with a natural airway and in those who are intubated or subjected to tracheostomy not dependent upon mechanical ventilation:
- -
Peak expiratory pressure (PEmax): This parameter measures the maximum pressure generated by the expiratory muscles. The measurement is made following a maximum inspiration coming as close as possible to total lung capacity. However, consensus is lacking regarding the cutoff point of this parameter in defining whether cough is effective or not. On the other hand, peak expiratory pressure has limitations resulting from the measuring process used.2,7,14
- -
Gastric pressure during cough maneuvering (PGA-cough): This parameter estimates the force generated by the expiratory muscles in the expulsive phase; the normal values for adults are >175cmH2O in men and > 100cmH2O in women. The technique is invasive, however, and there are moreover technical problems for implementing the procedure.2–14
- -
Maximum insufflation capacity measure (MIC): This parameter corresponds to the maximum intrathoracic volume which the patient is able to reach. In adults it has been estimated that the minimum value for ensuring flows that avoid the retention of secretions through spontaneous cough is 1500ml, versus 1000ml in the case of patients with assisted cough.14,15
- -
Peak flow during cough (PFC): This is the parameter that best determines the capacity to eliminate respiratory secretions during cough. The PFC values show good correlation to the conventional lung and muscle function test results in both healthy individuals and in patients with neuromuscular diseases. The parameter is easy to determine with portable systems that moreover allow us to measure peak expiratory flow. The minimum effective PFC for mobilizing airway secretions is ≥2.7l/s; lower PFC values have been associated to increased mortality in patients with neuromuscular diseases, and to failed definitive tracheostomy closure.3,14,16,17
The clinical practice guides referred to patients with neuromuscular diseases recommend the chronic use of mechanical mucociliary clearance techniques in individuals with PFC≤2.7l/s (160l/min). In patients with PFC≤4.5l/s (270l/min), such techniques are advised during exacerbations or processes that increase the production of bronchial secretions.6,18–20
Furthermore, if peak expiratory flow is measured, the association between this parameter and PFC is useful for assessing the degree of bulbar involvement in neuromuscular disease, since a PFC value equaling peak expiratory flow indicates that glottic closure is difficult or impossible.
Other related explorations- -
Basic spirometry: A vital capacity of <50% the theoretical value in adults is generally considered to be insufficient. Forced and non-forced vital capacity can be measured with a simple electronic spirometer, a Wright spirometer, or conventional spirometry. Furthermore, basic spirometry can complete the study of the background respiratory disease of the patient.15
- -
In patients with suspected diaphragmatic dysfunction of any origin, the measurement of forced vital capacity in the sitting position and in dorsal decubitus with a reduction of >25% (dorsal decubitus) is diagnostic of severe diaphragmatic dysfunction. The ultrasound study of diaphragm kinetics allows us to identify the existence of unilateral or bilateral involvement. In this regard, severe dysfunction of the evaluated hemidiaphragm is defined by echographic caudal displacement during non-forced respiration of ≤10mm in men and ≤9mm in women.21–23 Diaphragmatic dysfunction secondary to prolonged mechanical ventilation is quite prevalent in the UCI, and ultrasound is an accessible and useful technique for establishing an early diagnosis of this disorder.24
- -
Pleuropulmonary ultrasound (PPU): This technique is of growing importance in the management of critical patients, due to its non-invasiveness, validity and applicability at the patient bedside. It facilitates the detection of pleural effusion and pneumothorax, and allows the diagnosis and follow-up of pneumonic condensation and atelectasis. Furthermore, PPU complements the hemodynamic information provided by echocardiography with the assessment of extravascular lung water, and offers information on pulmonary aeration in a range of diseases. In patients with ineffective cough, protocolized PPU (exploration of 8 thoracic areas according to the international recommendations) allows us to monitor the presence of atelectasis and its response to mechanical cough, as well as to ensure the early detection of serious complications such as nosocomial pneumonia, and avoid unnecessary radiation exposure. This complementary technique in turn allows the prediction of extubation success in patients subjected to mechanical ventilation.25,26
Ineffective cough is an indication for the use of techniques that either facilitate or replace cough—improving inspiratory capacity or mobilizing the secretions to where they can be cleared by the patient or by physical means.3,8–10
Conventional initial management in such situations seeks to reduce the viscosity of the secretions in order to facilitate their elimination through natural cough. In addition to opportune medical treatment (antibiotics, mucolytic agents, bronchodilators, etc.), postural drainage, respiratory physiotherapy and cough incentivizing (contraindicated in situations of instability or postural intolerance) are also indicated. These techniques, together with early patient mobilization, suffice in most cases.27 In patients with very dense secretions, active humidification systems may prove useful.2,28
When these measures are not enough, noninvasive assist techniques should be considered for the management of secretions, including manual cough assist, mechanical cough assist (mechanical insufflation–exsufflation therapy [MI–E] or MC) and intrapulmonary percussive ventilation (IPV).2,5–7,29–32 In the studies published to date (mostly involving patients with neuromuscular diseases), mechanical mucociliary clearance techniques have been found to reduce the number of pulmonary infections and moreover tend to improve lung function. Patients admitted to the ICU and with prolonged mechanical ventilation develop conditions comparable to neuromuscular diseases in terms of muscle weakness, atrophy and fatigability,24 and these techniques have been shown to offer benefits when included in the mechanical ventilation weaning protocols of critical patients—even in individuals with severe restrictive problems in which other weaning regimens have failed.10–13,33
These therapies are effective and safe, and are often used in our Unit, applied indistinctively by experienced physicians and nurses (following a protocol prescribed by the supervising physician and detailed in a specific treatment sheet). In other centers such techniques are indicated and programmed by rehabilitation specialists and physiotherapists (fundamentally in spinal cord injury units). However, due to the physiopathological characteristics inherent to critical patients, such procedures in the ICU should be known and supervised by intensivists—in contrast to chronic treatments or stable patients, which are not contemplated in this review.
Mechanical cough assistMechanical cough assist (MC) or mechanical insufflation–exsufflation therapy (MI–E) basically involves the mechanical generation of a transthoracic pressure gradient sufficient to produce an expiratory flow capable of mobilizing the bronchial secretions.
A number of devices can be found on the market for MC.32,34,35 The MI–E systems are portable electronic devices designed for discontinuous use that simulate cough through positive pressure followed by a proximal negative pressure. Both pressure settings are previously selected (equipotent or not) in order to secure an effective expiratory flow. These systems can generate maximum positive and negative pressures of up to ±70cmH2O (total pressure gradient of up to 140cmH2O), though in general it is not advisable to exceed ±40cmH2O, due to the risk of barotrauma. This treatment is moreover regulated according to the inspiration/pause/expiration times, and can be applied to the patient using a mask or mouthpiece, or through the endotracheal tube/tracheotomy cannula. Supplementary oxygen can also be administered, if necessary.32,34,35
Indications of mechanical cough assistThe indications of MC can be summarized from two perspectives: physiological and clinical.36–43
Physiological indications of mechanical cough
- -
Incapacity to generate cough flow peaks of >4.2l/s (250l/min).
- -
In relative terms, a vital capacity of <50% of the theoretical value.
- -
In absolute terms, a vital capacity of <1500ml (in adults).
- -
Clinical indications of mechanical cough
- -
Neuromuscular disease/polyneuromyopathy.
- -
Thoracic diseases (combination of restrictive disorders with incapacity to perform correct diaphragmatic movements).
- -
Diaphragmatic dysfunction with and without inspiratory support (mechanical ventilation).
- -
Traumatism or postoperative period of thoracoabdominal/abdominal surgery.
- -
Prolonged mechanical ventilation.
- -
Specifically, it is necessary to assess cough efficacy in:
- -
Neuromuscular diseases, where MC has been shown to improve mobilization of the secretions and lessen the need for inspiratory assist (mechanical ventilation); furthermore, it reduces respiratory infections and the need for hospitalization.2
- -
Thoracic diseases with secondary restrictive pulmonary disorders. Cough efficacy also must be assessed in patients in which improved management of secretions is observed, despite a lack of improvement in lung mechanics that is nevertheless observed in other types of patients.41,42
- -
Patients with diaphragmatic dysfunction, especially patients with high spinal cord injuries characterized not only by inspiratory weakness but also expiratory problems associated to thoracic and abdominal mechanical alterations (compliance).41,42
- -
Patients with diminished respiratory muscle strength and an increased production of secretions.41,42,44
- -
Patients subjected to prolonged invasive mechanical ventilation, where in most cases the indication is based on a combination of respiratory muscle weakness, fatigue and atrophy of multifactorial origin, making it impossible for the patient to cough effectively.2,24
- -
Mechanical cough assists have also been proposed in polytraumatized patients or individuals in the perioperative period of thoracoabdominal surgery, where surgical muscle dissection or inadequate analgesia precludes adequate cough function.38
Patients with PFC reduction <2.7l/s secondary to high spinal cord injuries, neuromuscular deficiencies or severe fatigue associated to intrinsic pulmonary disease.34,35
The contraindications are: a history of bullous emphysema, known susceptibility to pneumothorax or pneumomediastinum, and recent barotrauma.34,35
Mechanical cough application techniqueMechanical cough assist using MI–E devices can be carried out in the presence of an artificial airway, with a sealed tube cuff (tracheal cannula or tube), or with the natural airway using a face mask or mouthpiece (nasal obturator)—in which case due patient instruction is required.34,35 In patients with an artificial airway, the technique can be used with or without patient collaboration; MC therefore can be employed in patients with different degrees of sedoanalgesia. In patients dependent upon mechanical ventilation, the volumes generated by MI–E (with or without supplemental oxygen) substitute mechanical ventilation for the duration (normally 60–90s) of the procedure.
The following disposable materials are needed: an antibacterial filter, simple tubing (no more than 1.5m), an oxygen outlet adaptor (if needed), and an interface (oronasal mask, mouthpiece with nasal obturator or connector adaptable to the artificial airway [22/15mm]).
The usual MI–E models can be used manually—in which case the adjustable parameters are not operative—or in automatic mode, which requires the prior programming of several parameters:
- -
Inspiratory time (Ti), in seconds: the time needed to reach the established inspiratory pressure.
- -
Pause time (Tp), in seconds: the transition time from the point where positive inspiratory pressure is reached to start of the application of proximal negative pressure.
- -
Expiratory time (Te), in seconds: the time needed to reach the pre-established maximum negative pressure.
- -
Maximum inspiratory (Pi) and expiratory pressure (Pe), expressed in cmH2O. The I-E pressure is the maximum insufflation pressure limit; the expiration pressure is equivalent but of a negative value.
- -
Positive and negative pressure balance regulator: this allows partial lowering of the negative pressure with respect to the positive pressure.
- -
Inspiratory flow: this regulates insufflation flow between 600 and 200l/min (high/low).
Utilization of the more recently marketed systems allows us to use digital commands to select the following parameters with greater precision than the analogical devices:
- -
Inspiration pressure: from 0 to 70cmH2O, in increments of 1cmH2O.
- -
Inspiration flow: high/medium/low.
- -
Ti: from 0 to 5s, in increments of 0.1s.
- -
Pe: from 0 to 70cmH2O, in increments of 1cmH2O.
- -
Te: from 0 to 5s, in increments of 0.1s.
- -
Tp: from 0 to 5s, in increments of 0.1s, only adjustable in automatic mode with deactivated cough-trak.
- -
Cough-trak: a trigger system that starts inhalation (positive pressure) when patient inspiration is detected, thereby facilitating adaptation to MC and improving efficacy in delivering pressures when used in patients with a natural airway.
- -
Oscillation: flow oscillation improves mobilization of the secretions by lowering their viscosity and clearing them from the lower airway. The frequency can be regulated between 1 and 20Hz and the amplitude between 1 and 10cmH2O; activation can be made during Ti or Te.45,46
These systems moreover allow the following:
- -
Pre-determination of three MC programs can be made with different parameters, for different indications in the same patient.
- -
Adjustment of the parameters can be blocked, in order to prevent them from being modified by untrained persons.
- -
Cycle-by-cycle monitoring of the efficacy of MC, controlling both the volume of air exhaled by the patient and the peak expiration flow.
- -
Monitoring of SpO2 can be incorporated to the MC system by means of an adequate device.
The sum of the times Ti+Tp+Te is referred to as the cough cycle. A cough series or sequence is the continued application of several cough cycles (generally 8–12 cycles). It is advisable to leave an interval of 2–3min between sequences. Mechanical cough assist should be applied at least three times a day in a programmed manner, and whenever required by the patient.
In order to avoid frequency disadaptation in patients with spontaneous respiration, the times are adjusted to the basal respiratory frequency of the patient, causing the system to apply a frequency (of cough cycles) equal to or greater than that frequency. The distribution of times is made according to the predominant background disease condition. Thus, in patients with restrictive predominance Te is shortened, while in patients with obstructive predominance Te is prolonged. In turn, Ti and Tp are adjusted to secure the desired cough cycle frequency.47,48
The maximum Pi and Pe administered are intended to reach an intrapulmonary volume of close to 70% of the vital capacity of the patient. These pressures should not be reached in the first attempts, during the patient adaptation period. Adaptation is to be started applying pressures of ±20cmH2O, with increments of ±5cmH2O every 2–3 cycles, conditioned to patient wellbeing. The pressures usually needed to ensure effective cough vary between ±30 and ±40cmH2O. In some cases we can reach pressures of up to ±60cmH2O, with effective differential pressures of 60, 80 and up to 120cmH2O, respectively. In general, Pe is kept equivalent (equal to Pi in absolute value), but negative.47–50
It may be useful to lower Pi and preserve tolerated Pe modifying the balance of pressures, when the secretions are retained in the glottic–subglottic region.
A high inspiratory flow is selected when the system is used with an artificial airway, while a low flow is selected in the presence of a natural airway.
The patient should be calm in the Fowler position (recumbent semi-decubitus). If the patient is conscious and cooperative, we should explain the technique and its purpose, as well as alert the patient to the sensations that may be produced. The efficacy of MC improves if the patient contracts the abdominal muscles during the expiratory phase, or if manual abdominal compression is applied during this phase (some series describe good results with electrical stimulation of the abdominal muscles).44
The application times of the technique should be spaced at least 2h after and about 30min before meals. If aerosol therapy is required, it should be administered approximately 30min before MC.47
Application to patients with natural airwayIn order for the procedure to be effective in patients with a natural airway,50,51 the glottis must be kept open during the application of each cough cycle. If the patient is unable to keep the glottis open, we can cause reflex opening by positioning a piece of corrugated tube (22mm) to be grasped with the lips, or by training the patient (pronounce the vowel “O”, misting a mirror with the breath, etc.). This step is very much simplified thanks to the cough-trak system. However, the patient may voluntarily close the glottis on noticing the air flow of the inspiratory cycle of MC; it is therefore still sometimes necessary to train the patient to keep the glottis open.
The more frequently used oronasal mask is applied with the same precautions and technique as manual ventilation manual with a pneumatic bag.
As has been commented above, for the starting parameters we begin with low pressures until the values affording efficacy and patient wellbeing are reached, adjusting the time of each cycle to the base respiratory frequency and the predominant respiratory disorder. Application always must be made with a low inspiratory flow in order to minimize aerophagia.
Application to patients with artificial airwayThe procedure is simplified in patients with an artificial ariway,50,51 since in this case there is no concern about whether the glottis is open or not. The system is directly fitted to the trans-laryngeal tube or tracheotomy cannula using an ISO 22/15 adaptor. If necessary, an adaptor can be used to supply oxygen to the system.
The same adjustment of parameters described above for the natural airway is used, with the exception that in this case we can apply high or medium inspiratory flows, conditioned to patient wellbeing.
Complications of mechanical cough assistMost of the complications are very infrequent (different studies involving over 500 MC series have reported no significant complications).50–54 A recent Canadian national survey specifically focusing on critical patients found the most frequent complications to be chest pain (manual MC) and hypotension, reported by 17% of those surveyed in patients subjected to MC with recruitment intention.11 The potential complications are:
- -
Pneumothorax or pneumomediastinum (predisposing disease).
- -
Chest wall pain with or without respiratory muscle injury, fundamentally in patients subjected to chronic mechanical ventilation or with long-evolving neuromuscular diseases, with a greatly diminished vital capacity. This complication can be avoided by slow and progressive insufflation pressure increments.
- -
Hemoptoic sputum secondary to mucosal damage on clearing firmly adhered secretions.
- -
Nausea, secondary to gases, and abdominal bloating due to aerophagia. This can be improved by lowering the insufflation pressures and with training (open glottis or extended neck).
- -
Bradycardia-hypotension, particularly in patients with acute spinal cord injury.
- -
Early collapse of the lower airway. This happens in patients with important air flow limitation, causing MC to be ineffective. This problem can be minimized by applying the technique passively, with no accompanying expiratory effort on the part of the patient.
- -
In patients with advanced bulbar amyotrophic lateral sclerosis, MC produces hypopharyngeal collapse that prevents the air from exiting the larynx – thereby contraindicating the technique. This adverse effect can be minimized by applying MC with the new devices that allow Pe to be programmed independently of inspiratory pressure – thereby making it possible to lower the former until hypopharyngeal collapse is avoided.38
The physiological effects of MC with regard to the elevation of central venous pressure and intracranial pressure, alterations in heart rate or increased intragastric pressure are similar to those caused by natural cough, though to a lesser extent.
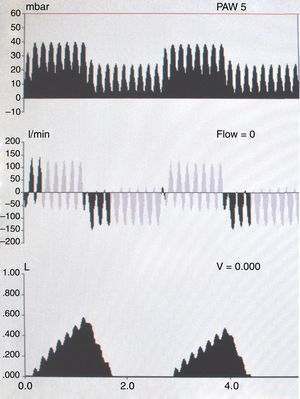
Intrapulmonary percussive ventilationIntrapulmonary percussive ventilation (IPV) is an airway secretions clearance technique derived from high-frequency ventilation. It involves the constant administration of a pulsatile gas flow with low successive tidal volumes (TVs) in the patient airway, with high frequency through an open respiratory circuit—this affording successive TVs with low pressure and high flow, creating a positive transpulmonary pressure gradient. The insufflated gas volumes are small (between 1ml and 300ml), and are administered on a continuous basis, superimposed or not upon spontaneous ventilation of the patient (Fig. 1). Expiration is achieved passively.54
The frequency of the percussions in most of the marketed devices varies between 80 and 650cycles/min. The circuit used is continuously open, regardless of whether applied on a mask, mouthpiece or tracheostomy cannula. The circuit includes a Hudson type high-flow pneumatic nebulizer.
Mobilization of the respiratory secretions from the lower toward the upper airway can cause obstruction of the trachea and main bronchi. This technique therefore must be completed with immediate evacuation of the secretions, using instrumental methods or sequentially applying MC in patients with ineffective cough. The manufacturers recommend a total treatment time of up to 20min. There is a risk of causing hyperventilation, however; the time therefore should be individualized according to clinical and laboratory test criteria. On the other hand, dead space can be added to the circuit with tubing, in order to control the hyperventilation induced by treatment with IPV.6,53,55
Intrapulmonary percussive ventilation is applied by means of a series of devices that include:
- -
Gas source (most devices incorporate an internal compressor).
- -
Phasitron®: a part that modifies delivery of the compressed air-gas to the patient, with the aim of converting TV of high pressure and low flow into TV of low pressure and high flow in a dynamic manner, according to the pulmonary mechanics of the patient.
- -
Nebulizer: connected in parallel to Phasitron®.
- -
Interface.
This technique is indicated for the mobilization of bronchial secretions in the context of the management of peripheral obstructions and ventilation disorders in obstructive or restrictive diseases characterized by hypersecretion, independently of the degree of patient collaboration, since it is superimposed upon patient respiration. The technique can be applied in adults and children, and in both acute and long-term treatments. This therapeutic modality in turn can be applied in the presence of an artificial airway or a natural airway using a face mask or mouthpiece.6,53–55
The conditions in which most experience has been gained are:
- -
Cystic fibrosis43,56
- -
Neuromuscular diseases57–59
- -
Chronic obstructive pulmonary disease31,60
- -
Neonates61,62
However, experience with IPV has also been gained in patients with muscle weakness acquired in the ICU, obese individuals, burn victims, etc. In the last few years in which extracorporeal oxygenation techniques have become increasingly used as life support systems, treatment combined with IPV has been shown to reduce the extracorporeal membrane oxygenation (ECMO) times in patients with acute respiratory distress syndrome (ARDS).63–67
The outcomes of IPV can be optimized by combining it with physiotherapy, postural drainage, cough assist, MC and any other type of respiratory physiotherapy technique.
Contraindications53,55:
- -
Absolute: non-drained pneumothorax.
- -
Relative: Lyell syndrome, severe hemoptysis, coagulopathy-anticoagulation, epilepsy, peak flow <3l/s (180l/min), provided there is no available artificial airway aspiration system or MC device in patients without an artificial airway.
As a general rule, we use this technique53,55 in patients subjected to mechanical ventilation. It can be applied in series with the mechanical ventilator in the different ventilation modes (both controlled and spontaneous). The main parameters when applying IPV are:
- -
Operating pressure (OP). This is the pressure generated by the compressor; its adjustment determines the flow rate feeding the respiratory circuit (Phasitron®). The value ranges between 0.8 and 3.6mbar. Low OP (<2mbar) favors vibratory effect, while higher OP implies a greater ventilatory effect.
- -
Percussion frequency. This is the number of percussions or cycles administered to the patient per minute. It varies between 75 (±10) and 400 (±20) cycles per minute or 11.2–6.7Hz. High frequencies of >300cycles/min improve the percussive or vibratory effect, while low frequencies of <200cycles/min imply a greater recruitment and ventilatory effect.
- -
I:E ratio. This is the ratio between the increment and decrement times of the administered percussion pressures. The ratio varies between 1:2.5 and 1.5:1, and is modified according to the respiratory pattern of the patient and the background disease condition.
- -
Proximal pressure (PP). This is the pressure measured in the proximal airway, and is determined by the frequency, OP, flow transmitted to the patient, the pulmonary resistances, thoracopulmonary compliance and the inspiratory and expiratory flows generated by the patient. This parameter is displayed on the data screen. When the pressure limit of 40cmH2O is reached, the system interrupts IPV.
The parameters that determine IPV are interdependent: a change in adjustment of one parameter implies the modification of other parameters and therefore also of the effect upon the patient. The selection of parameters is made taking into account the age, disease and clinical condition of the patient. In this regard, it must be considered that an OP between 0.8mbar and 2mbar favors the vibratory effect—thereby causing increased mobilization of the secretions—while higher OP settings favor the ventilatory and recruitment effects of the technique. High frequencies (250–400cycles/min) generate a considerable vibratory effect that facilitates the clearance of secretions, while lower frequencies (75–250cycles/min) enhance the ventilatory effect of IPV.
It is advisable for the first treatment of the patient to be made with a low OP setting and high frequency, in order to allow improved adjustment to therapy. In practice, once the base OP and frequency have been established, it is advisable to vary the percussion frequency, alternating its predominantly vibratory effect with the predominantly ventilatory effect.53,55,68,69
Although it is advisable for the patient to be in recumbent semi-decubitus for this treatment, in some cases IPV can be applied adopting passive lung drainage techniques that require different patient positions.53
Complications and side effects of intrapulmonary percussive ventilationIntrapulmonary percussive ventilation53,55 is a safe technique: the different series published to date do not report serious complications. Indeed, most studies describe IPV as a well tolerated technique. Some of the described complications are:
- -
Intercostal pain.
- -
Activation of pharyngeal reflexes (nausea, vomiting, etc.).
- -
Elevation of body temperature (mobilization of bronchial secretions).
- -
Dry mouth.
- -
Dyspnea.
- -
Headache (hyperventilation, sinus disorders).
- -
Conjunctivitis (defective mask fitting).
- -
Tracheal stoma discomfort (mobilization of the cannula).
- -
Aerophagia.
- -
Dizziness (hyperventilation).
- -
Muscle cramps or paresthesias of the extremities (hyperventilation).
- -
Hypoventilation.
- -
Desaturation (inappropriate adjustment, closing of the glottis, insufficient oxygen supply, mobilization of an excessive amount of bronchial secretions).
Both MC and IPV are safe and effective techniques that require specific equipment that is easy to use by trained staff.
These techniques could improve the extubation protocols and avoid reintubations in critical patients with ineffective cough.
Further controlled studies involving the different mechanical mucociliary clearance modalities in the critical care setting would be advisable.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Fernández-Carmona A, Olivencia-Peña L, Yuste-Ossorio ME, Peñas-Maldonado L y Grupo de Trabajo de Unidad de Ventilación Mecánica Domiciliaria de Granada. Tos ineficaz y técnicas mecánicas de aclaramiento mucociliar. Med Intensiva. 2018;42:50–59.