The new drug treatments for different hematological neoplasms have improved the prognosis of these diseases by expanding the available therapeutic options. However, these drugs are not free from side effects that can be potentially life-threatening for the patient. Considering the potential hazards, it is necessary to determine whether the use of such drugs requires protocolized admission to the Intensive Care Unit (ICU) of patients considered to be at risk, in order to guarantee strict vigilance and monitoring.
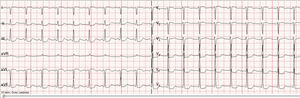
We present the case of a 56-year-old male with multiple myeloma, no other clinical antecedents of interest, and no previous heart disease who suffered cardiac arrest following the administration of chemotherapy with carfilzomib (Kyprolis®). The patient was diagnosed with multiple myeloma (IgG, initial stage IIIA, ISS-I) in 2003 and received radiotherapy, chemotherapy, rescue therapy with hematopoietic stem cells, and posterior consolidation in the form of autogenous transplantation. Two transthoracic echocardiographic studies (in 2011 and 2012) revealed mild tricuspid and mitral valve insufficiency, with no other relevant alterations. Following complete remission, biological progression was observed in 2012. Despite administration of a new chemotherapy cycle, a progressive increase in the monoclonal component was noted, with multiple hypermetabolic foci corresponding to tumor infiltration. Therapy was started with pomalidomide, dexamethasone and cyclophosphamide. Since this treatment proved ineffective and disease progression was observed, alternative treatment was considered in the form of carfilzomib, lenalidomide and dexamethasone (KRd). Treatment with carfilzomib was started with no prior echocardiographic study. The dosing scheme was 20mg/m2 on days 1 and 2 of the cycle, and 27mg/m2 on days 8, 9, 15 and 16. The first cycle proved uneventful, though during infusion of the second cycle, 28 days later, the patient suffered sudden dyspnea and febrile syndrome with the second dose, requiring admission to the Department of Hematology. Under conditions of hypotension, poor perfusion and acute respiratory failure, a brain CT scan and thoracic angioCT study were made, which discarded pulmonary embolism and brain disease. Echocardiography in turn revealed mild-moderate mitral valve insufficiency and mild tricuspid valve insufficiency, with the estimation of a systolic pulmonary artery pressure of 37mmHg. Empirical antibiotic treatment was started, with fluid therapy and oxygen therapy, followed by clinical improvement. However, 3h later the patient presented acute lung edema followed by cardiac arrest. The usual cardiopulmonary resuscitation maneuvers were applied during 5min, with the administration of 1mg of adrenalin, after which rhythm recovery in atrial fibrillation was observed, and the patient was moved to the ICU. The chest X-rays confirmed the diagnosis of acute lung edema, exhibiting a “butterfly wing” pattern, with perihilar redistribution and cardiomegalia. The ECG tracing showed ST-segment depression on the inferolateral aspect (Fig. 1), with no significant enzyme changes (troponin T 53ng/l). Coronary angiography was not performed, since the patient referred no chest pain suggestive of ischemic heart disease, and the electrocardiographic changes were set in the context of cardiac arrest. Treatment was provided in the form of furosemide, corticosteroids and antibiotics, followed by good neurological, hemodynamic and respiratory recovery. Monitoring revealed no new electrocardiographic changes, and discharge was therefore decided 48h after admission. The cardiac event was considered to probably constitute a side effect of carfilzomib. Over the subsequent days the patient again suffered clinical worsening due to progression of his hematological disease. The patient rejected the continuation of medical treatment and died in the Hematology ward 10 days later.
Carfilzomib, authorized in 2012, is a potent second-generation 20S proteasome chymotrypsin type activity inhibitor used to treat refractory multiple myeloma and other diseases such as Waldenstrom's macroglobulinemia, lymphoma amyloidosis and certain autoimmune diseases.1 The drug is administered via the intravenous route in an interval of about 10min. Dexamethasone premedication is advised in order to minimize the intensity of frequent symptoms such as fever, nausea, fatigue or headache.2 Carfilzomib has a broad range of adverse effects, from transfusion reactions to both hematological (thrombocytopenia and anemia) and non-hematological complications.3 The PX-171-007 trial reported that a high dose of carfilzomib results in greater efficacy than the approved dose4 of 27mg/m2, though higher doses also increase the risk of cardiovascular adverse events. Carfilzomib is characterized by low peripheral neuropathy rates (such complications being common with other therapeutic regimens based on bortezomib), though recent case series and studies indicate that treatment with proteasome inhibitors can be associated to serious cardiac events.5 The initial clinical trials recorded episodes of heart problems with congestive heart failure, lung edema and a decrease in left ventricular function in 7% of the patients–the most frequently described adverse events being arrhythmias, most of which were of a benign nature and of supraventricular origin.6 More recent publications report a large number of cardiac complications. Danhof et al.7 described serious adverse effects in 50% of the patients, with left heart failure in 23%. The risk of cardiac adverse events was higher in patients concomitantly receiving doxorubicin or thoracic radiotherapy, as in our case. Careful patient selection is therefore advised, with close monitoring of the population at risk. Table 1 describes the adverse effects and risk factors for cardiac toxicity.
Cardiovascular adverse effects of carfilzomib and cardiac toxicity risk factors.
| Adverse events, percentage | Cardiac toxicity risk factors |
|---|---|
| Cardiovascular | |
| Cardiac arrhythmia (13.3%) | Previous cardiovascular disease |
| Heart failure (7.2–23%) | Combination treatment with doxorubicin |
| Coronary disease (3.4%) | Thoracic radiotherapy |
| Myocardiopathy (1.7%) | |
| Dyspnea (42.2%) | |
| Hematological | |
| Anemia (46.8%) | |
| Thrombocytopenia (36.3%) | |
| Neutropenia (20.7%) | |
| Others | |
| Pneumonia (12.7%) | |
| Serum creatinine elevation (24.1%) | |
| Acute renal failure (5.3%) | |
| Hyperglycemia (12%) | |
| Water-electrolyte alterations: hypopotassemia (14%), hypomagnesemia (14%), hypercalcemia (11%) | |
It has been postulated that the cardiovascular effects can be mediated by different mechanisms derived from proteasome inhibition. The most important of these mechanisms is the accumulation of damaged and non-degraded proteins within the myocytes, which may prove toxic for the cells.8 In animal models the drug has been shown to induce ventricular dysfunction, and at structural level cardiomyocyte enlargement and vacuolization have been observed, as well as mitochondrial pleomorphism, perivascular interstitial fibrosis and the induction of apoptosis.9 Furthermore, proteasome inhibition generates changes in endothelial nitric oxide synthase (eNOS) activity and in nitric oxide levels–this in turn leading to vasodilatation and endothelial dysfunction associated with arterial hypertension and coronary disease, among other conditions. Other drugs are also under study, such as apremilast, which exerts a protective effect against carfilzomib-induced cardiotoxicity, and thus plays a key role in the modulation of oxidative stress.10
Oncohematological patients are fragile and require very complex management. Preventing serious adverse events in patients at risk though admission to intensive care for electrocardiographic and cardiorespiratory monitoring could prove necessary and constitute a new healthcare offer on the part of ICUs. This would require the introduction of protocols involving multiple disciplines (Clinical pharmacology, Hematology and Intensive Care Medicine) in order to select those patients considered to be at high risk and who could benefit from such monitoring, in view of the limited number of ICU beds available.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Rodríguez-García R, Espina MJ, Viña L, Astola I, López-Amor L, Escudero D. Tratamiento con carfilzomib. ¿Deberían estos pacientes ingresar en la unidad de cuidados intensivos? Med Intensiva. 2018;42:60–62.