On 31 December 2019, the Health Commission of Hubei Province of China first unveiled a group of unexplained cases of pneumonia, which WHO subsequently defined as the new coronavirus of 2019 (SARS-CoV-2). SARS-CoV-2 has presented rapid person-to-person transmission and is currently a global pandemic. In the largest number of cases described to date of hospitalized patients with SARS-CoV-2 disease (2019-nCoViD), 26% required care in an intensive care unit (ICU). This pandemic is causing an unprecedented mobilization of the scientific community, which has been associated with an exponentially growing number of publications in relation to it. This narrative literature review aims to gather the main contributions in the area of intensive care to date in relation to the epidemiology, clinic, diagnosis and management of 2019-nCoViD.
El 31 de diciembre de 2019, la Comisión de Salud de la provincia China de Hubei, dio a conocer por primera vez un grupo de casos inexplicables de neumonía, que posteriormente la OMS definió como el nuevo coronavirus de 2019 (SARS-CoV-2). El SARS-CoV-2 ha presentado una transmisión rápida de persona a persona y actualmente es una pandemia mundial. En la mayor serie de casos descrita hasta la fecha de pacientes hospitalizados con enfermedad por SARS-CoV-2 (2019-nCoViD), el 26% requirió atención en una unidad de cuidados intensivos (UCI). Esta pandemia está provocando una movilización de la comunidad científica sin precedentes, lo que lleva asociado un numero exponencialmente creciente de publicaciones en relación con la misma. La presente revisión bibliográfica narrativa, tiene como objetivo reunir las principales aportaciones en el área de los cuidados intensivos hasta la fecha en relación con la epidemiología, clínica, diagnóstico y manejo de 2019-nCoViD.
Back in December 31st, 2019, the Health Commission of the Chinese province of Hubei, reported for the first time a group de inexplicable cases of pneumonia.1 It was disclosed that some patients had developed pneumonic processes with radiographic changes that produced ground-glass opacities, lymphopenia, and thrombopenia, hypoxemia, and kidney and liver function alterations.2 The Chinese health authority announced that patients initially tested negative for respiratory viruses and common bacteria. Later on, these patients tested positive for a new coronavirus.3 The virus was named as new coronavirus SARS-CoV-2 by the WHO. This virus was responsible for the COVID-19 disease (new CoronaVirus Disease). On March 13th, 2020, the Health Secretary of the Spanish Government announced 4209 cases, with a cumulative incidence of notified confirmed cases/100 000 inhabitants of 8.994 (Table 1).
Epidemiological summary of confirmed cases reported as of March 13th, 2020.
| Overall | China | Italy | Iran | South Korea | Spain | |
|---|---|---|---|---|---|---|
| Number of cases (CI) | 132 567 | 80 981 | 15 113 | 10 075 | 7979 | 4209 |
| Number of deaths (L) | 4947 (3.7) | 3173(3.9) | 1016(6.7) | 429(4.3) | 67(0.8) | 120(2.9) |
CI, cumulative incidence of confirmed cases reported/100 000 inhabitants; L, lethality, percentage of deaths among the confirmed cases reported.
SARS-CoV-2 is very similar to bat coronaviruses, and it has been postulated that they are the source of coronavirus pneumonia. Although the origin of COVID-19 is still under study, current tests suggests that spread to humans occurred through transmission from wild animals illegally sold in the Huanan Seafood Wholesale market.5,6
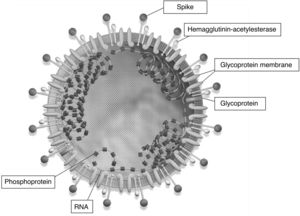
Complete genome sequencing and phylogenic analysis indicated that SARS-CoV-2 was different from the beta-coronavirus associated with the human severe acute respiratory distress syndrome (SARS) and the Middle East respiratory syndrome (MERS). SARS-CoV-2 has characteristics that are typical of the coronavirus family and it has been classified as part of the lineage of beta-coronavirus 2b. Coronaviruses are RNA viruses and have largest genome among the RNA viruses known to this day. The single-stranded, helical genome is enveloped together with a protein (nucleocapsid). There are at least 3 protein structures in the viral envelope: membrane M protein, E protein whose main function is viral assembly and the spike (S) protein involved in virus penetration to attack host cells (Fig. 1).7
Both, SARS-CoV and SARS-CoV-2, enter the cell through the angiotensin-converting enzyme 2 receptor (ACE2). SARS-CoV-2 first infects the lower respiratory airways and binds to the ACE2 receptor in the alveolar epithelial cells. Both viruses are powerful inflammatory cytokine inducers. The «cytokine storm» or «cytokine cascade» is the mechanism that has been postulated as responsible for organ damage. The virus activates immune cells and induces the secretion of inflammatory cytokines and chemokines in pulmonary vascular endothelial cells.8
Compared to previously isolated SARS-CoV strains, SARS-CoV-2 (2019) probably uses the human ACE2 less efficiently than SARS-CoV-2002 but more efficiently than el SARS-CoV-2003. Since the ACE2 binding affinity has proven to be one of the most important determinants of SARS-CoV infectivity, it could be argued that SARS-CoV-2 has evolved in its ability to infect and spread among humans.9
The epidemiological criteria to define a suspected case, at the end of January 2020, were the following: history of a trip to Wuhan or direct contact with Wuhan patients who had a fever or respiratory symptoms within 14 days before the onset of the disease. A confirmed case was defined as a case with respiratory samples that tested positive for SARS-CoV-2 in. at least, 1 of the following 3 methods: isolation of SARS-CoV-2 or, at least, 2 positive results in real-time on the reverse transcriptase polymerase chain reaction (RT-PCR) test for SARS-CoV-2 or a genetic sequence matching SARS-CoV-2.10
The estimated mean incubation time is approximately 5 days, similar to the mean values already known for the incubation period of SARS and MERS. In addition to proving empirically the comparability of SARS-CoV-2 with other disease-causing coronaviruses, the 95th percentile of the incubation period is between 10 to 14 days, indicative that a 14-day quarantine period would guarantee, to a great extent, the absence of the disease among exposed healthy individuals.11
COVID-19 has showed quick person-to-person transmission capabilities. Based on the estimation of the basic reproductive number (R0), indicative of the number of individuals that 1 affected case infects during the course of their disease in a population of people who were previously infection-free and have not been vaccinated; R0 for COVID-19 is, on average, 2.2 individuals, that is, every patient has been spreading the disease, on average, to > 2 people. One of the reasons that explains the quick spread may have to do with the atypical symptoms seen during the early stages of the disease in some infected patients.6 However, it is well-known that the infection seems to have been transmitted during the incubation period too. The fact that asymptomatic patients are potential sources of infection could justify a reassessment of the transmission dynamics of the current outbreak.12,13
The best example in this regard was described in a 5-member family that developed pneumonia due to SARS-CoV-2 in Anyang, China. Before showing any symptoms, they had been in contact with yet another asymptomatic family member who had just returned from the Wuhan epidemic center. The chain of events described suggests that coronavirus can be transmitted by an asymptomatic carrier. The incubation period for this patient was 19 days, which is long, but within the reported range of 0 to 24 days. His first RT-PCR results tested negative; false negative results have been reported as dependent on the quality of the testing kit used, the sample collected or the very performance of the test. The RT-PCR test has been widely used in diagnostic virology and it has yielded few false positive results. This means that it was unlikely that his second RT-PCR result may have been a false positive, so it was used to define the COVID-19.
The triage of critically ill patients during the current pandemicTriage protocols are designed to allocate scarce resources in a fair and transparent manner and in such a way that, by definition, some groups of people are excluded from access to healthcare. The current COVID-19 pandemic has taught us that bed availability at the ICU setting can be insufficient. Also, it has suggested some triage protocols for the intensive care setting,14 and extraordinary measures for a better use of resources by mitigating and controlling its effects.15,16
Although some triage protocols focus on excluding some patients to increase availability of ICU beds, such availability is not an end in itself.17 The implicit (and sometimes explicit) intention of classification protocols should aim at the «public good» of maximizing the survival of the population.
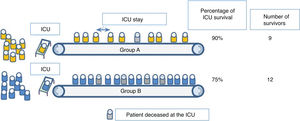
But it is incorrect to assume that this public good is achieved by maximizing survival among those who receive intensive care. Although many triage protocols acknowledge this when trying to exclude patients who do not need it at all (those who are «too healthy») and those with the least chances to benefit from it (those who are «too sick»), they do not pay enough attention to the inter-group differences regarding the duration of the intensive care needed to obtain results. Fig. 2 illustrates this point.
Many classification protocols would favor the admission of group A over group B because patients in group A are more likely to survive if they receive intensive care. However, in this example, patients from group B require shorter ICU stays compared to patients from group A. Therefore, the allocation of intensive care resources to group B actually leads to a larger absolute number of survivors, because more people have access to life-saving care. If the objective of triage is to improve the survival of the population with scarce resources, then, this scarce resources are not beds per se, but bed days; it is not ventilators, but ventilation time.18
From this we can deduce that triage will not be effective as long as, in the intensivist’s assessment, cases are not discriminated correctly, and it is accepted that most people who require intensive care have similar chances of survival and ICU stays of a similar length.
Clinical signs and laboratory testsCommon symptoms at the onset of the disease were fever, dry cough, myalgia, fatigue, dyspnea, and anorexia. However, a significant percentage of patients initially showed atypical symptoms, such as diarrhea and nausea. The complications of coronavirus related pneumonia during hospitalization included acute respiratory distress syndrome (SARS), arrhythmia, and shock. The bilateral distribution of irregular parenchymal condensation and ground-glass opacities have been the distinctive trademark typically seen on the computed tomography scan performed during the management of coronavirus related pneumonia. In the patients with the most serious disease who developed SARS and required admission to the ICU and oxygen therapy, the time elapsed between hospital admission and SARS was only 2 days. The main clinical characteristics an the most important complications associated with the data published in the Chinese population6,19–22 are shown on Table 2.
Clinical characteristics and complications associated with the main studies already published.
| Huang et al.9N = 41 | Chen et al.6N = 99 | Song et al.20N = 51 | Chen et al.21N = 29 | Wang et al.22N = 138 | |
|---|---|---|---|---|---|
| Mean clinical age | 49 (41−58) | 55 ± 13 | 49 ± 16 | 56 (26−79) | 56 (42−68) |
| Fever (98%)Cough (76%)Dyspnea (55%)Myalgia or fatigue (44%)Expectoration (28%)Headache (8%)Hemoptysis (5%)Diarrhea (3%) | Fever (83%)Cough (82%)Dyspnea (31%)Myalgia (11%)Confusion (9%)Headache (8%)Rhinorrhea (4%)Chest pain (2%)Diarrhea (2%)Nausea and vomit (1%) | Fever (96%)Cough (47%)Myalgia (31%). Anorexia (18%)Headache (16%)Dyspnea (14%)Diarrhea (10%)Nausea (6%) | Fever (97%)Expectoration (72%)Dyspnea (59%)Myalgia (41%). Diarrhea (14%)Headache (7%) | Fever (98.6%)Fatigue (69.6%)Dry cough (59.4%)Anorexia (39.9%)Myalgia (34.8%)Dyspnea (31.2%)Expectoration (26.8%)Diarrhea (10.1%)Nausea (10.1%). Dizziness (9.4%)Headache (6.5%)Vomiting (3.6%)Abdominal pain (2.2%) | |
| Complications | SARS (29%)Anemia (15%)CHF (12%)CKD (7%)Infection (10%)Shock (7%) | SARS (17%)CKD (3%)Septic shock (4%)MVAP (1%) | Shock (8.7%)CHF (7.2%)Arrhythmia (16.7%)SARS (19.6%)CKD (3.6%) |
CHF, congestive heart failure; CKD, chronic kidney disease; MVAP, mechanical ventilator-associated pneumonia; SARS, acute respiratory distress syndrome.
Most critically ill patients were old and had more underlying conditions compared to patients not admitted to the ICU.22 This suggests that age and prior comorbidities can be risk factors conditioning worse outcomes. Compared to the symptoms of patients who were not admitted to the ICU, the most common symptoms of patients in critical condition were dyspnea, abdominal pain, and anorexia. It is possible that the appearance of these symptoms could help identify patients with worse prognosis.
The other great COVID-19 focus of infection to this date, Italy, reported 12 462 cases on March 11, and 827 deaths. Only China had reported more deaths due to this new SARS-CoV-2 outbreak. The mean age of the deceased in Italy was 81 years and over two thirds of these patients had diabetes, heart conditions or cancer or were former smokers. Therefore, it is true that these patients had underlying health conditions, but it is also worth pointing out that they had pneumonia related SARS and SARS-CoV-2 related SARS. Of the patients who died, 42.2% were between 80 and 89 years old, 32.4% were between 70 and 79 years old, 8.4% were between 60 and 69 years and 2.8% of the patient were between 50 and 59 years.23
The most common laboratory anomalies observed have been lymphopenia, prolonged prothrombin time, and high lactate dehydrogenase levels. These laboratory anomalies are similar to those previously seen in patients with MERS and SARS infections. In non-survivors, the neutrophil count, D dimer, blood urea, and creatinine levels continued to increase, and the lymphocyte count continued to decrease until the patients died. On the other hand, neutrophilia can be associated with the cytokine storm induced by the viral invasion, the activation of coagulation may have been associated with a sustained inflammatory response, and acute kidney injury may have been associated with the direct effects of the virus, hypoxia, and shock. The 3 pathological mechanisms may be associated with the death of patients with coronavirus induced pneumonia.11
A recent meta-analysis shows that high procalcitonin levels is associated with a 5-time higher risk of severe infection due to SARS-CoV-2 (adjusted odds ratio [OR]: 4.76; 95%CI: 2.74–8.29).24 Although the total number of patients with COVID-19 with higher procalcitonin values seems limited,25 the results of this meta-analysis suggests that serial measures of procalcitonin levels can play a role when it comes to predicting progression towards a more severe form of the disease. It is well-known that the production and release of procalcitonin into circulation is higher during bacterial infections. However, the synthesis of this biomarker is inhibited by interferon-gamma whose concentrations increase during viral infections. Therefore, it is not surprising to see that procalcitonin levels remain within the reference range in patients with uncomplicated SARS-CoV-2 infections. It is not surprising to see either that a substantial increase of this levels is suggestive of bacterial co-infection in patients who develop severe forms of the disease, thus complicating the clinical condition.26 On the other hand, the significant increase of C-reactive protein levels in blood has become an important marker of COVID-19.27 As a matter of fact, significantly high C-reactive protein levels have been associated with a higher risk of developing SARS.28
It has been recently reported how 4 patients with COVID-19 who met the criteria for hospital discharge or interruption of quarantine in China (no clinical symptoms and radiological anomalies and 2 negative results on the RT-PCR test later tested positive on the RT-PCR between 5 and 13 days later. These findings are suggestive that, at least, a percentage of recovered patients can still be carriers of the virus. Although no family member was infected, all of the patients described were health professionals who were especially serious about their home quarantine. Perhaps it will be necessary to reassess the current criteria for hospital discharge or quarantine interruption as well as the patients’ continuous treatment.29
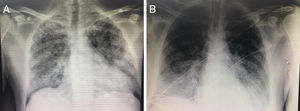
Key points of radiology assessmentsThe images of pneumonias caused by virus are varied and unspecific, and they can be seen in other pulmonary infectious or inflammatory processes.30,31 The simple chest X ray shows an interstitial pattern in over 85% of the cases, and it occurs bilaterally in over 75% (Fig. 2). On the other hand, greater damage in the simple X ray has been associated with the need for ICU admission.32
The computed tomography (CT) images were used in the screening and selection of suspected cases in hospitals with high volumes of this entity.26 Subsequent studies have showed the great utility of this imaging modality, even compared to molecular studies with findings suggestive of disease in patients with negative microbiological results.33 The most common findings are images of ground-glass opacities associated or not with parenchymal condensation with air bronchogram; lung fissure thickening; peripheral distribution, and predominant damage of more than 1 lobe, usually bilateral. Less common are images of cavitation, pleural effusion, lung nodules or signs of lymphadenopathy. The presence of parenchymal consolidation and interlobular septal thickening has been associated with more severe cases that required ICU admission. These findings compel physicians to make differential diagnoses with other pneumonic processes of viral origin. Compared to other viruses of the same family, there seems to be a greater tendency to bilateral damage compared to SARS and no specific distribution pattern similar to MERS.24,26,34–37
However, it is possible that no specific findings are found on the imaging modalities, so it is not a valid imaging modality to rule out patients with the disease, although normal CT images have been reported in a few cases even during the early stages of the disease.12,39
However, the CT scan has certain disadvantages since the availability of these machines can be insufficient, the patient needs to be transferred from the critical care unit, which can cause crossed infections. Viral pneumonia related injuries usually start in the terminal alveoli that are close to the pleura and can be seen clearly on a pulmonary ultrasound. In the clinical practice, for those patients who cannot be transferred or cannot be examined through an X-ray and in case of quick disease progression, quick bedside assessments are essential to be able to adjust the treatment plan.
The pulmonary ultrasound has advantages over the CT scan and the X-ray machines regarding disinfection and the smaller area of contact with the patients. In particular, the 9−15 MHz high-frequency linear-array transducer is clearly capable of showing the morphology and changes of subpleural injuries, and changes of air and water content in consolidated peri-pulmonary tissues. The ultrasound images of lung disease due to SARS-CoV-2 were significantly different from other injuries, such as bacterial pneumonia, lung abscesses, tuberculosis, compression and obstructive atelectasis, pneumothorax, benign and malignant tumors, etc.40
Microbiological diagnosisAchieving a sensitive and specific laboratory diagnosis is crucial for the correct identification of cases. In this regard, use of viral cultures is not practical as a diagnostic method because results are obtained rather late. On the other hand, the tests used for the detection of antigens and antibodies are not validated. For all these reasons, the RT-PCR test conducted in respiratory specimens is the most useful test for laboratory diagnostic purposes.41
Different techniques have been gradually developed with greater sensitivity and specificity to achieve microbiological diagnoses. However, the latest techniques available have revealed a high percentage of patients not diagnosed with the usual techniques used to this day.41 The presence of false negatives makes it necessary to perform the tests once or twice on the same paciente.42 The specimen obtained through bronchoalveolar lavage is considered the most adequate one and probably associated with a greater viral load seen in the specimens obtained from the lower respiratory tract. The chances of finding positive results is greater when the specimens are collected from the lower respiratory tract through bronchoalveolar lavage in male patients with fever and advanced age.43,44 Nevertheless, for the time being there are no data available on the exact rate of false negatives. This can be associated with the absence of a gold standard to achieve microbiological diagnosis.
Severe acute respiratory distress syndrome due to SARS-CoV-2 infectionOver the last few years, the use of molecular techniques has increased the detection of viral infections in patients admitted to the UCI due to respiratory infection. Its prevalence is between 17% and 53% depending on the series. Also, patients with severe viral infections who are eligible for ICU admission often show hypoxemic respiratory failure. Although the management of patients with SARS due to viral infection is similar to the management of SARS due to other etiologies, there are some differences that should be taken into account.45
Regarding non-invasive mechanical ventilation (NIMV) the studies conducted to this day have focused on influenza virus related infections. In a multicenter study conducted among patients with influenza virus related respiratory failure, it was reported that 56.8% of the patients required invasive mechanical ventilation (IMV), and that this fact was associated with a higher mortality rate at the ICU setting46 (Fig. 3). Regarding infections due to MERS a multicenter study published back in 2019 revealed that NIMV was started in 35 % of the patients. However, 92.4 % of them required IMV, and in this case no association with the ICU mortality rate was seen at the 90-day follow-up.47
There are studies where the use of high-flow nasal cannulas (HFNC) reduced the need for intubation.48 However, patients who were ventilated with HFNCs should be monitored and assisted by experienced personnel capable of performing endotracheal intubations of the patient’s clinical situation deteriorates rapidly or does not improve after a short period of time (approximately 1 h).32 In this context, the utility of the ROX index could be assessed ([SatO2/FiO2 ratio] × RR) to predict early the need to increase respiratory support if it remains <3.85 after 12 h.49
Currently, the recommendation established for the use of NIMV in patients with severe viral infections is to select patients properly since those with mild-to-moderate forms of hypoxemic respiratory failure can benefit themselves.50 The protocol published by the Spanish Secretary of Health in collaboration with numerous Spanish medical societies (SEMICYUC included) recommends limiting the use of NIMV in very specific patients while taking into account the history of a high rate of failure in MERS induced infections.51
Recently, an analysis of patients admitted to a Wuhan hospital with a diagnosis of severe respiratory distress syndrome due to COVID-19 revealed that 63.5% of the patients were treated with HFNCs, 56% of the patients received NIMV, and 42% required IMV. However, the crossing rate of the patients who were treated with HFNC/NIMV and then with IMV52 was not reported.
Regarding the prone maneuver, a study of patients infected with the influenza A virus already confirmed its utility improving oxygenation, maintaining it after going supine, and reducing pCO2 levels.53
In unpublished data about the management of patients in Italy, we see that in most cases patients show compliant lungs, often ventilated with high PEEP and maintaining protective plateau pressure and driving pressure. On the other hand, the most effective maneuver for treatment purposes is the prone position in several cycles and prolonged between 18 and 24 h.54
In this series of patients infected with SARS-CoV-2, the prone maneuvers was performed in 6 patients (11.5%), 4 of them died. Of the total number of patients, 6 (11.5%) required oxygenation with extracorporeal circulation, 5 of them died. According to the protocol published by the Spanish Secretary of health, the use of extracorporeal membrane oxygenation (ECMO) is advisable after having started protective ventilation and performed prone maneuvers without achieving improvements, at least, 20% in hypoxemia in patients with refractory hypercapnia to treatment with CO2 elimination systems.
The role of corticoids to treat severe respiratory failure of viral origin has been widely studied in patients with infection due to SARS and MERS, and today they are also being used in patients with SARS-CoV-2 infection.
Corticoids were used to treat SARS and MERS infections since the histology of the lungs revealed inflammation and diffuse alveolar damage,55 and a case suggestive of hemophagocytic syndrome.56 In this context, the role of corticoids as modulators of inflammatory response could be interesting. Although clinical improvement was seen in some patients, a retrospective study on the Hong Kong cohort of patients with SARS infection showed that the use of corticoids was associated with a higher 30-day mortality risk (OR, 26.0; 95%CI, 4–154.8).57 In another retrospective study of patients with MERS infection, the use of corticoids was associated with a higher risk of requiring mechanical ventilation, vasopressors, and renal replacement therapies.58
According to the current guidelines published by the WHO, the systematic use of corticoids is ill-advised except if they are used for something different than SARS-CoV-2 infection or in clinical trials.59
However, a recent study on risk factors associated with SARS and death in patients with pneumonia due to the 2019 coronavirus outbreak declared in Wuhan, China showed that among SARS patients, treatment with methylprednisolone reduced mortality rate (OR, 0.38; 95%CI, 0.20−0.72), neutrophilia, and organ and coagulation dysfunction.28
Antiviral treatmentCurrently, there is no evidence from randomized controlled studies to support specific pharmacological treatment against COVID-19 in suspected or confirmed cases. The atomized-inhalation of interferon-alpha can be considered in adults, but it has a weak degree of recommendation (5 million units in sterile water/twice a day); the use of oral lopinavir/ritonavir (2 capsules/twice a day) has a weak degree of recommendation too.60
A recent systematic review showed that early therapy with lopinavir/ritonavir improved the anti-coronavirus effect in terms of patient mortality and lower use of glucocorticoid. However, beyond this early treatment window, no significant effect has been found in its late application.61
Currently, the most commonly used antivirals in clinical trials include remdesivir, ASC 09, lopinavir/ritonavir, arbidol, as well as traditional biological agents such as interferon-alpha and thymosine alpha-1. At the same time, 3 studies with glucocorticoids are in the pipeline, 2 of them with patients with severe pneumonia. Also, traditional antimalarial drugs are being widely studied: chloroquine and hydroxychloroquine.62 There is already in vitro evidence of the greater potential of hydroxychloroquine over chloroquine.63Table 3 shows the therapeutic options available.
Drugs used and drugs in the pipeline to treat infections due to SARS-CoV-2.
| Drug | Dose | Adverse effects |
|---|---|---|
| Lopinavir/ritonavir | 400/100 mg/12 h | Diarrhea, nausea, long QT, P450 CYP3A inhibition |
| Lopinavir/ritonavir + subcutaneous INF-β1b | 400/100 mg/12 h250 µg/mL | Avoid INF-β1b in psychiatric patientsFever, headache, leukopenia, hypertonia |
| Lopinavir/ritonavir + nebulized INF-α2b | 400/100 mg/12 h | Avoid INF-α2b in heart disease, liver or kidney dysfunction |
| Remdesivira | Load: 200 mg/24 hMaintenance: 100 mg/24 h/for 5−10 days | |
| Hydroxychloroquine | Load: 400 mg/12 hMaintenance: 200 mg/12 h/for 4 days | Retinopathies |
AEMPS, Spanish Agency of Medicines and Medical Devices; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2.
In an analysis conducted in critically ill patients hospitalized due to MERS, 18% of them had bacterial co-infection and 5% viral co-infection.
In a register of 68 patients from Northern China, the rate of co-infection went from 20% to 80% from one region to the next, being the being influenza A and B virus, Mycoplasma pneumoniae and Legionella pneumophila the most commonly associated pathogens.64
However, according to the protocol published by the Spanish Secretary of Health, although the current recommendations do not support the use of antibiotics, their use should be considered in critically ill patients with pneumonia of unknown origin, sepsis or suspected of bacterial over-infection.
Safety against the pandemicThe keys that the WHO65 has given on this regard are based on several key points:
Early identification and control of the sourceStrict adherence to prevention protocols. Identifying and isolating quickly patients with acute respiratory symptoms that can be potentially attributed to COVID-19. It is necessary to bear in mind that, during times of pandemic, approximately 20% of the patients with SARS will require ICU admission. These are patients who will need individual rooms with proper negative pressure ventilation (≥ 160 L/s/patient). Also, it is important to establish predetermined routes if the patient has to be transferred or moved in order to minimize transit in the room (both from personnel and visitors), and use FFP2 or FFP3 masks.12,66,67
Standard precautionsThe staff in contact with infected patients must wear protocolized personal protective equipment (PPE): mask (FFP2: for general care and tests [hemogram, biochemical] or FFP3: if specimens with risk of generating aerosols are going to be obtained), gloves, eye protection, long sleeve, unsterile protective gowns, aprons, and footwear that can be decontaminated. Equipment should be worn only once, although if reused, sterilization must be conducted correctly (for example, with 70% ethylic alcohol). Hand washing is essential as well as the application of an alcohol solution if no organic remains are visible. Special care should be paid to airway procedures or those that expose the staff to respiratory secretions, as well as to the collection and transportation of respiratory specimens. Cleaning the surfaces with bleach is effective and good enough.14,68
Perhaps one of the key elements is remembering to always put on and above all take off the personal protective equipment step by step (Table 4).69
Summary of the protocol for donning and doffing personal protective equipment.
| Donning | Doffing |
|---|---|
| Make sure that you are wearing work clothes (scrubs and closed-heeled footwear), and not carrying personal items (cell phones, pens), hair tied up and off your neck and short fingernails. Hydrate appropriately and use the washroom. | Remove your PPE in the patient’s roo, by the door.There will be a chair and a black container by the door for hand sanitation. |
| 1. Hand hygiene. With hand sanitizer and air drying.2. High boot covers. Wear mid-calf boots. Tie the straps.3. Waterproof gown. Wear it down to your mid-calf, loose-fitting, tie around your neck and waist.4. FFP3 mask. Secure upper strap around the crown of your head and the lower strap right below ear level. Adjust the nose piece. Check the adjustment: out both hands covering the front. When breathing out hard it will tend to separate from your face. When inhaling it will collapse on your face; if not there is lateral leak and should be readjusted.5. Hood. Extend borders on the front and back of your head thus reducing the opening on the face to the minimum for appropriate vision.6. Face shield or goggles. Adjust the strap to put it into place. Wearing goggles and shields at the same time is ill-advised.7. Nitrile gloves. Gloves should cover the cuffs of the gown. | 1. Gloves. Remove them making sure not to touch the outside of the gloves and throw them into the black container.2. Perform hand hygiene.3. Face shield or goggles. Flexing the head slightly, grab the strap around the back of your head, pull upwards and away without touching the front and dispose.4. Perform hand hygiene.5. Hood. Flexing your head slightly, grab with one hand by its highest part and slowly pull away from your face, dispose.6. Perform hand hygiene.7. Waterproof gown. Release the ties around your neck and waist. Grab by the neck ties and pull the gown carefully down and away from your body. Remove the gown by folding the inside onto the outside and dispose. 8. Perform hand hygiene.9. High boot covers. Sit on a chair and release the covers. Remove by turning them inside out and down. Dispose in container.10. Perform hand hygiene and leave the patient’s room.Outside the room, by the door there will be a black container for hand hygiene.11. FFP3Mask. Flexing your head slightly grab the lower strap with both hands and raise it to the level of the upper strap. Grab both straps and pull mask upwards and away from your face and dispose in the container without touching its frontal side.Perform final hand hygiene with alcohol-based hand sanitizer. |
If a decision is made to start respiratory support with HFNC, it is advisable to put a surgical mask on the patient’s face to limit the production of aerosols. Similarly, when dealing with NIMV support, closed masks should be worn since they adapt very well to the patient’s face and avoid accidental leaks. Double-limb respiratory circuits with antibacterial filters in both are recommended and should be seriously taken into consideration.70
Intubation is perhaps the moment that involves the greatest risk of transmission. Therefore, it is a scenario that should be considered properly to just have the exact and necessary number of people in the room (the fewer number of people in contact with the patient the lower risk of transmission) and, of course, to have all the necessary material inside the room (to avoid having to enter or exit the room again). It is advisable that the most experienced health professional in the management of rapid sequence intubations should be the one who performs this technique to minimize the number of attempts so that it becomes an elective non-emergent intubation. Similarly, ambu-bag ventilation should be avoided to avoid generating aerosols. To perform a laryngoscopy, it is better to use a video-laryngoscope with a separate screen to keep the longest distance possible from the patient.71
Administrative controlsIt is necessary to train and educate the staff in charge of the patient and his caregivers and implement mechanisms of improvement regarding care as questions keep arising. Also, appoint a person in charge of observing how the staff works and establish the proper feedback for infection control purposes.
The proper use of masks depends on regular supply, proper training, hand hygiene, and attitude of the healthcare personnel. It is useful to keep a registry of people getting in and out of the patient’s room. The increasing the physician/patient and nurse/patient ratios should also be assessed to provide better care.12,15
Psychological impactOne dimension that has been poorly explored among the huge healthcare problems that involves in the existence of a pandemic which, in general, affects all patients who require isolation, their families and friends, and healthcare workers confined for safety reasons and who have to remain living in this situation for a long time.
In the city of Wuhan, China health professionals have been facing enormous pressure, including a high risk of infection and inadequate protection against the virus, excessive workloads, frustration, discrimination, isolation, patients with negative emotions, lack of contact with their families, and physical and mental exhaustion. This situation produces health professionals with stress, anxiety, depressive symptoms, insomnia, denial, anger, and fear. These become problems that not only affect healthcare workers, but also impact patients and the quality of healthcare provided the same way. Acknowledging contagion as an occupational disease and having more staff available are key actions, as well as implementing proper rest and renewal systems for the health workers who are «at the frontline of healthcare». Teams of psychologists have been made available both for healthcare workers and patients alike with good results.72
AuthorsAlejandro González-Castro: Study design. Selection of the references on epidemiology, clinical signs, and laboratory. Final writing of the manuscript.
Patricia Escudero-Acha: Reference search and writing of the SARS section. Final writing of the manuscript.
Yhivian Peñasco: Reference search and writing of the radiology section.
Oihana Leizaola: Reference search and writing of the treatment section.
Victoria Martínez de Pinillos Sánchez: Reference search and writing of the safety section.
FundingThis manuscript received no funding whatsoever.
Conflicts of interestAll authors declared no conflicts of interest whatsoever.
Please cite this article as: González-Castro A, Escudero-Acha P, Peñasco Y, Leizaola O, Martínez de Pinillos Sánchez V, Garcia de Lorenzo A. Cuidados intensivos durante la epidemia de coronavirus 2019. Med Intensiva. 2020;44:351–362.