To study the correlation between intraabdominal and intrathoracic pressures in patients with suspected intraabdominal hypertension.
DesignA prospective, observational cohort study.
SettingPolyvalent intensive care unit of a University hospital.
PatientsTwenty-seven medical-surgical patients dependent upon controlled mechanical ventilation due to acute respiratory failure and with several risk factors for intraabdominal hypertension (IAH).
Main variablesIntraabdominal (IAP), esophageal (Peso) and airways pressures were measured under static (st) and dynamic (dyn) conditions. Respiratory system (Crs), lung (Clu) and chest wall compliance (Ccw) were calculated.
ResultsIn 10 patients IAP>12mmHg (IAH, IAPst, 14±2 [12–21] mmHg), while in the rest the pressure proved normal (n=17; IAPst, 8±2 [3–11] mmHg). Pesost was 11±5 (2–27) and Pesodyn 7±4 (2–24) cmH2O. Depending on the presence or absence of IAH, Pesost was 9±4 vs 7±3cmH2O (P=.2) and Pesodyn 6±2 vs 4±3cmH2O (P=.3), respectively. The correlation between Pesost and dyn with IAPst was 0.5 (P=.003) and 0.4 (P=.03), respectively. The compliance components were decreased (Crs, 31±8; Clu, 52±22 and Ccw, 105±50ml/cmH2O); Ccw was significantly lower in patients with IAH (81±31 vs 118±55ml/cmH2O; P=.02). The correlation coefficient between IAPst and Ccw was −0.7 (P<.001), and −0.5 (P=.002) with respect to Crs.
ConclusionsA stiffer chest wall was observed in patients with IAH. In patients with risk factors for IAH, pressures in these compartments were highly variable.
Analizar la correlación entre la presión intraabdominal e intratorácica en pacientes con sospecha de hipertensión intraabdominal (HIA).
DiseñoEstudio prospectivo observacional de una cohorte.
ÁmbitoUnidad de medicina intensiva polivalente de un hospital universitario.
PacientesSe incluyó a 27 pacientes medicoquirúrgicos dependientes de ventilación mecánica controlada por fallo respiratorio agudo y con factores de riesgo de hipertensión intraabdominal.
Principales variablesMedimos las presiones intraabdominal (PIA), esofágica (Peso) y de la vía aérea en condiciones estáticas (est) y dinámicas (din). Calculamos la distensibilidad del sistema respiratorio (Csr), pulmón (Cp) y pared torácica (Cpt).
ResultadosEn 10 pacientes la PIAest fue mayor de 12mmHg (HIA, PIAest, 14±2 [12-21] mmHg) y en el resto fue normal (n=17; PIAest, 8±2 [3-11] mmHg). La Pesoest fue 11±5 (2-27) y Pesodin, 7±4 (2-24) cmH2O. Considerando la presencia o no de HIA, Pesoest fue 9±4 vs. 7±3cmH2O (p=0,2) y Pesodin, 6±2 vs. 4±3cmH2O (p=0,3), respectivamente. La correlación de Pesoest y din con PIAest fue 0,5 (p=0,003) y 0,4 (p=0,03), respectivamente. Los componentes de la distensibilidad del sistema respiratorio estaban disminuidos (Csr, 31±8; Cp, 52±22; Cpt, 105±50ml/cmH2O), Cpt fue significativamente más baja en los pacientes con HIA (81±31 vs. 118±55ml/cmH2O; p=0,02). El coeficiente de correlación entre la PIAest y Cpt fue –0,7 (p<0,001) y de –0,5 (p=0,002) con Csr.
ConclusionesLa pared torácica es más rígida en pacientes con hipertensión abdominal. En presencia de factores de riesgo de HIA las presiones en estos compartimentos son muy variables.
Intensive resuscitation with fluids, abdominal surgery, ileus, etc., produces intraabdominal hypertension (IAH) in 30%–80% of all critical patients.1 The incidence of IAH is thus seen to be highly variable, in the same way as the intraabdominal pressure (IAP) levels, and this situation has clinical implications.2 On the other hand, clinical exploration alone is unable to determine the presence or absence of IAH,3 and the measurement of intravesical pressure is recommended.4,5 These factors complicate the management of these patients, though if IAP elevation is not considered, it will have an impact upon the rest of the behavior of the organism–favoring polycompartmental syndrome and multiorgan failure.1,6
The transmission of abdominal pressure to the chest compartment increases thoracic rigidity,7 compresses the lungs,8 and elevates airway pressure. Determination in a patient subjected to mechanical ventilation of whether the high pressures in the respirator are due to thoracic or pulmonary rigidity cannot be made through clinical exploration, particularly in cases of acute respiratory distress syndrome (ARDS).9 Such an evaluation requires the measurement of abdominal and esophageal pressures, which thus has diagnostic and therapeutic implications. These measurements make it possible to distinguish thoracic from pulmonary rigidity, modify the respirator parameters–particularly positive end-expiratory pressure (PEEP) – and to adopt measures designed to reduce abdominal pressure. Therefore, it seems necessary to evaluate the effect of intraabdominal pressure upon the respiratory system.2 However, esophageal pressure is not measured in routine practice,10 as this requires additional equipment or respirators with sophisticated monitoring systems. In effect, such measurements are usually made on an isolated basis in research studies.
The present study examines the correlation between the abdominal and thoracic pressures in patients with suspected abdominal hypertension, without the use of devices additional to those commonly employed in critical patients.
Patients and MethodsWe studied 27 patients with acute respiratory failure subjected to controlled mechanical ventilation, with deep sedation (6 points on the Ramsay scale), and occasionally muscle relaxation. Measurements were made of abdominal and esophageal pressures due to the risk of abdominal hypertension and difficulties with mechanical ventilation. The study was authorized by the Ethics Committee of the hospital, and the routine care of these patients was not interfered with in any way.
Intraabdominal Pressure (IAP)The measurements were made following the recommendations of the recent consensus conference.11 The intraperitoneal pressure was estimated from the bladder pressure measured with the Foley catheter, connected by means of a T-valve to a syringe and to a pressure transducer (D-85716, Edwards Lifesciencies, Unterschlessheim, Germany), and to the bedside monitor (Marquette Hellige Solar 8000. Medical System, Milwaukee, USA). The measurements were made in supine decubitus, with the transducer on the axillary midline and after injecting 20ml of 0.9% saline solution. Correct transducer measurement was confirmed with slight suprapubic pressure, which induces an oscillation in the pressure curve. The monitor cursor was used to measure the pressure at the end of expiration (static pressure) (IAPst), together with the respiratory oscillation (dynamic pressure) (IAPdyn), which estimates abdominal compliance,12 as the difference between the end of inspiration and expiration. The mean value of at least three measurements was calculated, with a variability of less than 10%.
Intrathoracic PressuresEsophageal pressure was measured with the patient nasogastric tube used for feeding or gastric drainage (Levin type 125cm, 14–16F, Unomedical, Denmark), without additional equipment.13 We measured the pressure transmitted by fluid,14 in a way similar to the technique used to measure bladder pressure. The tube was connected via a T-valve to a syringe and a transducer. Since the material was made of transparent plastic, the presence of bubbles or other material within it could be evaluated. This method has been validated in experimental15 and clinical studies.16 For confirming the position of the tube within the esophagus it is not possible to perform an occlusion test, due to the absence of respiratory effort. We therefore used the chest X-ray to check the position of the distal tip, applying slight epigastric compression. Slow withdrawal was carried out until the pressure change and transmission of the heart beat were observed; the first tube mark (37cm) is always visualized.17 Prior to a new saline purge, we gently aspirated the air or liquid contained in the esophagus. The measurements were made at the raised patient bedside, and with the transducer on the axillary midline. When the pressure curve showed a stable registry, a rapid transducer washing test was performed, checking the response to the pressure increase and recovery of the previous pressure value. The monitor cursor was used to measure the pressure at the end of expiration (static pressure) (Pesost) and the respiratory oscillation (dynamic pressure) (Pesodyn), established as the difference between the end of inspiration and expiration. As a reference we used a middle point of the oscillation of the heart beat. The mean value of at least three measurements was calculated, with a variability of less than 10%.
The compliance of the respiratory system (Crs), chest wall (Ccw) and lungs (Clu) was assessed as the ratio between the tidal volume and the airway pressure delta (plateau pressure – total PEEP), esophageal pressure delta (change in esophageal pressure between the end of inspiration and expiration) and airway pressure delta minus the esophageal pressure delta, respectively. The respirator was in volume controlled mode, constant flow and an inspiratory pause of 0.1–0.2s. The airway pressures were measured from the respiratory screen in cmH2O, and the monitor measures in mmHg were transformed into cmH2O (1mmHg=1.36cmH2O).
Statistical AnalysisThe normal distribution of the quantitative variables was checked with the Kolmogorov–Smirnov test. The descriptive results relating to the quantitative variables were expressed as the mean±standard deviation, with the interval (range) in the parameters of interest. The comparison of means between the patients with and without abdominal hypertension was based on the Student t-test. Correlations between quantitative variables (Pesost, Pesodyn, IAPst, IAPdyn) were established with the Pearson test. Statistical significance was accepted for P<.05.
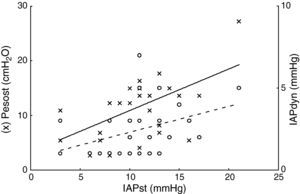
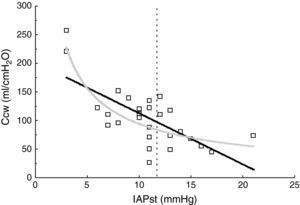
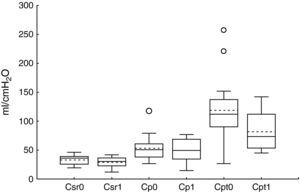
ResultsThe patient characteristics and ventilation parameters are reported in Tables 1 and 2. In 10 of the 27 patients the IAPst was>12mmHg (37%). The correlation coefficient between the static (IAPst, 10±33–21mmHg) and dynamic intraabdominal pressures (IAPdyn, 2.4±1.61–7) was 0.4 (P=.04); 0.5 in the group with abdominal hypertension (IAPst, 14±212–21mmHg) and 0.3 in the patients without IAH (n=17; IAPst, 8±23–11 mmHg) (Fig. 1). IAPdyn was independent of the presence or absence of IAH (2.2±1.71–7mmHg without IAH vs 2.8±1.41–5mmHg with IAH; P=0.4). The static esophageal pressure was 11±5 (2–27) cmH2O, while the dynamic esophageal pressure was 7±4 (2–24) cmH2O. Considering the presence or absence of IAH, Pesost was 9±4 vs 7±3cmH2O (P=0.2) and Pesodyn 6±2 vs 4±3cmH2O (P=0.3), respectively. The correlation of abdominal static pressure (11±52–27cmH2O) with esophageal pressure was 0.5 (P=.003), and 0.4 with Pesodyn (P=.03). The correlation of IAPst with chest wall compliance was −0.69 (P<.001), and −0.56 (P=.002) with respiratory system compliance. The respiratory, pulmonary and thoracic compliance values were diminished (31±8, 52±22 and 105±50ml/cmH2O, respectively). The correlation coefficient between IAPst and Ccw using an exponential equation (y=a×b) increased to −0.81 (Fig. 2), and was not modified between IAPst and static or dynamic esophageal pressure. In the patients with IAH, chest wall compliance was significantly lower than in the patients without IAH (81±31 vs 118±55ml/cmH2O; P=.02). The compliance values of the respiratory and pulmonary systems were not significantly lower (Fig. 3) (Crs, 28±9 vs 33±7cmH2O and Clu, 50±21 vs 54±23). Static esophageal pressure was not correlated to chest wall compliance (r=0.01).
Ventilation parameters.
| TV (ml) | 582±55 (480–700) |
| Fr (rpm) | 16±2 (10–22) |
| V’I (l/m) | 42±6 (33–60) |
| Pimax (cmH2O) | 38±9 (27–72) |
| Ppl (cmH2O) | 26±8 (18–55) |
| FiO2 (%) | 55±15 (40–100) |
| PEEP (cmH2O) | 6±2 (3–12) |
FiO2: fraction of inspired oxygen; Fr: respiratory frequency; Pimax: maximum inspiratory pressure; PEEP: positive end-expiratory pressure; Ppl: plateau pressure; TV: tidal volume; V’I: inspiratory flow.
The data express the mean±standard deviation (range).
Relationship between static intraabdominal pressure (IAPst, x-axis) and static esophageal pressure (Pesost, axis and left, circles in black). Continuous line linear regression fit, Pesost, 3.2±0.7·IAPst and relation to dynamic intraabdominal pressure (IAPdyn, axis and right, circles in white); broken line fit, IAPdyn, 0.7±0.15·IAPst. The increase in abdominal pressure was related to thoracic and abdominal rigidities, though there was important variability between patients, which possibly explains the weak correlation. In practice, within the pressure range studied, when abdominal palpation proves abnormal (IAPdyn, estimator of abdominal compliance), we cannot infer the change in intraabdominal or intrathoracic pressure.
In 6 patients ventilated with PEEP≥10cmH2O, the pressures in the abdomen and chest tended to be higher, though not significantly so vs the patient with PEEP<10cmH2O (IAPst 13±3 vs 10±3mmHg; Pesost 9±2 vs 8±4mmHg).
DiscussionThe results of our study show that in patients with risk factors for abdominal hypertension, the intraabdominal and intrathoracic pressures are highly variable. We found abdominal hypertension in one-third of the cases, these being patients in which the intrathoracic pressures are higher, and particularly chest rigidity is greater.
Bladder pressure is the standard5 for estimating IAP, though it is necessary for the abdominal contents to act as a single compartment in order for bladder pressure to reflect IAP.7,18 If this is not the case then intraorgan pressure (bladder, gastric, etc.) will be variable,7,18 and bladder pressure may not reflect intraperitoneal pressure.19. The IAP values, which we measured, were higher than those reported in patients subjected to mechanical ventilation (5–7mmHg),11 but similar to those described in the presence of risk factors for IAH (14±120, 11±021mmHg) or IAH (15±3mmHg1,22). These higher IAP values facilitate abdominal hydraulic mechanics, and allow intravesical pressure to reflect intraperitoneal pressure.6 We found abdominal hypertension in approximately one-third of the studied patients, and the mean values varied greatly from one patient to another. This dispersion of values has been described by other investigators in patients with clinical disorders (8–36mmHg),1,3,6 surgical conditions, and in polytraumatized subjects (2–94mmHg).23 In addition, and coinciding with previous studies,21 abdominal distension evidenced by IAPdyn showed a weak correlation with abdominal pressure (IAPst). These factors indicate that in patients with risk factors for IAH it is necessary to measure IAP, since clinical assessment alone is insufficient–at least within the pressure range shown by the studied patients.
Esophageal pressure at the end of expiration has been little used for estimating pleural pressure, mainly because mediastinal weight influences the measurement. However, it has been shown that this artifact represents less than 5cmH2O,24 which is a small percentage within the range of static esophageal pressure described in these patients.2 Accordingly, the values we obtained are similar to those reported in patients with ARDS (10–12cmH2O),25 though higher values have also been described (17±5cmH2O).10 In contrast, with spontaneous breathing in the supine position, the pressure is lower (0.7–5.3cmH2O).26 This difference is not attributable to mechanical ventilation, since the latter does not modify the chest mechanics,27 and values of −0.8±1.9cmH2O have been reported.28 However, in critical patients subjected to mechanical ventilation, multiple factors affect the chest mechanics (obesity, edema, abdominal surgery, etc.), and the esophageal pressure range is very broad and unpredictable (4–32cmH2O2).
It is more common to use the respiratory variation of esophageal pressure (Pesodyn) to assess chest compliance.29 The values we obtained were higher than those previously described in patients with acute respiratory failure (4±4cmH2O),10 and in coincidence with other studies we found an elevation in IAP to produce an increase in chest wall rigidity17,29. Thus, Gattinoni et al.30 recorded a correlation coefficient of 0.84 in patients with IAP values of 5–35mmHg. However, the opposite results have also been described, with no correlation between chest compliance and IAP, exhibiting values of 16±3 and 19.3±7.8mmHg.17,31 This discrepancy has been attributed to the different IAP ranges shown by the patients of these studies, though possibly other factors may intervene32–fundamentally abdominal wall,33 thoracic34 and pulmonary compliances,35 which buffer the changes in thoracic pressure to 20mmHg of IAP.31,35 In this way, the transmission of IAP to the chest can vary between 25% and 80%,36,37 indicating that the mechanical interaction between the abdomen and chest is complex.38 In accordance with the above, our results show that an exponential model better fits the relationship between abdominal pressure and thoracic compliance.
We measured respiratory compliance without interrupting ventilation. Therefore, there is a resistive component in elastic pressure, due mainly to the viscoelasticity of the chest. However, this factor is of little importance, as has been demonstrated in patients with morbid obesity.39 The respiratory system compliance components which we measured are similar to those described in these patients.40 Chest wall compliance was 37% lower than in anesthetized patients (105±50ml/cmH2O vs 167ml/cmH2O; P=.001)41; thus, respiratory system compliance underestimates the pulmonary compliance. Thoracic compliance was not correlated to static esophageal pressure, as has also been reported by other authors,17 and has been attributed to the fact that thoracic compliance is influenced by ventilation volume and respiratory system compliance.42 But in patients of this kind, possibly the main factor is the weight of the abdomen upon the esophagus, which may increase static esophageal pressure without modifying thoracic compliance43 – as has been demonstrated in morbidly obese individuals.44 In our study the method used to measure esophageal pressure (involving a saline-filled catheter) may have exerted an influence. Although this method has been previously used in clinical studies,45 an artifact may be produced in the presence of a hydrostatic pressure gradient between the distal tip of the catheter and the transducer.46 This does not influence the reference method for measuring esophageal pressure (balloon catheter),47 though this technique is also not exact, due to the position and volume of the balloon.48
In sum, the results of our study indicate that in subjects with risk factors for abdominal hypertension it is necessary to measure abdominal pressure, since clinical assessment alone is insufficient, and the patients show decreased thoracic compliance. The measurement of esophageal pressure allows more adequate evaluation of respiratory mechanics and may possibly serve to optimize mechanical ventilation in patients with abdominal hypertension.
Please cite this article as: Ruiz Ferrón F, et al. Presión intraabdominal y torácica en pacientes críticos con sospecha de hipertensión intraabdominal. Med Intensiva. 2011;35:274–9.