In the present study, we aimed to compare in-hospital mortality and safety of intravenous beta-blockers and amiodarone in septic patients with new-onset atrial fibrillation (NOAF). The null hypothesis is that there is no significant difference in in-hospital mortality and safety of Beta-blocker (BBs) and amiodarone in treating NOAF in patients with sepsis.
DesignWe conducted a retrospective analysis based on the MIMIC-IV database. Septic patients with NOAF were screened.
SettingPatients admitted to adult mixed ICU for septic patients with NOAF.
PatientsA total of 34,789 patients were screened of whom 1394 patients were included for the analysis: 286 in the amiodarone group and 1108 in the BBs group.
InterventionsNone.
Main variables of interestCox proportional hazard model was used to examine the in-hospital mortality, ventilator-free days and duration of atrial fibrillation in patients receiving either amiodarone or intravenous BBs. Propensity score matching was applied to determine any association.
ResultsAfter Propensity Score (PS) matching, a total of 244 patients were included in both the BB and amiodarone groups. In this cohort, BBs was significantly associated with lower in-hospital mortality [adjusted hazard ratio (HR) of 0.70 (95% CI 0,54–0,91; P&#¿;=&#¿;0.008)]. On the other hand, patients who received amiodarone had a shorter duration of atrial fibrillation (54.17&#¿;h vs 72.81&#¿;h; P&#¿;=&#¿;0.003). There was no significant difference in ventilator-free days between the BB group and the amiodarone group.
ConclusionIn septic patients with NOAF, patients receiving BBs had lower in-hospital mortality than those who received amiodarone. On the other hand, amiodarone group had a shorter duration of atrial fibrillation. There was no significant difference in ventilator-free days between the BB group and the amiodarone group.
En el presente estudio, nuestro objetivo fue comparar la mortalidad hospitalaria y la seguridad de los beta-bloqueadores intravenosos y la amiodarona en pacientes sépticos con fibrilación auricular de inicio reciente (FAIR). La hipótesis nula es que no existe una diferencia significativa en la mortalidad hospitalaria y la seguridad del beta-bloqueador y la amiodarona en el tratamiento de la FAIR en pacientes con sepsis.
DiseñoRealizamos un análisis retrospectivo basado en la base de datos MIMIC-IV. Se seleccionaron pacientes sépticos con FAIR.
ÁmbitoPacientes admitidos en la UCI mixta para adultos con sepsis y FAIR.
PacientesSe seleccionaron un total de 34789 pacientes, de los cuales 1394 pacientes se incluyeron para el análisis: 286 en el grupo de amiodarona y 1108 en el grupo de BBs.
IntervencionesNinguna.
Variables de interés principalesSe utilizó el modelo de riesgos proporcionales de Cox para examinar la mortalidad hospitalaria, los días libres de ventilador y la duración de la fibrilación auricular en pacientes que recibieron amiodarona o beta-bloqueadores intravenosos. Se aplicó la correspondencia de puntuaciones de propensión para determinar cualquier asociación.
ResultadosDespués de la correspondencia de puntuaciones de propensión (PS), se incluyó un total de 244 pacientes en ambos grupos, tanto en el de BBs como en el de amiodarona. En esta cohorte, los BB se asociaron significativamente con una menor mortalidad hospitalaria [razón de riesgo ajustada (HR) de 0.70 (IC 95% 0,54–0,91; P&#¿;=&#¿;0.008)]. Por otro lado, los pacientes que recibieron amiodarona tuvieron una duración más corta de fibrilación auricular (54.17&#¿;horas vs 72.81&#¿;horas; P&#¿;=&#¿;0.003). No hubo una diferencia significativa en los días libres de ventilador entre el grupo de BB y el grupo de amiodarona.
ConclusiónEn pacientes sépticos con FAIR, los pacientes que recibieron BBs tuvieron una mortalidad hospitalaria más baja que aquellos que recibieron amiodarona. Por otro lado, el grupo de amiodarona tuvo una duración más corta de fibrilación auricular. No hubo una diferencia significativa en los días libres de ventilador entre el grupo BB y el grupo de amiodarona.
Across the globe, sepsis cause more than 11 million deaths per year. Septic shock, has associated with an in-hospital mortality rate approaching 40%.1 Atrial fibrillation (AF) is the most common arrythmia in septic patients.2 It may result in hemodynamic instability, high mortality rate, prolonged intensive care unit (ICU) stay and increased healthcare costs. The incidence of new-onset AF (NOAF) ranges from 4% to 9% in patients with sepsis and up to 46% in patients with septic shock.3,4 Regarding treatment, intravenous amiodarone and beta-blockers (BBs) are the most common agents used in practice.5
However, for septic patients with NOAF, it remains unclear which medication should be used. Amiodarone and BBs have been compared on several retrospective studies with inconsistent results regarding important outcomes such as in-hospital mortality, ICU length of stay, complications, pharmacological cardioversion rate, heart rate control, ventilator-free days and others.6 A large study reviewed 39,693 patients with sepsis treated with amiodarone or BB.7 After adjustment for confounding factors, BBs were associated with lower in-hospital mortality than amiodarone (RR 0.67, 95% CI: 0.59–0.77). Nonetheless, time of onset of AF was not documented and up to 40% of patients were not critically ill. There has been no systematic evaluation of the prognosis and safety of these two classes of medications in septic patients admitted to ICUs with NOAF.
In the present study, we aimed to compare in-hospital mortality, ventilator-free days and safety of intravenous BBs and amiodarone in septic patients with NOAF, utilizing the Medical Information Mart for Intensive Care (MIMIC)-IV database, MIMIC-IV is a publicly available database sourced from the electronic health record of the Beth Israel Deaconess Medical Center.
Materials and methodsStudy designWe conducted a retrospective analysis of the MIMIC-IV (2.2) database that includes comprehensive and high-granularity information of patients about well-defined and characterized patients admitted to ICU at Beth Israel Deaconess Medical Center between 2008 and 2019.8 One author (GH) obtained access to the database and was responsible for data extraction (certification numbers 10642176).
Population selection criteria and definitionsPreviously validated nurse-recorded rhythm status from the MIMIC-IV database was used to define NOAF (Patients admitted to ICU who occurrence of AF with a duration exceeding one hour, excluding those with a pre-existing history of AF).9 Inclusion and exclusion criteria for patients with NOAF were as follows: (1) diagnosis of sepsis based on the 3.0 definition; (2) NOAF during ICU stay; (3) prescribed intravenous amiodarone or a BB. If a patient had multiple admissions to ICU, only the first stay was analyzed. Patients with pre-existing AF, AF lasting less than 2&#¿;h, those with history of cardiovascular disease, patients receving both amiodarone and an intravenous BB and/or receiving amiodarone or a BB before ICU admission were excluded.
Patients in the MIMIC-IV who fulfilled the definition of sepsis were eligible for inclusion. Sepsis was diagnosed according to the sepsis-3 criteria.10 Infection was identified from the International Classification of Diseases code in the MIMIC-IV. Data on comorbidities including heart failure, valve disease and diabetes were also collected for analysis and based on the recorded ICD-9 code in the MIMIC-IV database.
Variable extractionThree sets of data were collected: baseline, daily observations, and outcome. The following data were extracted from the MIMIC-IV database on the first day of ICU admission: age, gender, weight, comorbidity, renal replacement therapy (RRT), Sequential Organ Failure Assessment (SOFA) score, Acute Physiology Score III (APS III) and need for invasive mechanical ventilation. Other relevant data including vital signs, laboratory measurements, and prescribed medication were obtained daily throughout the ICU stay. If a variable was recorded more than once on one ICU day, we used the value related to the greatest severity of illness.
Since the nurse-recorded heart rate was sampled once per hour, the during of AF from nurse documentation of AF, the accuracy of nurse charted AF and its temporal precision in critical care patients for manual review by board-certified cardiac electrophysiologists. We also included in the study cohort was the use of medications during the first nurse-recorded episode of AF and the last recording of AF.
Patients in whom a intravenous BB or intravenous amiodarone was administered at the time of the first presentation of AF and at the time of the last presentation of AF were divided into two groups: amiodarone group or BB group.
OutcomeThe primary outcome was in-hospital mortality. Secondary outcomes were number of recorded episodes of AF (the duration of AF), length of ICU stay, ventilator-free days, and ICU staytime.
Statistical analysisWe report the number and percentage of patients as categorical variables, and the median (first quartile [Q1]; third quartile [Q3]) for continuous variables. Categorical variables were compared using the Chi-squared test or Fisher’s exact test and continuous variables by Mann–Whitney U test. Outcomes for continuous variables are presented as the mean (and standard deviation), and for categorical variables as total number and percentage.
Propensity score matching (PSM) was used to control for confounding factors.11 The Propensity Score (PS) for initiating BB vs Amiodarone group, laboratory data were not included in the model because of the substantial proportion of missing information and laboratory date change that rapidly in critically ill patients. Patients in the Amiodarone group were 1:1 matched to those in the BB group based on their estimated PS using the nearest neighbor approach with a caliper width of 0.05 on the PS scale. PSM and propensity score-based inverse probability of treatment weighting (IPTW) were also used to adjust the covariates. After PSM and IPTW, the imbalance in the covariates between the Amiodarone and BB groups was significantly minimized.
To examine risks for particular types of patients, we incorporated subgroup analyses according to age, SOFA score, heart rate, previous cardiac surgery, heart failure, septic shock (including patients receiving dopamine, norepinephrine, dobutamine), use of antiplatelet agents (including patients receiving aspirin and clopidogrel) and use of anticoagulation (warfarin and low molecular weight heparin).
All analyses were performed using the Stata statistical software (version 13.0).
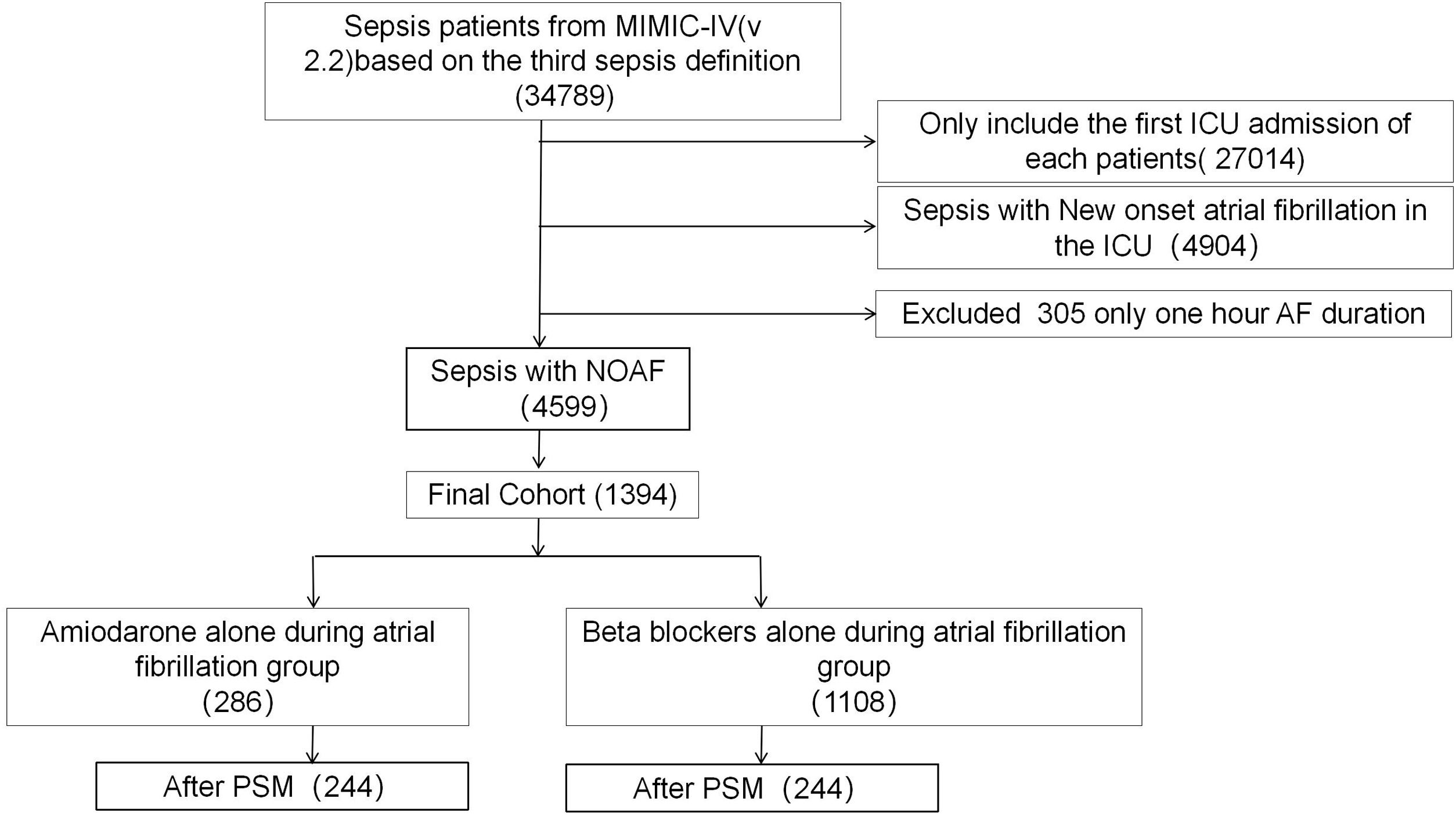
ResultsBasic characteristicsAfter reviewing the data of 34,789 septic patients, a total of 1394 were included in our study: 286 in the amiodarone group and 1108 in the BB group (1078 Metoprolol and 98 Esmolol, some of whom used both drugs). After PS matching there were 244 in the amiodarone group and 244 in the beta blocker group (Fig. 1).
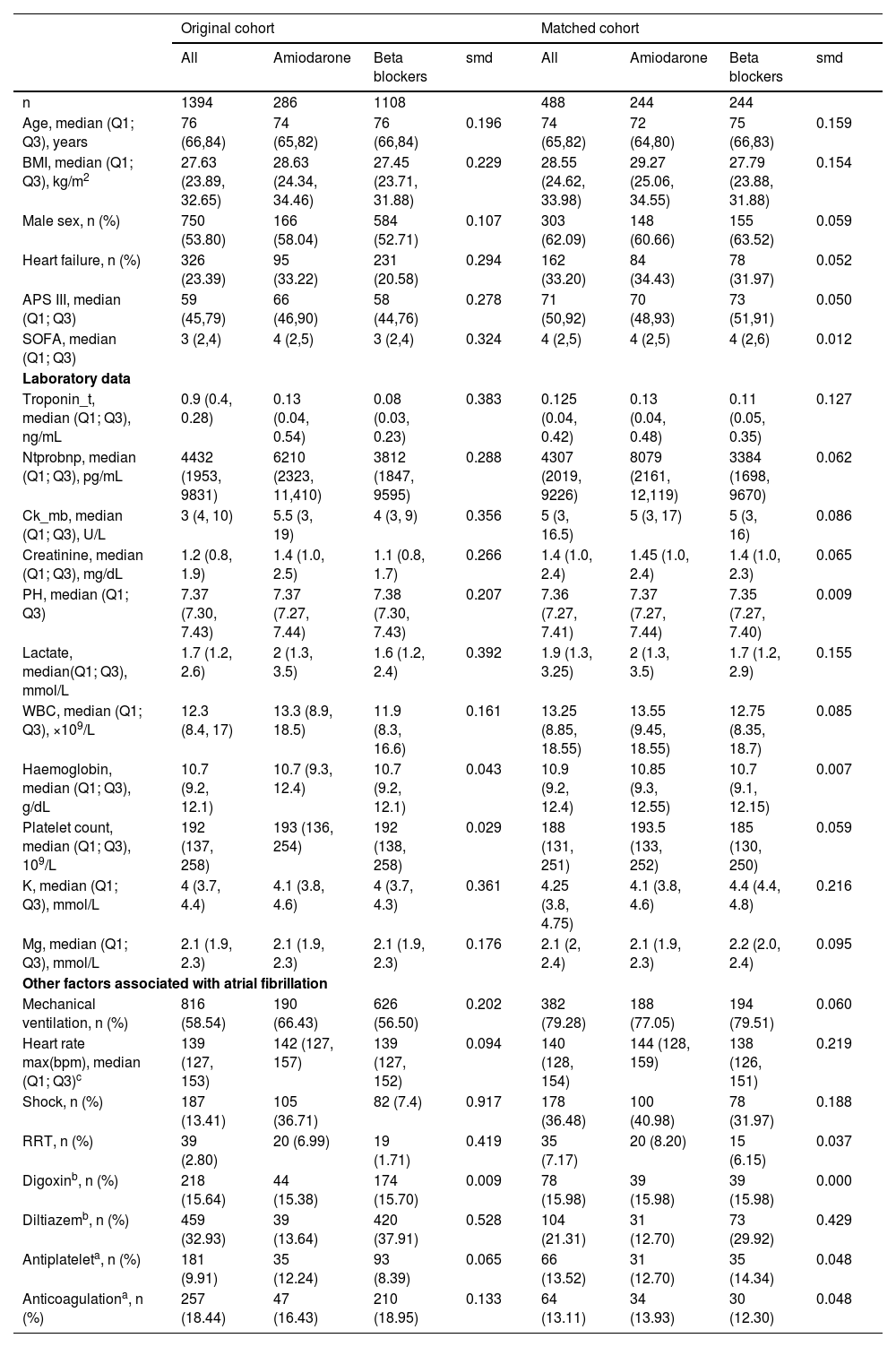
The median age, comorbidities, and SOFA score were significantly higher, while the APS III and Charison score significantly lower on admission in the amiodarone group compared with the BB group. Patients in the amiodarone group were more likely to require RRT within the AF (8.20% vs 6.15%), shock (40.981% vs 31.97%) than the BB group. Patients who received a BB were more likely to receive diltiazem (29.92% vs 12.70%) than the amiodarone group (all P&#¿;<&#¿;0.05). The baseline characteristics of the study patients are summarized in Table 1. Missing data were mainly laboratory results. Missing information is provided in Supplementary material: File 1; Table 1 and positive status of source of infection is shown in Supplementary material: File 1; Table 2. The types of admissions to the ICU is shown in Supplementary material: File 1; Table 3.
Characteristics of the study population on the day of atrial fibrillation onset.
| Original cohort | Matched cohort | |||||||
|---|---|---|---|---|---|---|---|---|
| All | Amiodarone | Beta blockers | smd | All | Amiodarone | Beta blockers | smd | |
| n | 1394 | 286 | 1108 | 488 | 244 | 244 | ||
| Age, median (Q1; Q3), years | 76 (66,84) | 74 (65,82) | 76 (66,84) | 0.196 | 74 (65,82) | 72 (64,80) | 75 (66,83) | 0.159 |
| BMI, median (Q1; Q3), kg/m2 | 27.63 (23.89, 32.65) | 28.63 (24.34, 34.46) | 27.45 (23.71, 31.88) | 0.229 | 28.55 (24.62, 33.98) | 29.27 (25.06, 34.55) | 27.79 (23.88, 31.88) | 0.154 |
| Male sex, n (%) | 750 (53.80) | 166 (58.04) | 584 (52.71) | 0.107 | 303 (62.09) | 148 (60.66) | 155 (63.52) | 0.059 |
| Heart failure, n (%) | 326 (23.39) | 95 (33.22) | 231 (20.58) | 0.294 | 162 (33.20) | 84 (34.43) | 78 (31.97) | 0.052 |
| APS III, median (Q1; Q3) | 59 (45,79) | 66 (46,90) | 58 (44,76) | 0.278 | 71 (50,92) | 70 (48,93) | 73 (51,91) | 0.050 |
| SOFA, median (Q1; Q3) | 3 (2,4) | 4 (2,5) | 3 (2,4) | 0.324 | 4 (2,5) | 4 (2,5) | 4 (2,6) | 0.012 |
| Laboratory data | ||||||||
| Troponin_t, median (Q1; Q3), ng/mL | 0.9 (0.4, 0.28) | 0.13 (0.04, 0.54) | 0.08 (0.03, 0.23) | 0.383 | 0.125 (0.04, 0.42) | 0.13 (0.04, 0.48) | 0.11 (0.05, 0.35) | 0.127 |
| Ntprobnp, median (Q1; Q3), pg/mL | 4432 (1953, 9831) | 6210 (2323, 11,410) | 3812 (1847, 9595) | 0.288 | 4307 (2019, 9226) | 8079 (2161, 12,119) | 3384 (1698, 9670) | 0.062 |
| Ck_mb, median (Q1; Q3), U/L | 3 (4, 10) | 5.5 (3, 19) | 4 (3, 9) | 0.356 | 5 (3, 16.5) | 5 (3, 17) | 5 (3, 16) | 0.086 |
| Creatinine, median (Q1; Q3), mg/dL | 1.2 (0.8, 1.9) | 1.4 (1.0, 2.5) | 1.1 (0.8, 1.7) | 0.266 | 1.4 (1.0, 2.4) | 1.45 (1.0, 2.4) | 1.4 (1.0, 2.3) | 0.065 |
| PH, median (Q1; Q3) | 7.37 (7.30, 7.43) | 7.37 (7.27, 7.44) | 7.38 (7.30, 7.43) | 0.207 | 7.36 (7.27, 7.41) | 7.37 (7.27, 7.44) | 7.35 (7.27, 7.40) | 0.009 |
| Lactate, median(Q1; Q3), mmol/L | 1.7 (1.2, 2.6) | 2 (1.3, 3.5) | 1.6 (1.2, 2.4) | 0.392 | 1.9 (1.3, 3.25) | 2 (1.3, 3.5) | 1.7 (1.2, 2.9) | 0.155 |
| WBC, median (Q1; Q3), ×109/L | 12.3 (8.4, 17) | 13.3 (8.9, 18.5) | 11.9 (8.3, 16.6) | 0.161 | 13.25 (8.85, 18.55) | 13.55 (9.45, 18.55) | 12.75 (8.35, 18.7) | 0.085 |
| Haemoglobin, median (Q1; Q3), g/dL | 10.7 (9.2, 12.1) | 10.7 (9.3, 12.4) | 10.7 (9.2, 12.1) | 0.043 | 10.9 (9.2, 12.4) | 10.85 (9.3, 12.55) | 10.7 (9.1, 12.15) | 0.007 |
| Platelet count, median (Q1; Q3), 109/L | 192 (137, 258) | 193 (136, 254) | 192 (138, 258) | 0.029 | 188 (131, 251) | 193.5 (133, 252) | 185 (130, 250) | 0.059 |
| K, median (Q1; Q3), mmol/L | 4 (3.7, 4.4) | 4.1 (3.8, 4.6) | 4 (3.7, 4.3) | 0.361 | 4.25 (3.8, 4.75) | 4.1 (3.8, 4.6) | 4.4 (4.4, 4.8) | 0.216 |
| Mg, median (Q1; Q3), mmol/L | 2.1 (1.9, 2.3) | 2.1 (1.9, 2.3) | 2.1 (1.9, 2.3) | 0.176 | 2.1 (2, 2.4) | 2.1 (1.9, 2.3) | 2.2 (2.0, 2.4) | 0.095 |
| Other factors associated with atrial fibrillation | ||||||||
| Mechanical ventilation, n (%) | 816 (58.54) | 190 (66.43) | 626 (56.50) | 0.202 | 382 (79.28) | 188 (77.05) | 194 (79.51) | 0.060 |
| Heart rate max(bpm), median (Q1; Q3)c | 139 (127, 153) | 142 (127, 157) | 139 (127, 152) | 0.094 | 140 (128, 154) | 144 (128, 159) | 138 (126, 151) | 0.219 |
| Shock, n (%) | 187 (13.41) | 105 (36.71) | 82 (7.4) | 0.917 | 178 (36.48) | 100 (40.98) | 78 (31.97) | 0.188 |
| RRT, n (%) | 39 (2.80) | 20 (6.99) | 19 (1.71) | 0.419 | 35 (7.17) | 20 (8.20) | 15 (6.15) | 0.037 |
| Digoxinb, n (%) | 218 (15.64) | 44 (15.38) | 174 (15.70) | 0.009 | 78 (15.98) | 39 (15.98) | 39 (15.98) | 0.000 |
| Diltiazemb, n (%) | 459 (32.93) | 39 (13.64) | 420 (37.91) | 0.528 | 104 (21.31) | 31 (12.70) | 73 (29.92) | 0.429 |
| Antiplateleta, n (%) | 181 (9.91) | 35 (12.24) | 93 (8.39) | 0.065 | 66 (13.52) | 31 (12.70) | 35 (14.34) | 0.048 |
| Anticoagulationa, n (%) | 257 (18.44) | 47 (16.43) | 210 (18.95) | 0.133 | 64 (13.11) | 34 (13.93) | 30 (12.30) | 0.048 |
ICU: ICU intensive care unit, Q1 first quartile, Q3 third quartile, BMI: body mass index, APS: acute physiology score, SOFA: Sequential Organ Failure Assessment, PaO2: partial pressure of arterial oxygen, FiO2: fraction of inspired oxygen, LMDH: low molecular weight heparin, ACEI: Angiotensin-Converting Enzyme Inhibitor, ARB: Angiotensin II Receptor Blocker.
Comorbidities: data extraction by ICD code.
Laboratory data: first data after ICU admission.
K, Mg: nearest data to the first AF recorded, within 24&#¿;h of the first AF recorded.
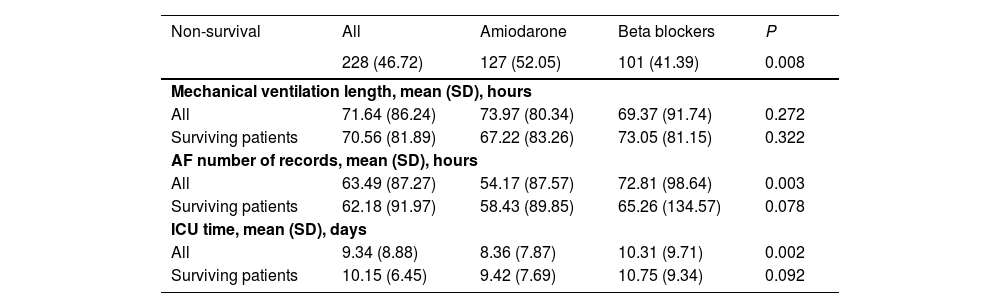
The Cox proportional hazard model was used to examine the mortality between the two groups. After PSM and IPTW, the imbalance in the covariates between the amiodarone group and the BB group was significantly minimized (Supplementary File 2, Fig. S1). In the matched cohort, patients who received a BB had a significantly lower in-hospital mortality compared with those in the amiodarone group, with statistically significant differences and an adjusted HR of 0.70 (95% CI 0.54–0.91, P&#¿;=&#¿;0.008). The primary are detailed in Table 2.
Primary and secondary outcomes.
| Non-survival | All | Amiodarone | Beta blockers | P |
|---|---|---|---|---|
| 228 (46.72) | 127 (52.05) | 101 (41.39) | 0.008 | |
| Mechanical ventilation length, mean (SD), hours | ||||
| All | 71.64 (86.24) | 73.97 (80.34) | 69.37 (91.74) | 0.272 |
| Surviving patients | 70.56 (81.89) | 67.22 (83.26) | 73.05 (81.15) | 0.322 |
| AF number of records, mean (SD), hours | ||||
| All | 63.49 (87.27) | 54.17 (87.57) | 72.81 (98.64) | 0.003 |
| Surviving patients | 62.18 (91.97) | 58.43 (89.85) | 65.26 (134.57) | 0.078 |
| ICU time, mean (SD), days | ||||
| All | 9.34 (8.88) | 8.36 (7.87) | 10.31 (9.71) | 0.002 |
| Surviving patients | 10.15 (6.45) | 9.42 (7.69) | 10.75 (9.34) | 0.092 |
In the matched cohort, patients in the BB group had significantly longer mean ICU stay (10.31 days vs 8.36 days, P&#¿;<&#¿;0.001), and number of AF episodes (72.81&#¿;h vs 54.17&#¿;h, P&#¿;=&#¿;0.003). Patients in the amiodarone group received RRT (15.67% vs 7.97%, P&#¿;<&#¿;0.001). However, the amiodarone group exhibited longer ventilator-free days compared to the BB group (73.97&#¿;h vs 69.37&#¿;h, P&#¿;=&#¿;0.272); however, the difference was not statistically significant. The secondary outcomes are detailed in Table 2.
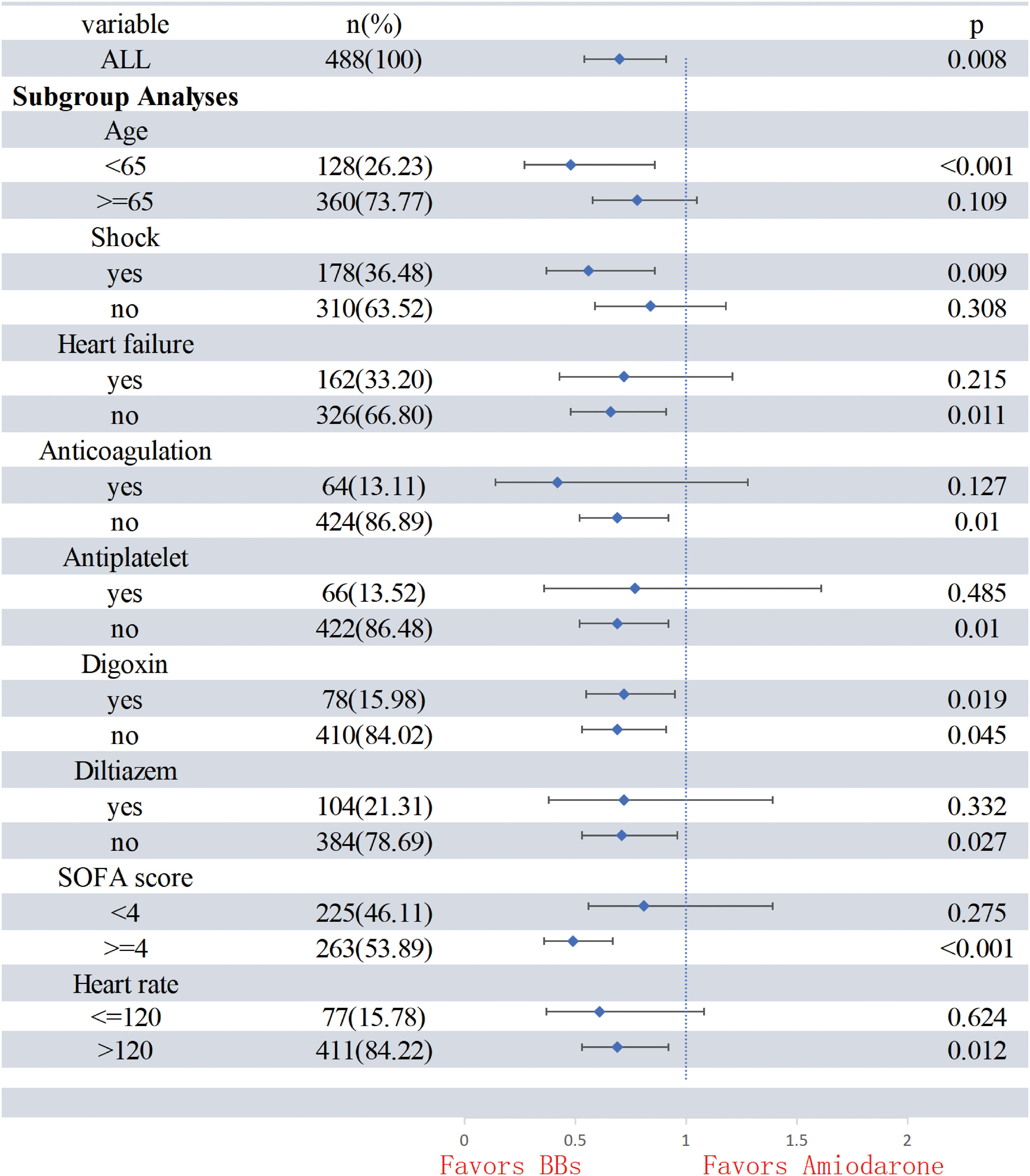
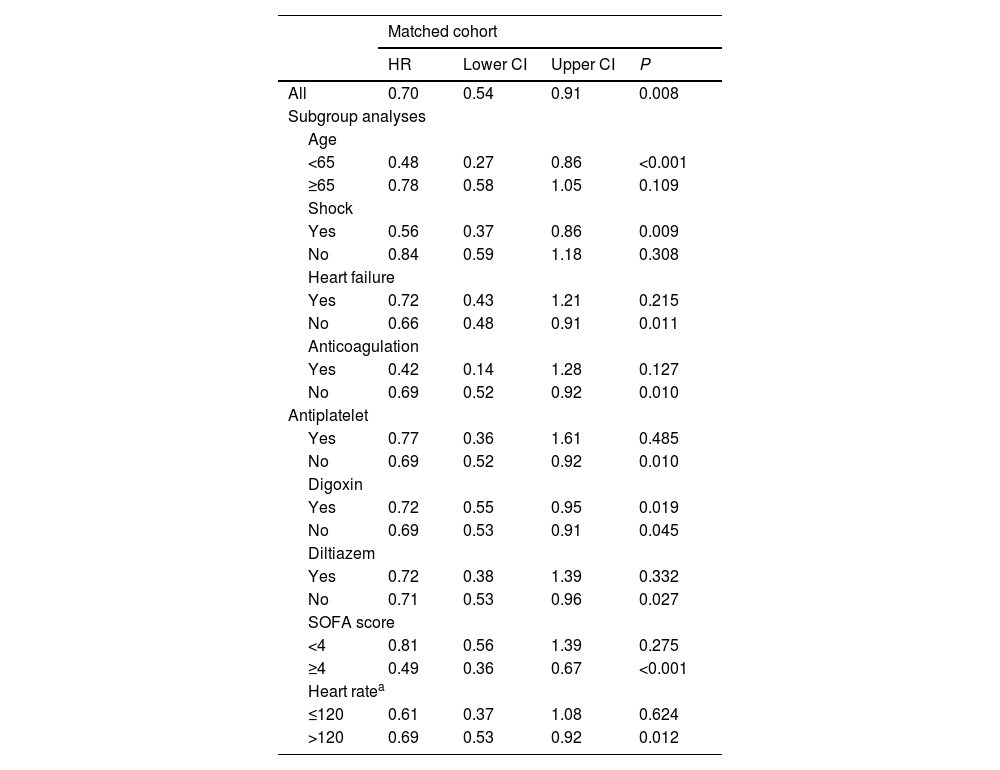
Subgroup analysisPropensity-adjusted analyses showed that patients in the BB group had lower in-hospital mortality in the following subgroups: (1) age <65 years, SOFA >4 and heart rate >120&#¿;bpm; (2) patients on vasopressors; and (3) patients without heart failure. However, the outcome was not observed in following subgroups: (1) patients with a heart rate <120&#¿;bpm, heart failure; (2) patients in whom diltiazem, antiplatelet and anticoagulation. The subgroup analysis is detailed in Table 3 and Fig. 2.
Subgroup analyses.
| Matched cohort | ||||
|---|---|---|---|---|
| HR | Lower CI | Upper CI | P | |
| All | 0.70 | 0.54 | 0.91 | 0.008 |
| Subgroup analyses | ||||
| Age | ||||
| <65 | 0.48 | 0.27 | 0.86 | <0.001 |
| ≥65 | 0.78 | 0.58 | 1.05 | 0.109 |
| Shock | ||||
| Yes | 0.56 | 0.37 | 0.86 | 0.009 |
| No | 0.84 | 0.59 | 1.18 | 0.308 |
| Heart failure | ||||
| Yes | 0.72 | 0.43 | 1.21 | 0.215 |
| No | 0.66 | 0.48 | 0.91 | 0.011 |
| Anticoagulation | ||||
| Yes | 0.42 | 0.14 | 1.28 | 0.127 |
| No | 0.69 | 0.52 | 0.92 | 0.010 |
| Antiplatelet | ||||
| Yes | 0.77 | 0.36 | 1.61 | 0.485 |
| No | 0.69 | 0.52 | 0.92 | 0.010 |
| Digoxin | ||||
| Yes | 0.72 | 0.55 | 0.95 | 0.019 |
| No | 0.69 | 0.53 | 0.91 | 0.045 |
| Diltiazem | ||||
| Yes | 0.72 | 0.38 | 1.39 | 0.332 |
| No | 0.71 | 0.53 | 0.96 | 0.027 |
| SOFA score | ||||
| <4 | 0.81 | 0.56 | 1.39 | 0.275 |
| ≥4 | 0.49 | 0.36 | 0.67 | <0.001 |
| Heart ratea | ||||
| ≤120 | 0.61 | 0.37 | 1.08 | 0.624 |
| >120 | 0.69 | 0.53 | 0.92 | 0.012 |
SOFA: sepsis-related organ failure assessment; HR: hazard ratio.
There is a lack of high-quality studies comparing the efficacy of amiodarone and BB in septic patients with NOAF in the ICU. Our study showed that BB use was associated with lower in-hospital mortality compared with amiodarone in septic patients with NOAF. But patients who received amiodarone had shorter during of AF. These results may be related to the different pharmacological mechanisms.
Our study revealed that prescription of a BB was significantly associated with lower in-hospital mortality compared with amiodarone. Consistent with our study, a previous retrospective study found that BBs appeared to lower in-hospital mortality compared with amiodarone.7 In critically ill patients with sepsis and NOAF, the lower mortality risk with BB could be explained by several mechanisms. In the management of AF in hospitalized patients, it is crucial to prioritize the identification and treatment of potential triggers. This is because attempts at rate and rhythm control may be less effective until the acute illness improves.12 During sepsis, elevated levels of circulating catecholamines predispose to AF by triggering electrical remodeling and also increase atrioventricular node conduction which leads to a rapid ventricular response.13 This leads to reduced diastolic filling time and an elevated risk of hemodynamic compromise. BBs exert their sympathetic blocking effects primarily by antagonizing beta1 receptors, resulting in reduced AV node conduction and reduced catecholamine effects on the myocardium.13 Administration of BBs can stabilize hemodynamics by enhancing ventricular filling, thereby improving cardiac output and the patient's prognosis. Notably, the use of BBs during sepsis also enhances arterial elasticity and ventricular-arterial coupling, potentially addressing another possible cause of mortality.14,15
Amiodarone is a class III antiarrhythmic drug that is widely available in the ICU. It is a complex iodinated compound that, along with its active metabolite N-desethylamiodarone, blocks IKr, INa, IKur, Ito, ICaL, IKAch, and If channels with noncompetitive antagonism of alpha and beta-receptors.16 Our study similarly showed that the amiodarone group had shorter AF recording times. Nonetheless amiodarone is distinguished by a long half-life of weeks that can lead to numerous side effects.
Several studies have shown the efficacy of amiodarone in the management of atrial fibrillation in critically ill patients, but have not explained this potentially serious complication.6 Although amiodarone rapidly terminates atrial fibrillation, the long metabolism time of amiodarone may contribute to the poor prognosis. Serum amiodarone accumulation frequently impacts the thyroid, lungs, and liver. There are also reports of amiodarone-associated acute bleeding,17 which may be related to the two factors: first, amiodarone hinders the activity of numerous Cytochrome P450(CYP) isozymes involved in the metabolism of aspirin and other anticoagulant drugs; second, serum amiodarone can accumulate in the liver affecting the function of the liver, an important coagulation factor synthesizing organ.18,19 However, the full impact of these findings on ICU patient care requires more extensive research.
To examine the risk in specific types of patients, we explored effect modification by age, heart failure, anticoagulation, antiplatelet, SOFA score, heart rate, septic shock, digoxin, diltiazem, and heart rate. In the subgroup of patients with septic shock, BBs had lower in-hospital mortality compared with patients of the amiodarone group. Similarly, a recent study proved the hemodynamic safety of the betablocker-derivative propafenone in patients with septic shock with AF, and the lower hospital mortality compared with patients prescribed amiodarone (36.5% vs 41%). It suggested that within a certain range of shock, BBs do not worsen a patient’s hemodynamics, this finding may indicate that is safe using BB in these group of patients.20 Due to the inability to accurately obtain information related to the dosage of vasoactive drugs, we did not conduct further analysis, considering the side effects of BBs, its use in patients with severe septic shock still needs to be carefully considered, which requires more clinical studies to prove its safety.21 In the heart failure subgroup, regardless of the presence of heart failure, the BB group had no significantly for in-hospital mortality. However, the pharmacokinetic advantages of BBs, particularly the ultrashort-acting agent esmolol, that allows for rapid titration and discontinuation, with a metabolism time of about 5&#¿;min and the potential for rapid recovery from drug-associated hypotension.22 This positions esmolol as a potentially valuable medication for treating acute atrial fibrillation in the future. Nonetheless although BBs showed an advantage in different heart rate subgroups, a statistically significant difference was noted only when heart rate exceeded 120&#¿;bpm, possibly because the fast ventricular rates associated with AF are an important contributor to poor prognosis but can be moderated by BBs in critically ill patients.23 In addition, the BB and amiodarone groups did not show statistically significant differences in the subgroups using diltiazem. This is likely because BBs and calcium channel blockers (CCBs), such as verapamil and diltiazem, have similar pharmacological effects, including on voltage calcium channels, reducing depolarization of the AV node and slowing heart rate.13
LimitationsFirst, it is difficult to balance the multiple confounding factors that are likely to affect the treatment of AF, including electrolytes, source of infection, body temperature, and positive end expiratory pressure. Despite propensity score matching to balance observed baseline characteristics between some groups, there may be unmeasured confounders that influenced the results. Second, we used nurse-recorded instances of NOAF and duration of atrial fibrillation: these nurse-recorded data, although validated, still have a percentage of bias. Third, medication times were derived from the time of prescription and do not reflect the true length of medication use. In addition, our study did not consider the effects produced by drug dose.
ConclusionBB was associated with lower in-hospital mortality compared with amiodarone in septic patients with NOAF. On the other hand, amiodarone group had a shorter duration of atrial fibrillation. There was no significant difference in ventilator-free days between the BB group and the amiodarone group. The risk-benefit balance should be taken into consideration based on different populations when clinicians choose between these two medications.
CRediT authorship contribution statementG.H., H.L. and F.S. contributed equally to this work. W.J. and B.H. designed the study. G.H. extracted the data from the MIMIC-&#¿; Database. H.L., C.Z., M.J., C.H. and Y.Z. participated in processing the data and doing the statistical analysis. W.J., G.H., and F.S. wrote the manuscript. B.H. planned the analyses and guided the literature review. All authors read and approved the final version of the manuscript.
Consent for publicationNot applicable.
Ethics approval and consent to participateThe establishment of this database was approved by the Massachusetts Institute of Technology (Cambridge, MA) and Beth Israel Deaconess Medical Center (Boston, MA), and consent was obtained for the original data collection. Therefore, the need for informed consent were waived for this manuscript. This study was reviewed and approved by the Guangdong Provincial People’s Hospital Ethics Committee (No.KY2023-881-01).
FundingThe authors received financial support for the research, authorship, and/or publication of this article from the National Key Research and Development Program intergovernmental key projects (2023YFE0114300), Zhongnanshan Medical Foundation of Guangdong Province (ZNSXS-20240052), Natural Science Foundation of Guangdong (2022A1515012428, 2024A1515011329), the Peking Union Medical Foundation-Ruiyi Emergency Medical Research Fund (R2021002) and the China International Medical Foundation-Clinical Development Research Fund (Z-2018–31-2102–2). The funding agents had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We thank Professor Chunquan Ou, Department of Biostatistics, School of Public Health, Southern Medical University, for her assistance in the preparation of this manuscript for statistical analysis.
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.