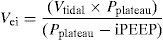COPD and asthmatic patients use a substantial proportion of mechanical ventilation in the ICU, and their overall mortality with ventilatory support can be significant. From the pathophysiological standpoint, they have increased airway resistance, pulmonary hyperinflation, and high pulmonary dead space, leading to increased work of breathing. If ventilatory demand exceeds work output of the respiratory muscles, acute respiratory failure follows.
The main goal of mechanical ventilation in these kinds of patients is to improve pulmonary gas exchange and to allow for sufficient rest of compromised respiratory muscles to recover from the fatigued state. The current evidence supports the use of noninvasive positive-pressure ventilation for these patients (especially in COPD), but invasive ventilation also is required frequently in patients who have more severe disease. The physician must be cautious to avoid complications related to mechanical ventilation during ventilatory support. One major cause of the morbidity and mortality arising during mechanical ventilation in these patients is excessive dynamic pulmonary hyperinflation (DH) with intrinsic positive end-expiratory pressure (intrinsic PEEP or auto-PEEP). The purpose of this article is to provide a concise update of the most relevant aspects for the optimal ventilatory management in these patients.
Los pacientes con EPOC y asmáticos utilizan una proporción sustancial de ventilación mecánica en la UCI, y su mortalidad global en tratamiento con ventilación mecánica puede ser significativa. Desde el punto de vista fisiopatológico muestran un incremento de la resistencia de la vía aérea, hiperinsuflación pulmonar, y elevado espacio muerto anatómico, lo que conduce a un mayor trabajo respiratorio. Si la demanda ventilatoria sobrepasa a la capacidad de la musculatura respiratoria, se producirá el fracaso respiratorio agudo.
El principal objetivo de la ventilación mecánica en este tipo de pacientes es proporcionar una mejora en el intercambio gaseoso así como el suficiente descanso para la musculatura respiratoria tras un período de agotamiento. La evidencia actual apoya el uso de la ventilación mecánica no invasiva en estos pacientes (especialmente en el EPOC), pero con frecuencia se precisa de la ventilación mecánica invasiva para los pacientes con enfermedad más severa. El clínico debe ser muy cauto de cara a evitar complicaciones relacionadas con la ventilación mecánica durante el soporte ventilatorio. Una causa mayor de morbilidad y mortalidad en estos pacientes es la excesiva hiperinsuflación dinámica pulmonar con presión positiva al final de la espiración (PEEP intrínseca o auto-PEEP). El objetivo de este artículo es proporcionar una concisa actualización de los aspectos más relevantes para el óptimo manejo ventilatorio en estos pacientes.
Chronic obstructive airway disease includes not only chronic obstructive pulmonary disease (COPD) and chronic asthma but also disorders such as bronchiectasis, pneumoconiosis and post-tuberculosis lung disease. COPD is a major health and economical problem of growing prevalence; in the year 1990 it represented the sixth most common cause of deaths worldwide, and by 2020 the disease is expected to become the third most common cause of death. Mortality associated to asthma is also considerable, with an estimated 1–8 deaths per 100,000 inhabitants and year. Adequate triage or screening for admission to the Intensive Care Unit (ICU) is therefore essential among patients at risk.1
Although a general indication for almost all conditions, patients of this kind should not receive invasive mechanical ventilatory support, since mortality increases significantly as a result. However, the failure of noninvasive mechanical ventilation (effective in at least 75% of the cases, particularly in COPD), or the prescription of surgery, can lead to a fatal outcome. Heterogeneity and the presence of mixed variants are the rule in patients of this kind, with the frequent combination of physiopathological characteristics of asthma (inflammation of the bronchial tree and bronchospasm), emphysema (loss of parenchymal compliance) and chronic bronchitis (presence of mucosal hypersecretion). The adoption of rigid general strategies for the ventilation of these patients therefore can lead to dangerous simplifications in management approach. Moreover, it must be remembered that patients with emphysema and chronic bronchitis typically have serious comorbidities that add further elements to be taken into account when planning treatment—our objective being to improve gas exchange and ensure recovery of the respiratory muscles. In addition to ventilatory support, immediate medical treatment should be started (Table 1). Oxygen must be administered to correct the hypoxemia in acute asthma, as well as in COPD—though in this case it is essential to avoid the increase in pCO2 and consequent hypercapnic narcosis; the correct titration of FiO2 is therefore essential,2 making sure that SaO2 does not exceed 90%. If worsening of the respiratory acidosis occurs despite these measures, then ventilation support is clearly indicated.3 Once such support has been started, a differential diagnosis should be established among the possible causes of respiratory failure (e.g., pulmonary thromboembolism, pneumothorax, and upper airway obstruction), with identification of the etiological factor triggering the episode. It should be remembered that heart failure (particularly right-side failure) is often present in decompensated COPD.
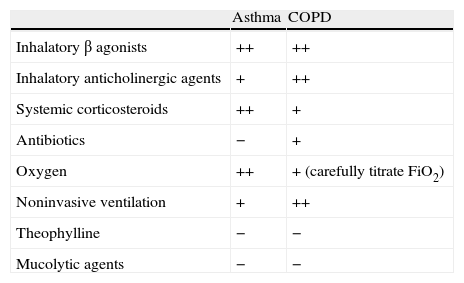
Medical treatment in asthma and COPD.
| Asthma | COPD | |
| Inhalatory β agonists | ++ | ++ |
| Inhalatory anticholinergic agents | + | ++ |
| Systemic corticosteroids | ++ | + |
| Antibiotics | − | + |
| Oxygen | ++ | + (carefully titrate FiO2) |
| Noninvasive ventilation | + | ++ |
| Theophylline | − | − |
| Mucolytic agents | − | − |
++: good level of evidence; +: relatively good level of evidence; −: without evidence.
No system has been developed allowing prediction of the survival or success/failure rate in the weaning of patients with COPD exacerbation. Thus, unless we have prior written patient rejection of intubation, ventilation support is to be provided when necessary. Respiratory arrest constitutes the terminal event of COPD and asthma, and is preceded by certain warning signs: lethargy, obnubilation, auscultation silence, bradycardia and hypotension. However, it is difficult to determine the severity of the process before arrest occurs; the medical history, clinical findings and initial response to treatment are therefore useful:
- –
The medical history will define the prognosis in acute COPD—fundamental considerations being the basal condition of the patient (evaluated by spirometry) and the presence of significant comorbidities. In asthma, antecedents of mechanical ventilation are indicative of a poor prognosis, in the same way as deterioration despite optimum treatment including oral corticosteroids and beta-agonists. It is important to point out that this latter disease can manifest gradually, with a slow and insufficient response to medical treatment and with important airway secretions and inflammation, or alternatively in a sudden manner, with a predominance of bronchospasm and progression towards respiratory arrest in a matter of minutes or hours (in rare cases).4
- –
The clinical findings in COPD (drowsiness, intercostal space retraction, paradoxical breathing, etc.) are fundamental for patient stratification, and respiratory acidosis is the best predictor of patient exhaustion. Arterial blood gas determinations are essential, as well as comparison with previous samples. Clinical signs of severe asthma are taken to be tachycardia, tachypnea, paradoxical pulse, incapacity to end a phrase in a single breath, and peak expiratory flow rate (PEFR)<50% of the theoretical normal or best normal value (<200l/min if not known). Signs of life-threatening situations are auscultation silence, cyanosis, confusion or coma, bradycardia and hypotension, and a non-quantifiable PEFR. It must be remembered that the measurement of PEFR may prove dangerous in severe and sudden onset asthma; although fatal worsening of bronchospasm has been described, it is very useful for assessing the efficacy of treatment once the acute phase has been passed.
- –
The initial response to treatment in asthma patients is an important prognostic factor. If the patient does not improve clinically, physiologically (blood gases) and mechanically (PEFR) within 1–2h, ventilation support should be considered, and it is essential to repeat evaluation of the patient (initially every hour).5 In COPD, the prognostic significance is not the same as in asthma, since the response to treatment is slower. These concepts are summarized in Table 2.
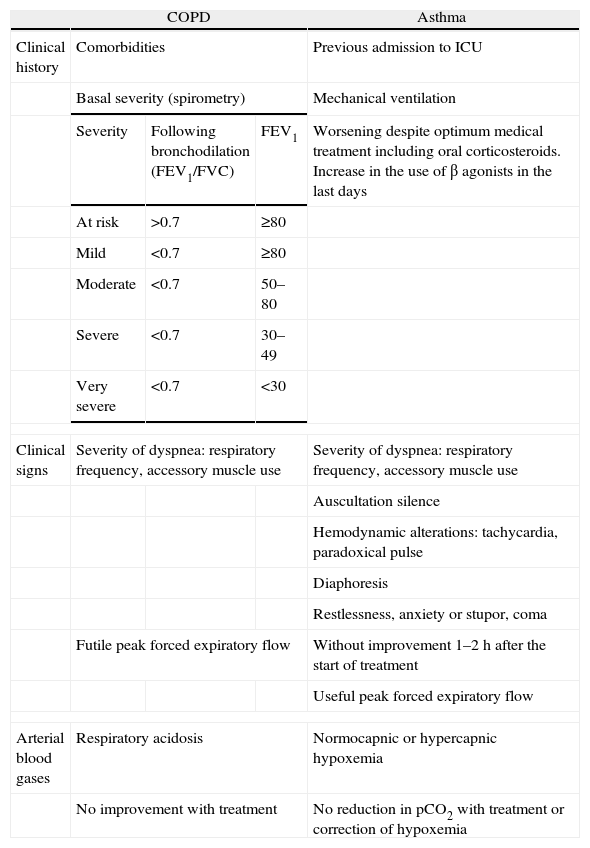
Table 2.Grading of the severity-risk association in respiratory failure.
COPD Asthma Clinical history Comorbidities Previous admission to ICU Basal severity (spirometry) Mechanical ventilation Severity Following bronchodilation (FEV1/FVC) FEV1 Worsening despite optimum medical treatment including oral corticosteroids. Increase in the use of β agonists in the last days At risk >0.7 ≥80 Mild <0.7 ≥80 Moderate <0.7 50–80 Severe <0.7 30–49 Very severe <0.7 <30 Clinical signs Severity of dyspnea: respiratory frequency, accessory muscle use Severity of dyspnea: respiratory frequency, accessory muscle use Auscultation silence Hemodynamic alterations: tachycardia, paradoxical pulse Diaphoresis Restlessness, anxiety or stupor, coma Futile peak forced expiratory flow Without improvement 1–2h after the start of treatment Useful peak forced expiratory flow Arterial blood gases Respiratory acidosis Normocapnic or hypercapnic hypoxemia No improvement with treatment No reduction in pCO2 with treatment or correction of hypoxemia FVC: forced vital capacity; FEV1: forced expiratory volume in 1s.
Patients of this kind present a series of coinciding factors, such as increased airway resistance (due fundamentally to edema, secretions, bronchospasm and collapse), pulmonary hyperinsufflation (reduction of lung and chest wall compliance), and a large physiological dead space—with the consequent increase in respiratory effort. If the ventilatory demand exceeds the capacity of the respiratory muscles, acute respiratory failure results.6 Although the lung mechanics are similar in both conditions, there are some physiopathological differences that should be mentioned. In effect, COPD is characterized by greater airway collapse, due fundamentally to destruction of the lung parenchyma (particularly in emphysema), as well as by a loss of elastic rebound of the lungs,7 while asthma is characterized by hypertrophy of the airway walls secondary to inflammation, lesser airway collapse despite the considerable decrease in airway caliber (central involvement versus peripheral involvement in COPD), and generally reversible obstruction—which can be minimal or lacking in long-evolving asthma.8 Likewise, and particularly in the asthmatic lung, the non-uniform distribution of the disease is a typical feature, together with important variability in the pulmonary distribution of obstruction within one same individual, who may experience substantial changes in a matter of minutes. It is necessary to take this element into account when selecting the ventilatory mode (volume control in favor of pressure control, fundamentally),9 since its consequences may be: (1) abnormal distribution of the ventilation/perfusion ratio due to the redistribution of blood towards normally ventilated zones; (2) increased risk of barotrauma; and (3) venous return difficulties with hemodynamic alterations, shock and cardiorespiratory arrest.10 Furthermore, a reduction in the diameter of the glottis during expiration may be added to all these conditions.11 It must be mentioned that in contrast to patients with COPD, patients with bronchial asthma typically present high peak inspiratory pressures (as a result of which the presence of pneumothorax, mucus plugs or acute lung edema should always be suspected), and usually require greater ventilation support to correct or improve their pH.
The limitation of airflow influences the efficacy of the respiratory mechanics in the following ways: (1) it increases the variation in pressure needed to overcome airway resistance for a given flow; (2) it causes hyperinsufflation, which partially counters the increase in resistance by expanding the airway diameter but also reduces the compliance of the system; (3) the hyperinsufflation (and destruction of the lung parenchyma) increases the ventilatory dead space, and therefore, also the minute-volume required for correct alveolar ventilation; (4) auto-PEEP or intrinsic PEEP (iPEEP) causes greater respiratory effort for activating the respiratory trigger; and (5) muscle capacity to cope with the increase in respiratory effort is altered (alteration of chest wall-diaphragm geometry, metabolic alteration due to hypoxemia-acidosis, cachexia in the COPD patient, etc.).
Mechanical Ventilation in COPD and AsthmaOnce the respiratory muscles are no longer able to meet the ventilatory demand, the patient gradually becomes exhausted. This is the point where ventilation support is indicated in order to disrupt the vicious circle. The purpose of mechanical ventilation is to offer some rest to the respiratory muscles until treatment is started to deal with the cause of the exacerbation and revert the bronchial obstruction.
In severe COPD exacerbation with respiratory acidosis, it is generally accepted that ventilation support initially must be provided on a noninvasive basis, as this has been shown to reduce the intubation rate, the duration of stay in intensive care, and patient mortality. Only when this measure fails (with exceptions, e.g., the performance of surgery) is patient intubation indicated.12 Conclusions based on a sufficient level of evidence have not been drawn in the case of asthma. Although there are no clearly defined criteria for starting such support in COPD and asthma, the indications could comprise at least two of the following conditions: (1) moderate–severe dyspnea, with use of the accessory muscles and abdominal breathing; (2) hypercapnic acidosis (pH<7.35); and (3) tachypnea (>25rpm). The most widely employed ventilatory modes are supportive pressure with PEEP and the BIPAP modes if the respiratory stimulus proves insufficient.13–16
There are no clearly defined criteria for the start of invasive mechanical ventilation in COPD and asthma. In COPD, the most widely adopted approach is patient intubation if noninvasive mechanical ventilation fails after 1h (clinical and blood gas worsening). In asthma, intubation should not be delayed for too long (always based on clinical criteria, e.g., diminished consciousness and respiratory frequency), since the main objective of mechanical ventilation is to maintain oxygenation and avoid respiratory arrest. Once the decision to intubate has been taken, full control of patient cardiorespiratory arrest is the priority concern, and the technique should be carried out by the clinician with the greatest experience—using the largest orotracheal tube diameter possible in order to reduce the resistances and optimize clearance of the secretions.17
The factors associated to ventilator-induced lung damage to be considered and avoided when initially programming the machine are volutrauma/barotrauma, atelectrauma and oxygen therapy-related toxicity (FiO2>0.5–0.6). In this sense it is important to underscore the concept of controlled hypoventilation as a general ventilatory strategy in application to both diseases – limiting the objectives to maintaining the oxygenation and minute-volume essential for avoiding severe acidosis. To this effect it is necessary to reach deep sedation, and (particularly in the case of asthma) the administration of short half-life neuromuscular relaxants entails a risk of myopathy in these patients (particularly when on corticosteroids); bolus dose administration with train of four (TOF) monitoring is therefore preferable.18
The initially programmed ventilator parameters can be very similar in both diseases, and we must choose a controlled or passive condition mode (there being no consensus regarding the concrete ventilatory mode)—attempting to prolong the expiratory time as far as possible by means of a low respiratory frequency (10–14rpm) and I:E ratio, in order to minimize iPEEP and air trapping. The general strategy is to combine a relatively low minute-volume (<115ml/kg) with a high inspiratory flow (80–100l/min) in order to ensure a short inspiratory time and therefore a low I:E ratio.19 No benefit has been demonstrated for an expiratory time of longer than 4s.20
Ventilation should be sufficient to maintain pH>7.15, with a low volume tidal (5–8ml/kg) in order to avoid plateau pressures>30mmHg. The indication of bicarbonate to resolve respiratory acidosis is questionable to say the least, and should be accompanied by caution—particularly if the patient suffers hemodynamic instability or intracranial hypertension. The guiding parameter is pH, not pCO2, avoiding alkalemia, particularly in chronic retainers.21 Adjustment of the trigger is essential for both diseases, usually adjusting by −1 to 2cmH2O for pressure and 2l/min for flow. Too sensitive a trigger will activate more cycles than necessary, giving rise to respiratory alkalosis, while too “hard” a trigger will increase respiratory effort. The benefits of flow versus pressure trigger are clear in the intermittent mandatory ventilation (IMV) mode, not so decisive in the pressure support ventilation (PSV) mode, and without differences in the rest of ventilatory modes.22 Titrating FiO2 not only serves to reduce toxicity associated to high oxygen concentrations but in the case of COPD also reduces the degree of respiratory center suppression. In this case FiO2 should be titrated to reach SatO2≥88%.
Dynamic Hyperinsufflation and Auto-PEEPDynamic hyperinsufflation appears when the pulmonary volume at the end of expiration is greater than the functional residual capacity (FRC) as a consequence of insufficient emptying of the lung, by starting inspiration before completing the preceding expiration.23 A vicious circle is created, and if the latter is not resolved, the patient gradually becomes exhausted until cardiovascular and respiratory collapse occurs in a way similar to that produced by tension pneumothorax. The only measure that has been shown to predict the complications of hyperinsufflation is the determination of end-inspiratory volume (Vei) over functional residual capacity, calculating the total volume of gas exhaled in a patient with muscle paralysis after 60s of apnea.24 In this context, Vei>20ml/kg is considered to be predictive of complications such as hypotension and barotrauma. Assuming that the compliance of the respiratory system remains constant, Vei could be calculated from the following formula, though the latter has not yet been validated:
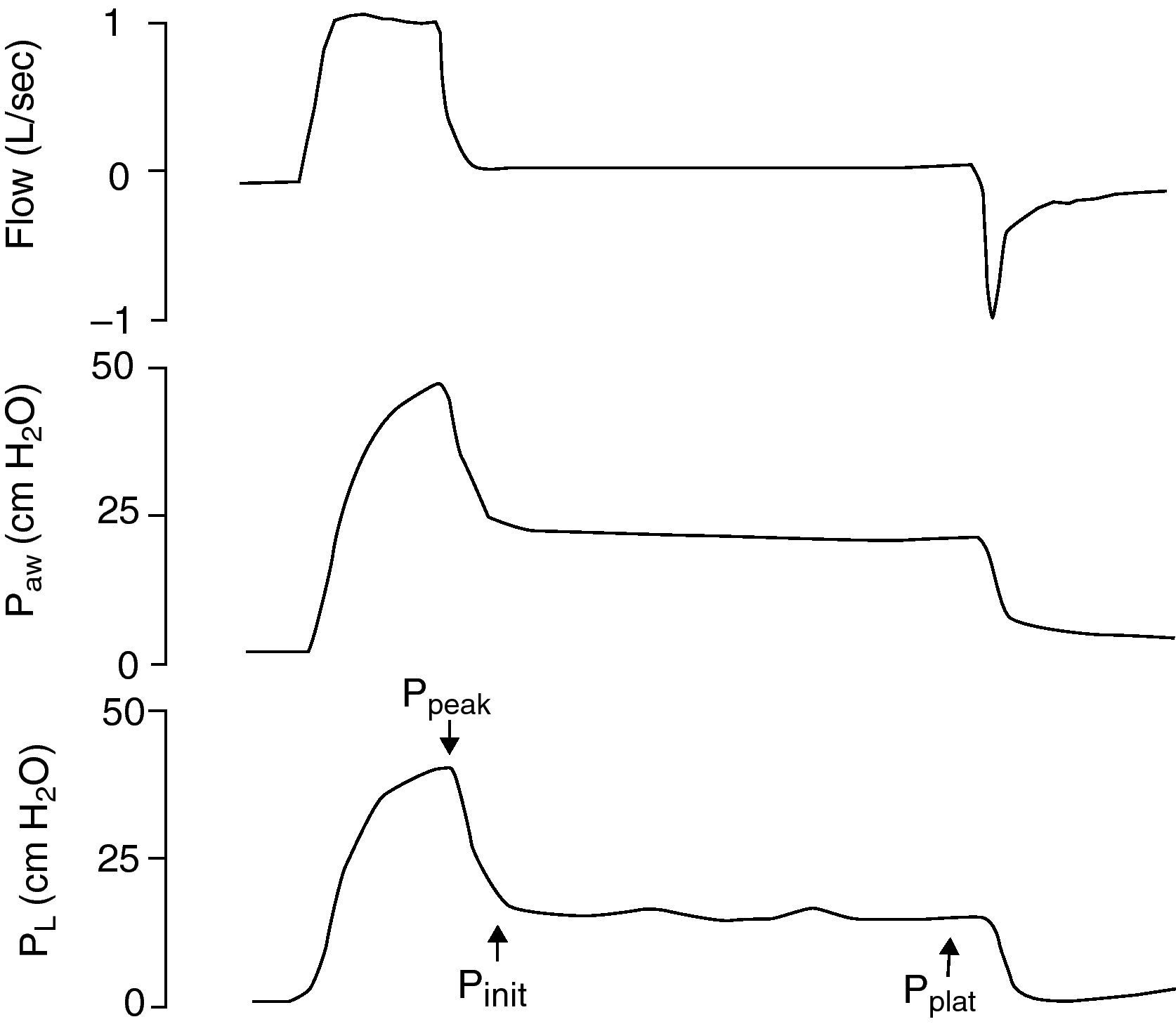
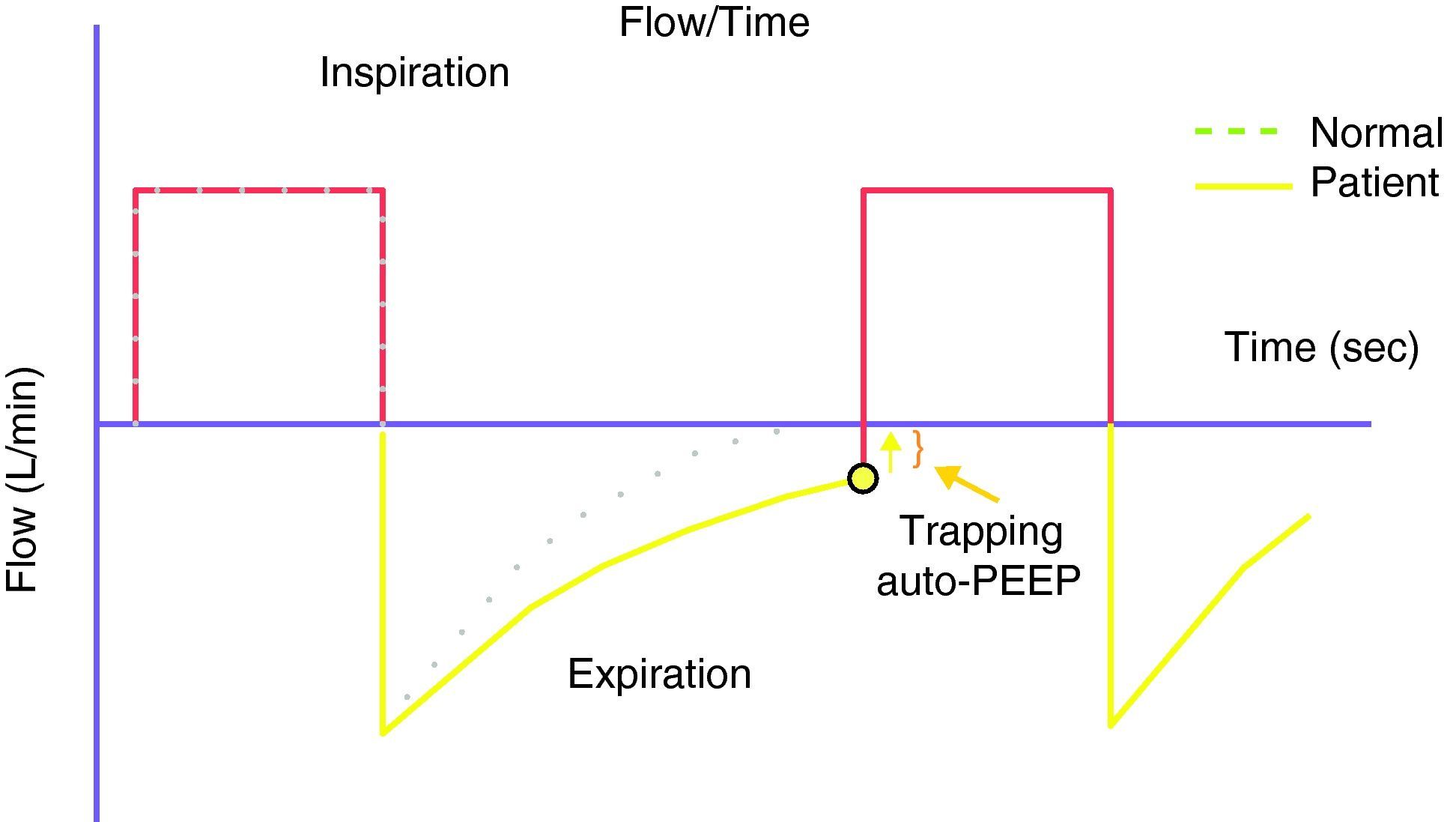
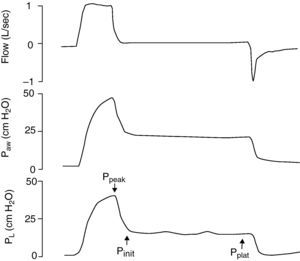
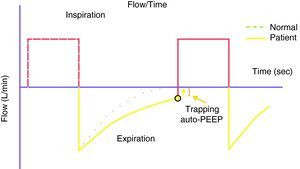
Because of the limitations in calculating Vei in clinical practice, iPEEP and Pplateau are used in its place, since both increase in situations of dynamic hyperinsufflation. Ppeak also increases, but lacks clinical value, since it increases with the rise in airway resistance and high inspiratory flows—with dissipation upon reaching the alveolus. iPEEP is defined as the spontaneous appearance of positive pressure at the end of expiration at alveolar level, due to insufficient expiratory time, and may appear with or without airflow limitation. The former group would include patients with asthma/bronchospasm and COPD, while in the absence of an increase in airway resistance a shortening of expiratory time due to disproportionate tachypnea can generate auto-PEEP (e.g., metabolic acidosis with compensatory hyperventilation, and primary respiratory alkalosis). It is the lowest mean alveolar pressure reached during the respiratory cycle, and is best measured during the sustained expiratory valve occlusion maneuver in the relaxed (not necessarily paralyzed) patient.25 During this maneuver there is no flow in the airway, and a pressure balance is found in the system, with determination of the mean end-expiratory alveolar pressure (Fig. 1). The presence of auto-PEEP can also be detected when the inspiratory efforts of the patient for activating the trigger are excessive or even ineffective26; when active expiration continues at the start of the next inspiration; and, using the flow-time plot of the ventilator, when expiratory airflow persists at the end of inspiration (Fig. 2).27 It can also be detected by applying successive increments of 3cmH2O of extrinsic PEEP, since at the point when the plateau and peak pressures increase, extrinsic PEEP will have exceeded auto-PEEP.7The calculation of iPEEP in a spontaneously breathing patient is much more complex and difficult to extend to clinical practice, due to the frequent contribution of the respiratory muscles during active expiration. Through the determination of esophageal pressure (Pesoph), an approximation can be obtained via the negative inflexion of Pesoph immediately before activating the trigger in each inspiration. The existence of hyperinsufflation is suspected, since inspiration begins before the expiratory flow returns to zero. The value calculated by this procedure is usually lower than that confirmed from sustained expiration on detecting the lowest iPEEP value, while the second yields the mean value.28 There is no iPEEP value predictive of barotrauma, though successive and increasing determinations of iPEEP reflect growing hyperinsufflation. There is general agreement that in order to minimize the risk of volutrauma and barotrauma, Pplateau must be kept below 30mmHg.15,16,29,30
The application of extrinsic or external PEEP (ePEEP) should be made whenever there is iPEEP, with the purpose of reducing the efforts for activating the trigger. The attempts to exceed iPEEP constitute one of the main causes of respiratory failure in COPD exacerbation, and moreover represent a major cause of failure in the weaning of patients with airflow obstruction. ePEEP can be useful for countering iPEEP. However, ePEEP should not be applied to patients with COPD while under controlled ventilatory modes with the purpose of maximizing exhalation and of preventing regional overdistension—reserving it for when the assisted mode is resumed, to thus reduce the patient triggering effort. ePEEP should not be used in severe asthma.31 It is not easy to calculate the adequate amount of ePEEP to be administered. Taking into account the waterfall principle, equaling ePEEP to iPEEP would imply a maximum decrease in inspiratory effort without the risk of hyperinsufflation.32 However, there are two reasons why ePEEP should be lower than iPEEP: (1) there may be situations characterized by the coexistence of a limitation in airflow and conditions without such limitation and (2) the distribution of iPEEP in COPD and asthma is usually not homogeneous, and in certain areas of the lung the effective iPEEP may be lower than the calculated static iPEEP. These two possibilities may give rise to an increase in total PEEP if ePEEP=iPEEP, with the consequent barotrauma. Although adequate scientific evidence is lacking, there is an agreement that ePEEP should be 80% of iPEEP.
If the above were not enough, there is still another problem: iPEEP varies in relation to the respiratory parameters (respiratory frequency and ventilation), the position of the patient, and the changes in respiratory mechanics related to the course of the disease and treatment response. Therefore, the values of iPEEP measured under controlled ventilation in a relaxed patient may be very different from those calculated under spontaneous ventilation—the situation in which we wish to apply ePEEP. In clinical practice it is useful to ask the patient about his or her degree of comfort and dyspnea while we very gradually increase ePEEP, so that the patient is personally aware of the relief in effort as a result of counterbalancing of the iPEEP until hyperinflation increases (the dyspnea will be proportional to Vei, as a result of which the patient can notice the increase in hyperinsufflation). Thus, in a patient with bronchial obstruction subjected to mechanical ventilation, we must determine auto-PEEP, apply different levels of ePEEP (=iPEEP), and observe the changes in total PEEP.
The concept of cardiopulmonary interaction is intimately linked to that of dynamic hyperinsufflation, since the lungs, heart and pulmonary circulation are located in the same compartment (chest cavity), with the consequence changes in cardiac function as a result of the variations in intrapleural pressure.33 Hyperinsufflation increases the positive intrathoracic pressure, accentuating the decrease in venous return and preloading of both ventricles, and increasing the pulmonary vascular resistances and thus right ventricle post-loading. Hypotension is the most common effect of diminished preload, and is accentuated by hypovolemia. In this context, situations of electromechanical dissociation have been described34; as a result, if hyperinsufflation is suspected in a patient subjected to mechanical ventilation, it is advisable to disconnect the respirator for 20–30s. Because of the increase in right ventricular post-loading, hyperinsufflation can give rise to acute failure with dilatation of the right ventricle – causing displacement of the interventricular septum and reduction of left ventricle filling, due to the interdependence between both ventricles. The intrathoracic pressure is partially transmitted to the circulation, and therefore influences the measured values of central venous pressure and wedge pressure—a fact that must be taken into account in hemodynamic monitoring.7
Weaning from Mechanical VentilationWeaning from mechanical ventilation is one of the main challenges for the intensivist. Prospective studies35 have shown the application of protocols to accelerate the process without increasing the failure rate. The use of merely intuitive clinical criteria is insufficient for taking decisions (sensitivity 35%; specificity 79%).36
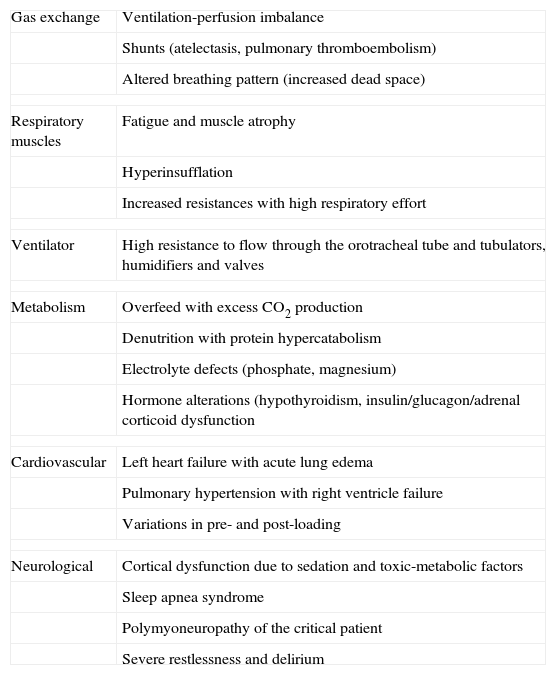
Mechanical ventilation is associated with many side effects. It is therefore advisable to start weaning as soon as possible, particularly in COPD. In order to assess the adequate moment for starting the process, due consideration is required of the possible causes of respirator dependency and of difficulties during weaning (Table 3), as well as of the factors allowing us to predict successful weaning (Table 4). Particularly in COPD and in difficult situations, the use of noninvasive mechanical ventilation is recommended after extubation, with a view to avoid the need for reintubation.37–39
Causes of respirator dependency and obstacles in weaning.
| Gas exchange | Ventilation-perfusion imbalance |
| Shunts (atelectasis, pulmonary thromboembolism) | |
| Altered breathing pattern (increased dead space) | |
| Respiratory muscles | Fatigue and muscle atrophy |
| Hyperinsufflation | |
| Increased resistances with high respiratory effort | |
| Ventilator | High resistance to flow through the orotracheal tube and tubulators, humidifiers and valves |
| Metabolism | Overfeed with excess CO2 production |
| Denutrition with protein hypercatabolism | |
| Electrolyte defects (phosphate, magnesium) | |
| Hormone alterations (hypothyroidism, insulin/glucagon/adrenal corticoid dysfunction | |
| Cardiovascular | Left heart failure with acute lung edema |
| Pulmonary hypertension with right ventricle failure | |
| Variations in pre- and post-loading | |
| Neurological | Cortical dysfunction due to sedation and toxic-metabolic factors |
| Sleep apnea syndrome | |
| Polymyoneuropathy of the critical patient | |
| Severe restlessness and delirium | |
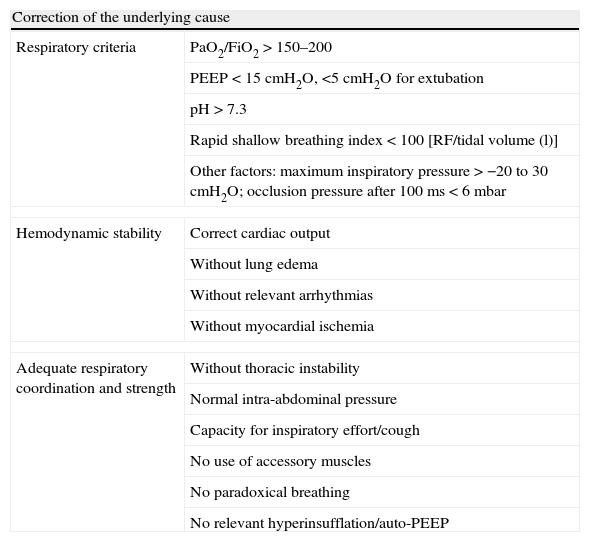
Weaning success prognostic factors.
| Correction of the underlying cause | |
| Respiratory criteria | PaO2/FiO2>150–200 |
| PEEP<15cmH2O, <5cmH2O for extubation | |
| pH>7.3 | |
| Rapid shallow breathing index<100 [RF/tidal volume (l)] | |
| Other factors: maximum inspiratory pressure>−20 to 30cmH2O; occlusion pressure after 100ms<6mbar | |
| Hemodynamic stability | Correct cardiac output |
| Without lung edema | |
| Without relevant arrhythmias | |
| Without myocardial ischemia | |
| Adequate respiratory coordination and strength | Without thoracic instability |
| Normal intra-abdominal pressure | |
| Capacity for inspiratory effort/cough | |
| No use of accessory muscles | |
| No paradoxical breathing | |
| No relevant hyperinsufflation/auto-PEEP | |
Apart from the new assisted ventilation systems offering potential advantages for these patients in the transition phase, such as proportional assisted ventilation (PAV) or the more recent neurally adjusted ventilatory assist (NAVA), a number of mechanical ventilation weaning methods are available. While the superiority of one concrete option over another has not been demonstrated, it is advisable to establish protocolized use of the selected method40:
- –
Progressive reduction of ventilatory support: FiO2, PEEP and the supportive pressure level can be gradually reduced, based on the respiratory pattern, the adequacy of gas exchange, and patient hemodynamic stability and comfort. It should be remembered that adjustment of the supportive pressure level to the respiratory frequency is dangerous, due to the great risk of over-assisting these patients and thus of promoting patient-ventilator synchrony and its complications—as has been recently demonstrated.41 It is interesting that the greater the level of assistance, the greater the incidence of patient failure to activate the trigger—probably as a result of hyperinsufflation, which can be reduced by applying ePEEP, though there is controversy in the literature regarding the application of this maneuver.42,43 In clinical practice, ineffective attempts to activate the trigger should not prevent us from continuing the weaning process, though an ePEEP addition test should be made, and it is even possible to speak with the patient to try and establish the ideal PEEP, using different levels of supportive pressure in the course of the day.
- –
Multiple daily t-tests: in the t-test the patient breathes spontaneously, and a success rate of 70% has been demonstrated after 30min of disconnection.44 It is not advisable to exceed 120min, in order to avoid fatigue, since the orotracheal tube increases breathing effort. The use of minimum supportive pressure (e.g., 5mmHg) is recommended to avoid tube resistance, though this technique has not been evaluated in any clinical trial to date.45,46 Another alternative is to use the ventilator without supportive pressure, preserving the alarm system as safety mechanism.
- –
SIMV (synchronized intermittent mandatory ventilation): the respiratory frequency and tidal volume/supportive pressure in controlled mode and supportive pressure/trigger level in spontaneous breathing are programmed in the respirator, with the gradual reduction of assistance. This method has revealed lesser efficacy in the recent literature and is now no longer recommended.40
If the weaning process fails, immediate re-evaluation of the possible causes is required (Table 3), with the adoption of specific measures47, and taking into account that with the exception of patients who are recovering from the effects of sedation, muscle relaxation, pneumothorax and atelectasis, respiratory alterations are only rarely resolved in a short period of time (e.g., several hours). The patients who do not pass the weaning test should have a rest period in a comfortable ventilation mode for at least 24h. The controlled modes are usually required to relax the patient, though use should be made of the assisted modes with high supportive pressure levels, because they allow better ventilator-patient synchrony and prevent muscle atrophy. After 24h of rest, a new weaning protocol should be reinitiated. When it becomes obvious that the patient will need long-term assisted ventilation, a tracheotomy should be considered. The impact of the timing of the latter upon the duration of mechanical ventilation remains unclear.48
Non-ventilatory Support and Management of ComplicationsDrug TreatmentAlthough in COPD the degree of reversibility of airflow obstruction is less than in asthma, the bronchodilators play an important role (representing first-choice treatment in asthma); in this context, inhalatory β2 agonists are recommended, in combination with anticholinergic drugs every 1–2h initially, followed by reduction according to the response obtained. Intravenous administration is not indicated in COPD (risk of arrhythmias, hypopotassemia, tremor and lactic acidosis), though it is accepted in asthmatics with a poor response to the inhalatory form. The usefulness of anticholinergic drugs in the asthmatic patient is less predictable than in COPD. Theophylline has shown minimal or no added efficacy, and adrenalin does not offer greater bronchodilating potency than the beta-mimetic drugs, and should not be used (either nebulized or via the systemic route), because of its many side effects. Both nebulizers and metered dose inhalers (MDIs) can be used for the administration of β2 agonists in patients subjected to mechanical ventilation, with grade A recommendation. The advantages of MDIs are their low cost, the absence of contamination, and improved dosing. The use of nebulizations is more comfortable and involves a lesser workload.49 The administration of aerosols in patients subjected to mechanical ventilation is problematic, since the medications tend to accumulate in the tubulators and in the endotracheal tube. This can be resolved by administering large amounts of bronchodilators with a nebulizer installed in the actual circuit—though the use of MDIs improves the efficiency of the procedure, and at a lesser cost. Four puffs elicit the maximum bronchodilator effect, though the technique must be correctly executed, i.e., we must ensure sufficient tidal volume (at least 500ml), an inspiratory time representing over 30% of the total cycle, correct ventilator-patient synchronization, activation of the device at the start of inspiration, and maintenance of an end-inspiratory pause of 3–5s, allowing passive expiration and repetition of the technique every 20–30s until the total dose is reached. It is worth mentioning that in the study published by Dhand et al.50, the drug dose deposited in the airway was found to be greater than with MDIs. The only study involving nebulizations in which the amount of drug deposited proved greater was carried out without a humidifier installed in the circuit.
Systemic corticosteroids have demonstrated their efficacy in severe COPD exacerbation characterized by a poor response to bronchodilators, and although there is no agreement on the dose to be administered, a regimen of 0.5mg/kg/6–8h of methylprednisolone is advised, with rapid reduction of the dose, and taking care not to exceed two weeks of treatment.51 Since inflammation of the airway wall is central to the pathogenesis of the disorder, corticosteroids constitute the first line of treatment in asthma; efficacy has been demonstrated both for hydrocortisone and for methylprednisolone, and a starting dose of the latter of 125mg/6–8h is advised in the maximum severity phase.
Regarding the use of other bronchodilators, opiates are not advised in induction and during the rest of the course—particularly morphic chloride (due to its associated histamine release and consequent bronchial constricting effect). In this sense the preferred drugs are propofol and ketamine, both of which have bronchodilating effects, though propofol causes principally hypotension (in some cases intense) and ketamine is associated with hallucinations, increased secretions, arterial and intracranial hypertension, and sometimes laryngeal spasms.52 Short-acting benzodiazepines (midazolam and lorazepam) are commonly used, though they have no bronchodilatory effects, and high doses are needed to suppress the respiratory stimulus. A number of randomized and controlled trials warrant the use of magnesium sulfate (even with normal serum magnesium concentrations) in acute severe asthma, due to its bronchodilator capacity secondary to inhibition of the calcium channels, at repeated doses of 2g/30min (maximum total dose 10g).53,54 The use of inhalatory anesthetics (halothane, isoflurane, etc.) has often been described in the literature, though they pose the inconveniences of rebound bronchospasm on interrupting administration, and the scarce flow which the anesthesia respiratory are able to deliver.55 Heliox (an 80:20 mixture of helium and oxygen) may be considered in patients in which the conventional treatments have failed, though there is no solid scientific evidence clearly supporting their use.56 Some studies have been published on the use of extracorporeal membrane oxygenation (ECMO) in severe asthma refractory to conventional treatment.57
Regarding anti-infectious treatment, it must be remembered that bronchial infections are the main cause of COPD exacerbation—a considerable number of these infections being produced by viruses (e.g., rhinovirus). Between 25 and 50% of these patients present bacterial colonization of the lower airways, associated to an increased inflammatory component. If the patient shows initial symptoms in the form of increased dyspnea and/or sputum secretion, or a worsening of their characteristics, active antibiotic treatment must be started against Haemophilus influenzae, Streptococcus pneumoniae, Moraxella catarrhalis and atypical bacteria, checking the immunization status of the patient. It should be remembered that the macrolides afford a greater antiinflammatory activity, improve action against Streptococcus pneumoniae, and inhibit the production of cytokines in the airway stimulated by rhinovirus overinfections. In contrast, in asthma antibiotic treatment should only be started if there is clear evidence of infection (despite the usual sputum purulence due to eosinophilia), since the triggering factors are not usually of an infectious nature. Mucolytic agents (e.g., N-acetylcysteine) are not used in the management of acute COPD and asthma.
Correction of Water–Electrolyte and Acid–Base ImbalancesThe chronic inflammatory component in patients with COPD and in asthmatics leads to lung remodeling, and ultimately to emphysema. Lung edema is more likely, due to the alteration of lymphatic drainage, even with moderate volume replacement. Although there may be coexisting metabolic acidosis (e.g., lactic acidosis), most patients with COPD exacerbation and asthmatics present respiratory acidosis. The rise in pCO2 implies an increase in brain blood flow and in intracranial pressure, cardiac output and circulating catecholamine concentrations, pulmonary artery pressure and right ventricle overload, as well as a decrease in peripheral vascular tone. As has been mentioned, the indication of bicarbonate administration is debatable, and it is preferable first to optimize mechanical ventilation and the cardiocirculatory condition of the patient. Iatrogenic respiratory alkalosis due to hyperventilation can worsen compensatory metabolic alkalosis in COPD with chronic hypercapnia subjected to mechanical ventilation. The symptoms appear with pH≥7.55, while above pH 7.7 the patient may experience seizures, coma, arrhythmias and cardiac arrest.58
Nutritional SupportWhile asthmatics rarely present nutritional deficits, COPD patients often suffer imbalances between energy intake and expenditure, due to the chronic inflammation and extra constant effort required, and moreover show a loss of muscle mass and even cachexia. Early nutritional support, preferably via the enteral route, aims to compensate the metabolic needs of these patients, though care is required not to administer excessive amounts of carbohydrate supplements, since they increase the production of CO2 and O2 consumption.
Treatment of ComplicationsSince these are inflammatory syndromes, fever is a common presence in COPD and asthma; infections and overinfections are further factors to be taken into account during patient admission. Most patients with COPD show bacterial colonization of the lower airways, and asthmatics in turn are frequently affected by sinusitis and the microaspirations secondary to gastroesophageal reflux. Logically, all other potential causes of fever must be excluded. Atelectasis persistently exerts a negative influence on the clinical course of these individuals. Apart from the hypoxemia resulting from the intrapulmonary shunt (abnormal ventilation/perfusion ratio), atelectasis poses an added risk of pulmonary infection. The causal mechanisms are the following: (1) chronic hyperinsufflation with the reduction of diaphragm function; (2) diminished lung and chest wall compliance due to general anesthesia; (3) alveolar collapse secondary to high concentrations of O2; (4) extrapulmonary compression (pneumothorax, pleural effusion, etc.), and (5) increased production and accumulation of bronchial secretions (this being the most common finding). In order to avoid atelectasis as far as possible, early weaning from mechanical ventilation should be started, and if this is not possible, then a spontaneous ventilation mode should be selected with a high tidal volume and PEEP, with the reduction of patient sedation. If the tracheobronchial secretions are abundant, endotracheal aspirations should be performed—attempting not to damage the tracheobronchial mucosa, oxygenating the patient before and after in order to avoid hypoxia, and taking care not to aspirate too often (loss of surfactant) or too little (aspiration atelectasis). Respiratory physiotherapy is advisable, with postural drainage, and in cooperative and non-intubated patients, forced spirometry and assisted cough are indicated. Optic fibrobronchoscopy with aspiration can prove harmful, since it may induce bronchial collapse and mucosal damage. It is not recommended in the presence of hemodynamic instability or severe hypoxia.59
Patients with COPD exacerbation or severe asthma are very susceptible to sinusal tachycardia and tachyarrhythmias as a result of hypoxia, stress, respiratory effort, sympathomimetic drug treatment, etc. The lung–heart interactions due to pulmonary hyperinsufflation affect cardiac output, resulting in hypotension and shock. In COPD, pulmonary hypertension usually implies cardiomyopathy, right heart failure and coronary insufficiency. Treatment is based on the control of these factors (sedation, titration of β2 agonists, avoidance of hyperinsufflation, the exclusion of ischemic heart disease, etc.).
Conflicts of InterestThe authors declare no conflicts of interest and assume responsibility for the data reflected in this manuscript.
Please cite this article as: García Vicente E, et al. Ventilación mecánica invasiva en EPOC y asma. Med Intensiva. 2011;35:288–98.