To analyze epidemiological data of catheter-associated urinary tract infection (CAUTI) in critically ill patients admitted to Spanish ICUs in order to assess the need of implementing a nationwide intervention program to reduce these infections.
DesignNon-intervention retrospective annual period prevalence analysis.
SettingParticipating ICUs in the ENVIN-UCI multicenter registry between the years 2007 and 2016.
PatientsCritically ill patients admitted to the ICU with catheter-associated urinary tract infection (CAUTI).
Main variablesIncidence rates per 1000 catheter-days; urinary catheter utilization ratio; proportion of CAUTIs in relation to total health care-associated infections (HAIs).
ResultsA total of 187,100 patients, 137,654 (73.6%) of whom had a urinary catheter in place during 1,215,673 days (84% of days of ICU stay) were included. In 4539 (3.3%) patients with urinary catheter, 4977 CAUTIs were diagnosed (3.6 episodes per 100 patients with urinary catheter). The CAUTI incidence rate showed a 19% decrease between 2007 and 2016 (4.69–3.8 episodes per 1000 catheter-days), although a sustained urinary catheter utilization ratio was observed (0.84 [0.82–0.86]). The proportion of CAUTI increased from 23.3% to 31.9% of all HAIs controlled in the ICU.
ConclusionsAlthough CAUTI rates have declined in recent years, these infections have become proportionally the first HAIs in the ICU. The urinary catheter utilization ratio remains high in Spanish ICUs. There is room for improvement, so that a CAUTI-ZERO project in our country could be useful.
Analizar los datos epidemiológicos de las infecciones del tracto urinario relacionadas con sonda uretral (ITU-SU) en pacientes críticos ingresados en UCI españolas para evaluar la necesidad de aplicar un programa de intervención a nivel nacional para disminuir dichas infecciones.
DiseñoAnálisis retrospectivo, no intervencionista, de prevalencia de periodo anual.
ÁmbitoUCI participantes en el registro multicéntrico ENVIN-UCI entre los años 2007-2016.
PacientesPacientes críticos ingresados en UCI con ITU-SU.
Variables principalesTasa de incidencia por 1.000 días de utilización de SU; ratio de uso de SU; proporción de ITU-SU con respecto del total de infecciones relacionadas con asistencia sanitaria (IRAS) controladas en el registro.
ResultadosSe han incluido 187.100 pacientes de los que 137.654 (73,6%) utilizaron SU durante 1.215.673 días (84,4% de los días de estancia en UCI). En 4.539 (3,3%) pacientes sondados se han diagnosticado 4.977 ITU-SU (3,6 episodios por 100 pacientes con SU). La tasa de incidencia de ITU-SU ha disminuido entre los años 2007 y 2016 un 19% (4,69 a 3,8 episodios por 1.000 días de SU), aunque se ha mantenido la ratio de uso de SU (0,84 [0,82-0,86]). La proporción de las ITU-SU ha aumentado desde el 23,3% al 31,9% del total de IRAS controladas.
ConclusionesAunque han disminuido las tasas de ITU-SU estas infecciones han pasado a ser, proporcionalmente, la primera de las IRAS en UCI. Persiste una elevada ratio de utilización de SU en UCI españolas. Existe un espacio de mejora, por lo que un proyecto ITU-ZERO podría ser útil en nuestro país.
Catheter-associated urinary tract infection (CAUTI) is a leading example of healthcare-associated infection (HAI).1 Its frequency is related to the duration of catheterization, the use of closed drainage systems, and the quality of care provided by the healthcare professionals in relation to catheter placement and maintenance.2,3 In critical patients admitted to the Intensive Care Unit (ICU), urinary catheters (UCs) are commonly used to monitor hourly diuresis as an indicator of patient hemodynamic status. As a result, CAUTI is one of the most common types of infection in this clinical setting.4,5
In Spain, HAIs have been monitored in patients admitted to the ICU since the year 1994 through the ENVIN-ICU registry.4 Catheter-associated urinary tract infections are included in this monitoring or surveillance process, along with bacteremia associated to vascular catheters (BVC) and ventilator-associated pneumonia (VAP). These are all infections acquired during admission to the ICU, and the assisting healthcare professionals are assumed to have a responsibility in the development of such disorders.4 For many years the objective of the ENVIN registry was to determine the frequency, etiology and multiresistance markers of each of the monitored infections. Since the year 2009, different safety projects have been implemented in order to favor safe practices in the management of invasive devices related to HAIs, with the intention of lowering the number of such infections. In this regard, the “Zero Bacteremia” (ZB)6 and “Zero Pneumonia” (ZP)7 projects have been able to reduce the Spanish national BVC and VAP rates by 50%, while the “Zero Resistance” (ZR)8 project has resulted in a 20% decrease in the number of patients acquiring a multiresistance bacterial infection during admission to the ICU (data not published).
To date, no Spanish national project has been developed with the aim of reducing the CAUTI rates in the critical care setting. The present study analyzes the evolution of the CAUTI rates, etiologies and multiresistance markers in the last 10 years, as well as the systemic response to these infections and the raw mortality figures during ICU stay in patients with CAUTI, with the purpose of assessing the need for a specific project destined to reduce these infections (the “Zero UTI” project).
Material and methodsDesign. A retrospective, multicenter observational annual period prevalence study was carried out.
Study patients. We analyzed all the patients admitted for over 24h to the ICUs participating in the ENVIN-ICU registry, with a diagnosis of CAUTI.
Analytical period. The study period was from April to June, covering the years 2007–2016 (10 years).
Characteristics of the ENVIN-ICU registry. The ENVIN (Estudio Nacional de Vigilancia de Infección Nococomial [National Nosocomial Infection Surveillance Study]) registry was developed by the Infectious Diseases Working Group of the Sociedad Española de Medicina Intensiva, Crítica y Unidades Coronarias (Spanish Society of Intensive and Critical Care Medicine and Coronary Units) (SEMICYUC) in the year 1994. Its aim was to record the infections associated to the use of invasive devices diagnosed during patient admission to the ICU. A database was designed for this purpose, and has evolved over the years. In the year 2004, changes were made to make the database compatible with the European project “Hospitals in Europe Link for Infection Control through Surveillance” (HELICS). At present, data collection is carried out using the ENVIN-HELICS software application that is accessed on the Internet (http://hws.vhebron.net/envin-helics/). Access is open (using an individual code) and free following identification and registry of the supervisors of each ICU. Participation in the registry is voluntary, and data compilation is performed on a longitudinal and prospective basis. Since its introduction, the incorporation of ICUs has increased, and in the year 2016 a total of 200Units were contributing data.4 As mentioned, data collection is carried out on a prospective basis, including all the patients admitted to the ICU for more than 24h in the course of a three-month period (from April to June). More than one day of admission was considered when the difference between the date of discharge from the ICU and the date of admission to the Unit was longer than 24h. Patients admitted more than once to the ICU were regarded as different cases; as a result, a given patient could be included several times in the same surveillance period. The duration of follow-up of the studied subjects was from admission to the ICU to discharge from the latter, with a maximum duration of 60 days. Two audits were made (in the years 2008 and 2010) that served to confirm the internal validity of the included information.9 The ENVIN registry has been approved by the Clinical Research Ethics Committee (CREC) of Hospital del Mar, among other hospital centers, and has been declared by the Spanish Ministry of Health, Social Services and Equality as a registry of interest for the National Health System.
Definition of catheter-associated urinary tract infection. The definition of CAUTI was based on the criteria of the European Center for Disease Prevention and Control (ECDC),10 which can be found in the ENVIN registry manual4 and include the confirmation of at least one of the following clinical signs or symptoms not present at the time of urinary catheter placement: fever over 38°C (excluding other possible causes), tension in the suprapubic zone or micturition urgency or pyuria (10leukocytes/ml or 3leukocytes/ml upon inspection of a non-centrifuged urine sample under high magnification), and one of the following microbiological criteria: (a) in patients without antibiotic treatment, a urine culture with the isolation of ≥105cfu/ml corresponding to no more than two microorganisms; or (b) in patients with antibiotic treatment, a urine culture with the isolation of <105cfu/ml corresponding to a single microorganism. If more than one microorganism was isolated from the urine culture, the sample was considered to be contaminated.
Study variables. Evaluation was made of those variables available in the ENVIN registry and which were of interest for the epidemiological analysis. With regard to the patients, we analyzed mortality in the ICU (defined as death occurring during ICU stay due to any cause) and the UC utilization ratio (defined as the proportion of days in which the patients used UC with respect to the total days of ICU stay). With regard to the infections, we analyzed the etiology, time of appearance with respect to the day of admission to the ICU (classified as ≤ or >4 days), multiresistance markers, and systemic response to the infection. The multiresistance markers were: Acinetobacter baumannii resistant to imipenem, Escherichia coli resistant to cefotaxime or ciprofloxacin, Klebsiella pneumoniae resistant to cefotaxime or ciprofloxacin, Pseudomonas aeruginosa resistant to imipenem, and Enterococcus spp. resistant to vancomycin. Systemic response in turn was evaluated using the definitions of the 1991 Consensus Conference11 and the modifications included in 2003,12 with joint expression in the text of severe sepsis and septic shock. Likewise, the number of nosocomial bacteremias secondary to urinary infection diagnosed from 48h of admission to the ICU were included.
Frequency measures. Use was made of the cumulative incidence, defined as the number of CAUTI episodes divided by the number of patients with UC per 100, and the infection rate expressed as the number of CAUTI episodes per 1000 days of UC. In order to calculate the impact of CAUTI with respect to the total HAIs controlled in the ENVIN registry, we calculated the proportion of each of them with respect to the total HAIs detected. The frequency of each of the pathogenic microorganisms (PMs) identified in the CAUTI was expressed as a percentage with respect to the total microorganisms identified. The multiresistance marker rate in turn was calculated as the ratio between the number of a given multiresistant PM divided by the total number of that PM identified per 100.
Statistical analysis. Data collection was carried out using the ENVIN-ICU software application, accessed on the Internet. The database (in SQL Server) is located in the same server. The program is equipped with security systems that obligatorily require completion of variables defined as basic and which impede the introduction of non-logical values. Qualitative variables were described with the percentage distribution of each of the categories. Quantitative variables in turn were reported as the mean and standard deviation (SD) in the presence of a normal data distribution, and as the median and percentiles 25 and 75 when otherwise. Analysis of the monotonic trends (ascending and descending) of the different indicators was based on the Mann-Kendall test. Statistical significance was considered for p<0.05.
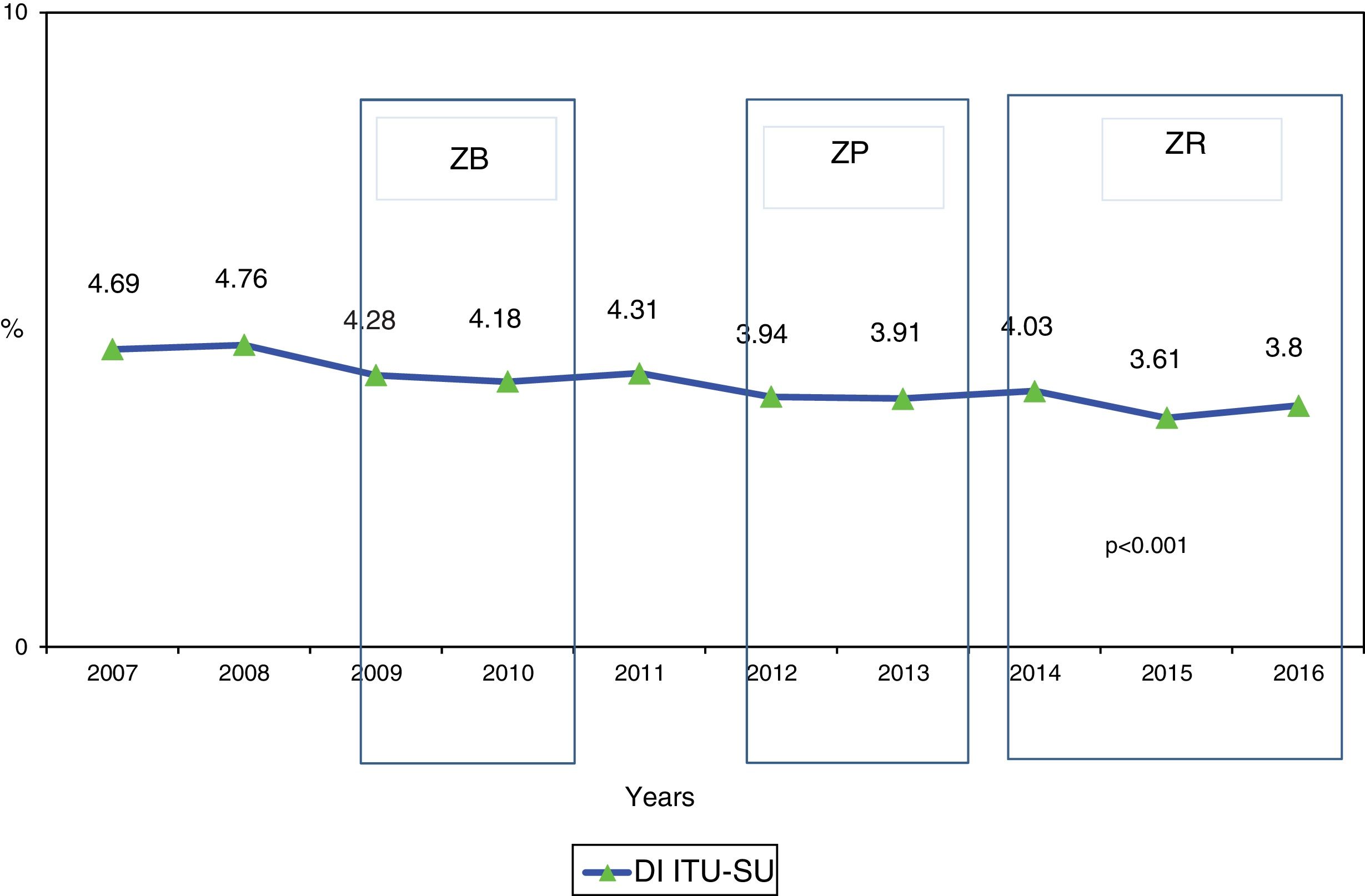
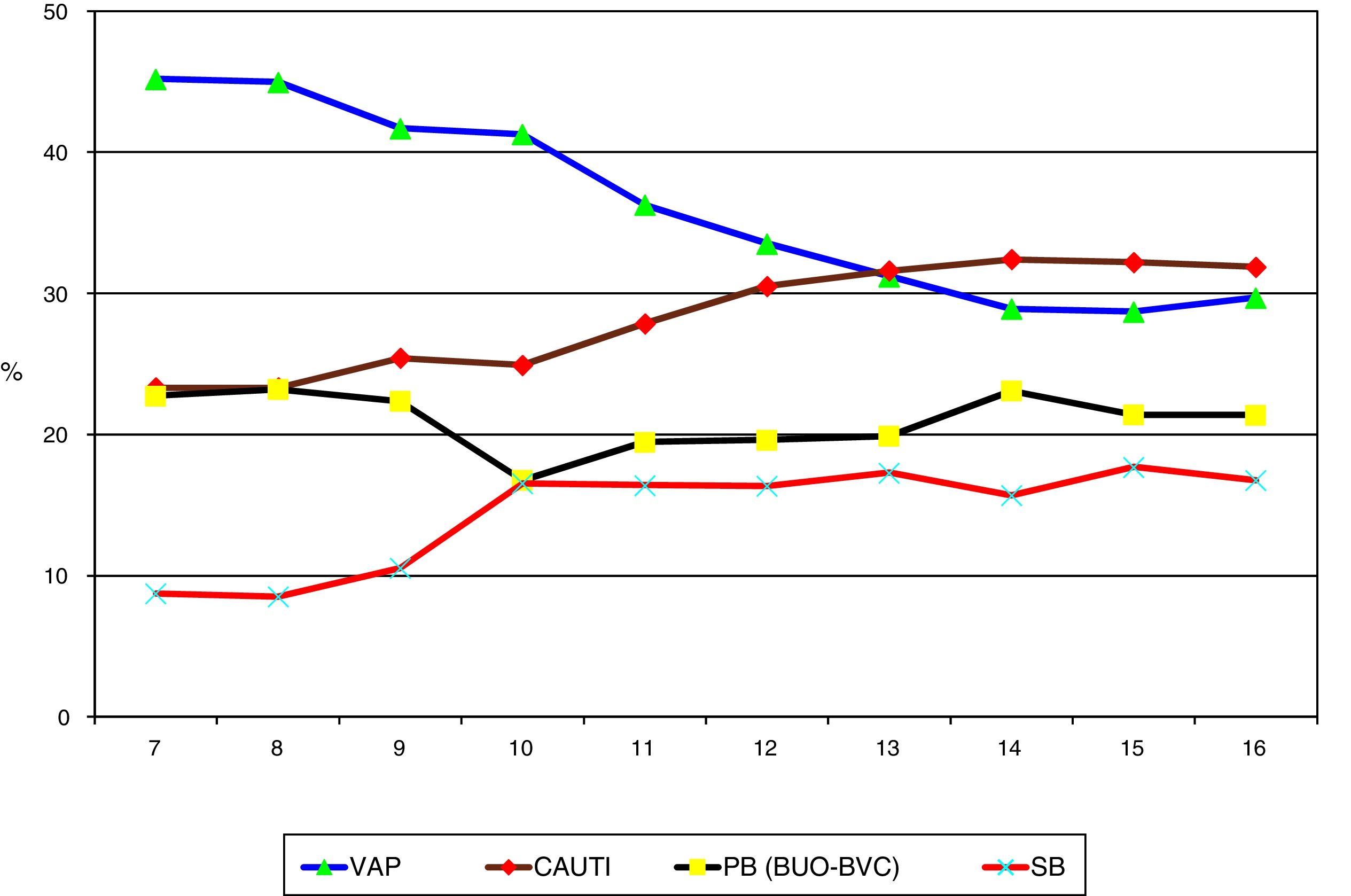
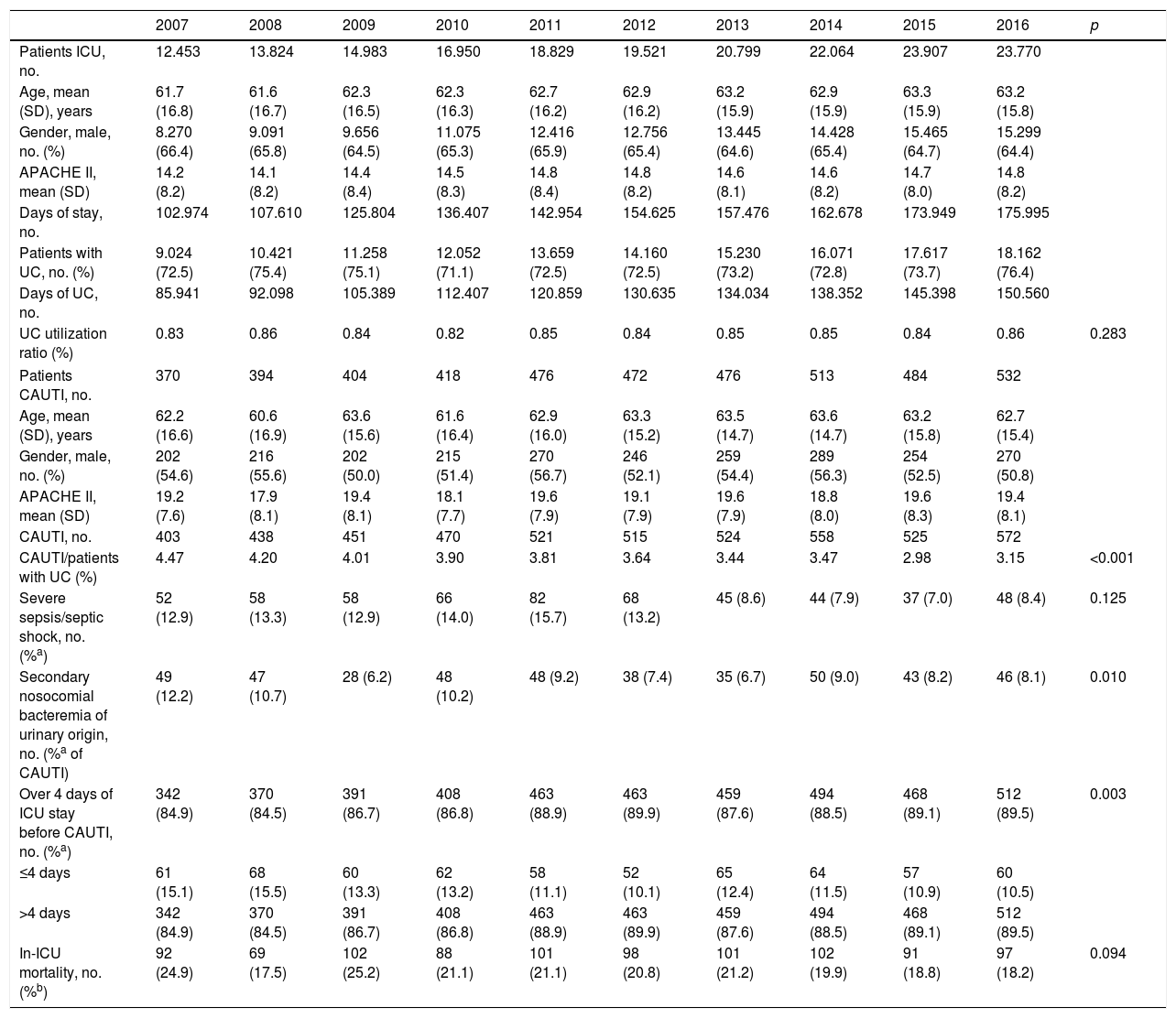
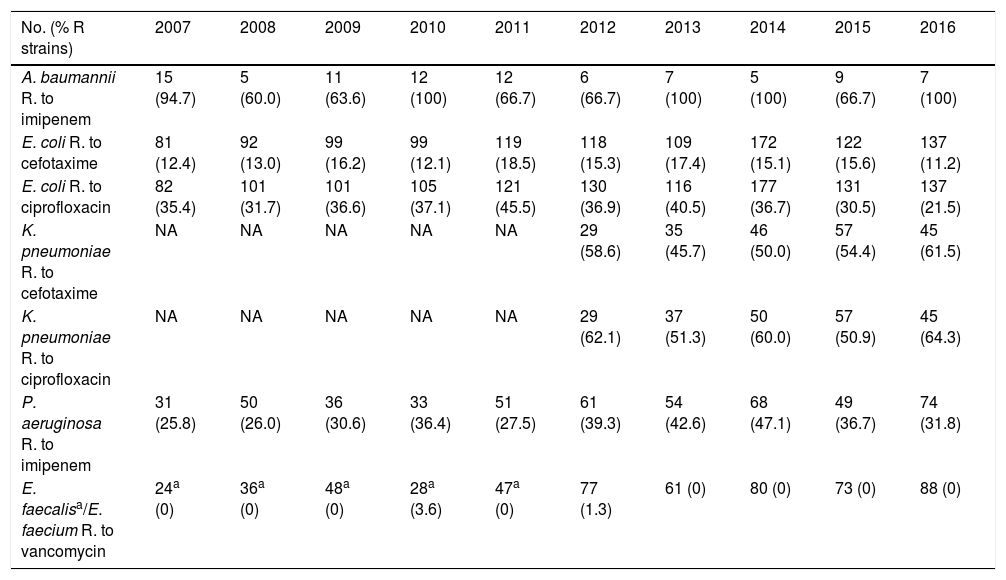
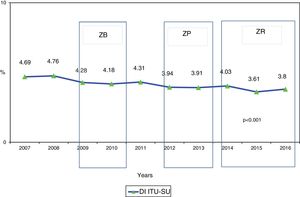
ResultsA total of 187,100 patients were included, of which 137,654 (73.6%) used UC for 1,215,673 days (84.4% of the days of ICU stay). A total of 4977 CAUTIs were diagnosed in 4539 catheterized patients (3.3%) (3.6 episodes per 100 patients with UC). Table 1 shows the number of patients included per year, as well as the days of stay, days of UC, the UC utilization ratio, number of CAUTIs diagnosed, and the CAUTI rate in relation to the number of patients with UC. Of note is the progressive increase over the years in the number of patients included in the registry, as well as in the days of stay and days of UC use. In contrast, stability was observed in terms of the UC utilization ratio (0.84 [0.82–0.86]), with a significant decrease in CAUTI rate in relation to the number of patients with UC, and a decrease in early CAUTIs (≤4 days ICU stay) (p=0.003). Fig. 1 reports the annual evolution of the CAUTI rate, expressed as infections diagnosed per 1000 days of UC over the analyzed years, and its correlation to the prevention projects ZB, ZP and ZR, applied in the Spanish ICUs. A progressive decrease was observed from 4.69 to 3.80 episodes per 1000 days of UC in the year 2016 (19% reduction) (p<0.001). Fig. 2 in turn includes the proportion of each infection controlled with respect to the total infections identified each year. Of note in this case is the progressive increase in the proportion of CAUTIs, which in recent years have ranked first among the HAIs related to the use of invasive devices.
Evolution of the epidemiological data referred to CAUTI identified in Spanish ICUs between the years 2007 and 2016.
| 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | p | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients ICU, no. | 12.453 | 13.824 | 14.983 | 16.950 | 18.829 | 19.521 | 20.799 | 22.064 | 23.907 | 23.770 | |
| Age, mean (SD), years | 61.7 (16.8) | 61.6 (16.7) | 62.3 (16.5) | 62.3 (16.3) | 62.7 (16.2) | 62.9 (16.2) | 63.2 (15.9) | 62.9 (15.9) | 63.3 (15.9) | 63.2 (15.8) | |
| Gender, male, no. (%) | 8.270 (66.4) | 9.091 (65.8) | 9.656 (64.5) | 11.075 (65.3) | 12.416 (65.9) | 12.756 (65.4) | 13.445 (64.6) | 14.428 (65.4) | 15.465 (64.7) | 15.299 (64.4) | |
| APACHE II, mean (SD) | 14.2 (8.2) | 14.1 (8.2) | 14.4 (8.4) | 14.5 (8.3) | 14.8 (8.4) | 14.8 (8.2) | 14.6 (8.1) | 14.6 (8.2) | 14.7 (8.0) | 14.8 (8.2) | |
| Days of stay, no. | 102.974 | 107.610 | 125.804 | 136.407 | 142.954 | 154.625 | 157.476 | 162.678 | 173.949 | 175.995 | |
| Patients with UC, no. (%) | 9.024 (72.5) | 10.421 (75.4) | 11.258 (75.1) | 12.052 (71.1) | 13.659 (72.5) | 14.160 (72.5) | 15.230 (73.2) | 16.071 (72.8) | 17.617 (73.7) | 18.162 (76.4) | |
| Days of UC, no. | 85.941 | 92.098 | 105.389 | 112.407 | 120.859 | 130.635 | 134.034 | 138.352 | 145.398 | 150.560 | |
| UC utilization ratio (%) | 0.83 | 0.86 | 0.84 | 0.82 | 0.85 | 0.84 | 0.85 | 0.85 | 0.84 | 0.86 | 0.283 |
| Patients CAUTI, no. | 370 | 394 | 404 | 418 | 476 | 472 | 476 | 513 | 484 | 532 | |
| Age, mean (SD), years | 62.2 (16.6) | 60.6 (16.9) | 63.6 (15.6) | 61.6 (16.4) | 62.9 (16.0) | 63.3 (15.2) | 63.5 (14.7) | 63.6 (14.7) | 63.2 (15.8) | 62.7 (15.4) | |
| Gender, male, no. (%) | 202 (54.6) | 216 (55.6) | 202 (50.0) | 215 (51.4) | 270 (56.7) | 246 (52.1) | 259 (54.4) | 289 (56.3) | 254 (52.5) | 270 (50.8) | |
| APACHE II, mean (SD) | 19.2 (7.6) | 17.9 (8.1) | 19.4 (8.1) | 18.1 (7.7) | 19.6 (7.9) | 19.1 (7.9) | 19.6 (7.9) | 18.8 (8.0) | 19.6 (8.3) | 19.4 (8.1) | |
| CAUTI, no. | 403 | 438 | 451 | 470 | 521 | 515 | 524 | 558 | 525 | 572 | |
| CAUTI/patients with UC (%) | 4.47 | 4.20 | 4.01 | 3.90 | 3.81 | 3.64 | 3.44 | 3.47 | 2.98 | 3.15 | <0.001 |
| Severe sepsis/septic shock, no. (%a) | 52 (12.9) | 58 (13.3) | 58 (12.9) | 66 (14.0) | 82 (15.7) | 68 (13.2) | 45 (8.6) | 44 (7.9) | 37 (7.0) | 48 (8.4) | 0.125 |
| Secondary nosocomial bacteremia of urinary origin, no. (%a of CAUTI) | 49 (12.2) | 47 (10.7) | 28 (6.2) | 48 (10.2) | 48 (9.2) | 38 (7.4) | 35 (6.7) | 50 (9.0) | 43 (8.2) | 46 (8.1) | 0.010 |
| Over 4 days of ICU stay before CAUTI, no. (%a) | 342 (84.9) | 370 (84.5) | 391 (86.7) | 408 (86.8) | 463 (88.9) | 463 (89.9) | 459 (87.6) | 494 (88.5) | 468 (89.1) | 512 (89.5) | 0.003 |
| ≤4 days | 61 (15.1) | 68 (15.5) | 60 (13.3) | 62 (13.2) | 58 (11.1) | 52 (10.1) | 65 (12.4) | 64 (11.5) | 57 (10.9) | 60 (10.5) | |
| >4 days | 342 (84.9) | 370 (84.5) | 391 (86.7) | 408 (86.8) | 463 (88.9) | 463 (89.9) | 459 (87.6) | 494 (88.5) | 468 (89.1) | 512 (89.5) | |
| In-ICU mortality, no. (%b) | 92 (24.9) | 69 (17.5) | 102 (25.2) | 88 (21.1) | 101 (21.1) | 98 (20.8) | 101 (21.2) | 102 (19.9) | 91 (18.8) | 97 (18.2) | 0.094 |
APACHE: Acute Physiology and Chronic Health Evaluation; SD: standard deviation; CAUTI: catheter-associated urinary tract infection; UC: urinary catheter; ICU: Intensive Care Unit.
Evolution of the proportion of each HAI with respect to the total HAIs controlled in the ENVIN registry-ICU. VAP: ventilator-associated pneumonia; CAUTI: catheter-associated urinary tract infection; PB: primary bacteremia; SB: secondary bacteremia; BUO: bacteremia of unknown origin; BVC: bacteremia associated to vascular catheters; HAI: healthcare-associated infection; ENVIN-ICU: National Nosocomial Infection Surveillance Study in Intensive Care Units.
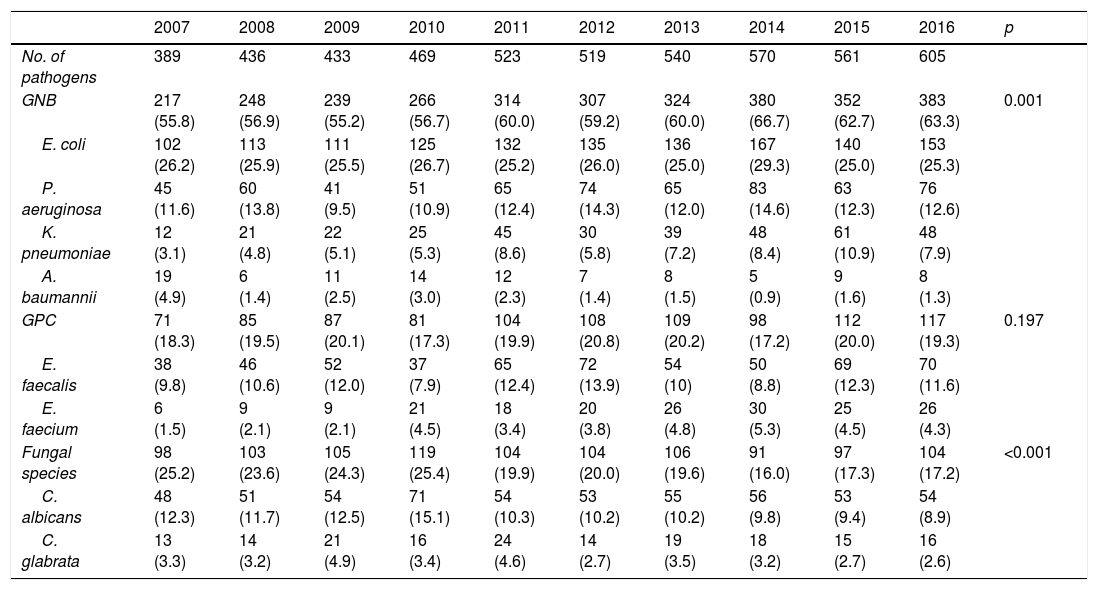
The evolution over the years of the etiology underlying CAUTI is reported in Table 2. Gramnegative bacteria (GNB) were seen to predominate, with an increase in their presence over the years (from 55.8% in the year 2007 to 63.3% in 2016; p=0.001). E. coli remained the most frequently identified pathogen (25%), followed by P. aeruginosa (12%). Of note was the gradual increase in K. pneumoniae in recent years (9%), and the decrease in A. baumannii (less than 2%). The frequency of grampositive cocci (GPC) was about 20%, with a predominance of E. faecalis. An increase was observed in the proportion of isolates corresponding to Enterococcus faecium, from 1.5% to over 4% of the total isolates. In contrast, fungi we seen to decrease their presence from 25.2% in the year 2007 to 17.2% in 2016 (p<0.001). This was particularly notorious in the case of Candida albicans, which decreased from 12.3% to 8.9%.
Evolution of the pathogenic microorganisms causing CAUTI in critical patients classified by groups.
| 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | p | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of pathogens | 389 | 436 | 433 | 469 | 523 | 519 | 540 | 570 | 561 | 605 | |
| GNB | 217 (55.8) | 248 (56.9) | 239 (55.2) | 266 (56.7) | 314 (60.0) | 307 (59.2) | 324 (60.0) | 380 (66.7) | 352 (62.7) | 383 (63.3) | 0.001 |
| E. coli | 102 (26.2) | 113 (25.9) | 111 (25.5) | 125 (26.7) | 132 (25.2) | 135 (26.0) | 136 (25.0) | 167 (29.3) | 140 (25.0) | 153 (25.3) | |
| P. aeruginosa | 45 (11.6) | 60 (13.8) | 41 (9.5) | 51 (10.9) | 65 (12.4) | 74 (14.3) | 65 (12.0) | 83 (14.6) | 63 (12.3) | 76 (12.6) | |
| K. pneumoniae | 12 (3.1) | 21 (4.8) | 22 (5.1) | 25 (5.3) | 45 (8.6) | 30 (5.8) | 39 (7.2) | 48 (8.4) | 61 (10.9) | 48 (7.9) | |
| A. baumannii | 19 (4.9) | 6 (1.4) | 11 (2.5) | 14 (3.0) | 12 (2.3) | 7 (1.4) | 8 (1.5) | 5 (0.9) | 9 (1.6) | 8 (1.3) | |
| GPC | 71 (18.3) | 85 (19.5) | 87 (20.1) | 81 (17.3) | 104 (19.9) | 108 (20.8) | 109 (20.2) | 98 (17.2) | 112 (20.0) | 117 (19.3) | 0.197 |
| E. faecalis | 38 (9.8) | 46 (10.6) | 52 (12.0) | 37 (7.9) | 65 (12.4) | 72 (13.9) | 54 (10) | 50 (8.8) | 69 (12.3) | 70 (11.6) | |
| E. faecium | 6 (1.5) | 9 (2.1) | 9 (2.1) | 21 (4.5) | 18 (3.4) | 20 (3.8) | 26 (4.8) | 30 (5.3) | 25 (4.5) | 26 (4.3) | |
| Fungal species | 98 (25.2) | 103 (23.6) | 105 (24.3) | 119 (25.4) | 104 (19.9) | 104 (20.0) | 106 (19.6) | 91 (16.0) | 97 (17.3) | 104 (17.2) | <0.001 |
| C. albicans | 48 (12.3) | 51 (11.7) | 54 (12.5) | 71 (15.1) | 54 (10.3) | 53 (10.2) | 55 (10.2) | 56 (9.8) | 53 (9.4) | 54 (8.9) | |
| C. glabrata | 13 (3.3) | 14 (3.2) | 21 (4.9) | 16 (3.4) | 24 (4.6) | 14 (2.7) | 19 (3.5) | 18 (3.2) | 15 (2.7) | 16 (2.6) |
GNB: gramnegative bacteria; GPC: grampositive cocci.
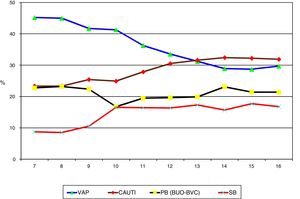
The evolution of the multiresistance markers corresponding to the main pathogens responsible for CAUTI is shown in Table 3. Of note is the high resistance rate of the A. baumannii isolates to imipenem-cilastatin over the years (between 60% and 100%) and of K. pneumoniae to cefotaxime (between 46% and 59%) and ciprofloxacin (between 50% and 62%). In contrast, there were very few vancomycin-resistant enterococcal strains.
Evolution of the resistance markers corresponding to the main pathogens causing CAUTI.
| No. (% R strains) | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 |
|---|---|---|---|---|---|---|---|---|---|---|
| A. baumannii R. to imipenem | 15 (94.7) | 5 (60.0) | 11 (63.6) | 12 (100) | 12 (66.7) | 6 (66.7) | 7 (100) | 5 (100) | 9 (66.7) | 7 (100) |
| E. coli R. to cefotaxime | 81 (12.4) | 92 (13.0) | 99 (16.2) | 99 (12.1) | 119 (18.5) | 118 (15.3) | 109 (17.4) | 172 (15.1) | 122 (15.6) | 137 (11.2) |
| E. coli R. to ciprofloxacin | 82 (35.4) | 101 (31.7) | 101 (36.6) | 105 (37.1) | 121 (45.5) | 130 (36.9) | 116 (40.5) | 177 (36.7) | 131 (30.5) | 137 (21.5) |
| K. pneumoniae R. to cefotaxime | NA | NA | NA | NA | NA | 29 (58.6) | 35 (45.7) | 46 (50.0) | 57 (54.4) | 45 (61.5) |
| K. pneumoniae R. to ciprofloxacin | NA | NA | NA | NA | NA | 29 (62.1) | 37 (51.3) | 50 (60.0) | 57 (50.9) | 45 (64.3) |
| P. aeruginosa R. to imipenem | 31 (25.8) | 50 (26.0) | 36 (30.6) | 33 (36.4) | 51 (27.5) | 61 (39.3) | 54 (42.6) | 68 (47.1) | 49 (36.7) | 74 (31.8) |
| E. faecalisa/E. faecium R. to vancomycin | 24a (0) | 36a (0) | 48a (0) | 28a (3.6) | 47a (0) | 77 (1.3) | 61 (0) | 80 (0) | 73 (0) | 88 (0) |
NA: not available; R: resistant.
The systemic response of these infections, as well as the raw mortality figures during admission to the ICU, showed a nonsignificant decrease over the years - the rate corresponding to CAUTI with severe sepsis or septic shock being about 10%, with an in-ICU mortality rate of approximately 19% (Table 1). Likewise, we recorded a decrease in nosocomial bacteremias of urinary origin acquired in the ICU from 12.2% to 8.1% during the studied period (p=0.01).
DiscussionThe present study recorded a 19% decrease in CAUTI rate at Spanish national level between the years 2007 and 2016. This decrease in CAUTI was accompanied by a delay in the diagnosis of the infection with respect to the day of admission to the ICU, and by a proportional increase in the participation of GNB in the etiology of infection, with a high prevalence of resistances among the most commonly identified GNB. The observed decrease in CAUTI rate was not associated to any specific intervention seeking to prevent such infections, though it coincided with the application at national level of the safety projects in critical patients (Zero Bacteremia, Zero Pneumonia and Zero Resistance). These projects placed special emphasis on the handling of invasive devices under conditions of asepsis. Furthermore, increased attention has been given to the concepts of safety in hospitalized patients – one of the main messages being that HAIs are to be regarded as adverse effects for which the healthcare professionals assisting the patients are responsible.
Despite the decrease in CAUTI observed in our study, the proportion of such infections in relation to the total infections associated to invasive devices in the ICU (controlled in the ENVIN registry) was seen to increase – to the point of becoming the most frequent of all such infections. This was attributable to the success achieved with the safety projects, which made it possible to reduce the BVC and VAP rates by half.6,7
Although by the year 2016 it proved possible to reduce the CAUTI rate to 3.8 episodes per 1000 days of UC, and the rates recorded in the first decade of the present century showed improvements,13 the frequency of such infections remains higher than in other recently published national epidemiological studies. The National Healthcare Safety Network Report, on occasion of its annual communication with the data contributed by American hospitals in the year 2013, presented rates of between 1.3 and 1.7 episodes per 1000 days of UC in medical-surgical ICUs and 4.5–5.3 episodes per 1000 days of UC in neurological, neurosurgical or burn patient ICUs.5 A recent interventional study involving over 900 hospitalization units (of which 40.3% were ICUs) in Columbia (USA) and Puerto Rico, reported CAUTI rates in the ICU of between 2.48 and 2.50 episodes per 1000 days of UC – the applied interventional program proving scantly effective in reducing such rates.13 In Germany, the Hospital Infections Surveillance System published the CAUTI rates in ICUs, differentiating among three periods between 1997 and 2005 – the diagnosed infections decreasing from 1.46 to 0.57 episodes per 1000 days of UC.14 In contrast, our results are better than those published by the International Nosocomial Infection Control Consortium report, which included data from ICUs in 50 countries between the years 2010 and 2015, with a global CAUTI rate of 5.1 episodes per 1000 days of UC,15 or those of the Italian Nosocomial Infection Surveillance in Intensive Care Units, with CAUTI rates in the years 2006–2007 of 6.6–12 episodes per 1000 days of UC.16 In our case, the ICUs participating in the ENVIN registry were mainly polyvalent Units with a predominance of medical-surgical patients, in which the best registered rates were lower than 2.5 episodes per 1000 days of UC.
The UC utilization ratio in our study was far higher than that reported by the National Healthcare Safety Network (NHSN) Report.5 While in the Spanish ICUs the utilization ratio was 0.84, in the American hospitals the ratio ranged between 0.53 and 0.68 in the medical-surgical Units and 0.50–0.75 in the neurological, neurosurgical or burn patient ICUs. Other studies have reported UC utilization ratios of over 90%.17 The reasons for these differences have not been studied, but may be related to the characteristics of the admitted patients (increased severity being associated with an increased need for monitoring urinary output), care pressure or burden (early discharges with few UC-free periods), or low adherence to the recommendation to suspend UC on an early basis. The UC utilization ratio in our patients identifies the suspension of UC as an element for improvement.
Gramnegative bacilli, particularly E. coli, are the predominant pathogens identified in most epidemiological studies.18 In our case E. coli was the also most commonly identified bacterium, though other studies have described a predominance of A. baumannii and P. aeruginosa.17 In recent years there has been a reported increase in GNB at the expense of K. pneumoniae, which has more than doubled its implication in these infections (from 4.5% to 10.2%). The presence of this pathogen has also been observed in other sites of infection, identifying it as an emergent pathogen in the endogenous flora of many ICUs. Likewise, CAUTIs due to GPC have exceeded infections due to fungal species, which have shown a diminished presence in recent years. Improved classification of asymptomatic candiduria possibly may have influenced this decrease in fungal presence. Nevertheless, Candida albicans continues to represent about 10% of all isolates in infections of this kind. Among the GPC, Enterococcus faecalis predominated, though the largest increment corresponds to E. faecium, which has doubled its presence.
It is known that the presence of multiresistant bacteria is increasing in CAUTI.18 In our study, the incidence of K. pneumoniae strains resistant to cefotaxime and ciprofloxacin exceeded 60%, while A. baumannii proved resistant to imipenem in 60–100% of the cases, and E. coli was seen to be resistant to ciprofloxacin in over 30%. This situation in many cases requires the use of alternative antibiotics as rescue therapy following a first ineffective empirical treatment.
The systemic impact of CAUTI and raw mortality in the ICU were low compared with other HAIs identified in critical patients.19 However, their presence has been associated to an increased risk of death, prolonged admission20 and an increased use of treatment resources.21,22 As a result, many healthcare institutions and scientific societies have published recommendations and promoted studies seeking to reduce their presence.13,14,18,23–27 The results of these experiences are controversial. While one-by-one application of the different preventive measures has not been shown to result in sustained reductions in CAUTI,28 the application of bundles of measures has been more effective, particularly in cases with a high starting point.27,29
The main limitation of our study is the fact that the information comes from a voluntary multicenter registry. This may result in selection bias, since not all the ICUs contributed information throughout the full period analyzed. On the other hand, given the difficulties of diagnosing CAUTI in critical patients, workshops were held during the analyzed period in order to homogenize definitions, and this may have modified the assessments of asymptomatic candiduria and bacteruria.
It can be concluded that CAUTI has become the most frequent HAI in Spanish ICUs following the sustained decrease in BVC and VAP achieved thanks to the ZB and ZP projects. This situation, associated with the high UC utilization ratio during admission and the presence of rates higher than those found in other ICU epidemiological registries, justifies the need for an intervention plan, following the structure of the safety projects in the critically ill, identifying bundles of measures to reduce the frequency to below 2.7 episodes per 1000 days of UC.
Contribution of the authorsThe article was drafted by FAL, and all the authors contributed to assessment of the contents, with the contribution of comments. All the authors form part of the guiding team of the ENVIN registry-ICU, and have contributed to the annual analyses of the information published on the website: http://hws.vhebron.net/envin-helics/.
Conflicts of interestThe authors declare that they have no conflicts of interest in relation to this article.
Thanks are due to all the professionals, physicians and nurses, that have collaborated between the years 2007 and 2016 in the ENVIN registry-HELICS, contributing the information that has been analyzed in this study. All of them are co-authors of this article, and their names are reflected in the annual reports of the ENVIN registry, accessible at: http://hws.vhebron.net/envin-helics/. Thanks are also due to Dr. Sonia Uriona and Dr. Susana Otero for administrative and secretarial contribution to the ENVIN registry-HELICS.
Please cite this article as: Álvarez Lerma F, Olaechea Astigarraga P, Nuvials X, Gimeno R, Catalán M, Gracia Arnillas MP, et al. ¿Es necesario un proyecto para prevenir las infecciones del tracto urinario en los pacientes ingresados en unidades de cuidados intensivos españolas? Med Intensiva. 2019;43:63–72.