Cardiac shock is the leading cause of death in patients with acute myocardial infarction. The objective of this systematic review and meta-analysis was to evaluate whether levosimendan, compared to any type of control, is associated with improved clinical outcomes in patients with cardiogenic shock complicating myocardial infarction.
Materials and methodsThe PubMed, EMBASE, Cochrane Central Register, and China National Knowledge Information databases were searched for pertinent studies published up until 1 May 2016. Randomized and non-randomized clinical trials comparing levosimendan to standard therapy or placebo, in adult patients with cardiogenic shock complicating myocardial infarction, and reporting at least one outcome of interest were included. The primary outcome was mortality, whereas secondary outcomes were length of ICU stay, SOFA score, cardiac index (CI), cardiac power index (CPI), ejection fraction (EF), end-systolic volume (ESV), mean blood pressure (MBP), pulmonary arterial pressure (PAP), mixed venous oxygen saturation (SvO2), pulmonary artery occlusion pressure (PAOP) and glomerular filtration rate (GFR). We pooled risk ratio (RR) and 95% confidence interval (CI) using fixed and random effects models.
ResultsThirteen studies comprising a total of 648 patients were included in the analysis. There was a nonsignificant reduction in mortality with levosimendan compared to the controls (RR=0.82 [0.65–1.01], P for effect=0.07, I2=0%). In the levosimendan group PAP and ESV were significantly reduced, while CI, CPI, EF, MBP and SvO2 were significantly increased. No differences in SOFA score, ICU days, PAOP or GFR were noted.
ConclusionsLevosimendan can improve hemodynamic parameters and cardiac function when compared with a control group, with no evidence of benefit in terms of survival.
El choque cardiogénico es la principal causa de muerte en pacientes con infarto agudo de miocardio. El objetivo de este metaanálisis y revisión sistemática fue evaluar si, en comparación con cualquier tipo de control, el levosimendán se asocia a mejores efectos clínicos en pacientes con choque cardiogénico que complique un infarto de miocardio.
Materiales y métodosSe realizaron búsquedas en las bases de datos PubMed, EMBASE, Cochrane Central Register y China National Knowledge Information para encontrar estudios pertinentes publicados hasta el 1 de mayo de 2016. Se incluyeron ensayos clínicos aleatorizados y no aleatorizados en los que se comparase el levosimendán con el tratamiento estándar o con un placebo en pacientes adultos con choque cardiogénico que complicase un infarto de miocardio, y que informasen sobre al menos una variable de interés. La variable principal fue la mortalidad, mientras que las variables secundarias fueron la duración del ingreso en la UCI, la puntuación SOFA, el índice cardíaco (IC), el índice de potencia cardíaca (IPC), la fracción de eyección (FE), el volumen sistólico final (VSF), la presión arterial media (PAM), la presión arterial pulmonar (PAP), la saturación venosa mixta de oxígeno (SvO2), la presión de oclusión de la arteria pulmonar (POAP) y la tasa de filtración glomerular (TFG). Agrupamos la razón de riesgos (RR) y el intervalo de confianza (IC) del 95% por medio de modelos de efectos fijos y aleatorios.
ResultadosSe incluyeron en el análisis 13 estudios que incluyeron un total de 648pacientes. Se observó una reducción no significativa de la mortalidad con levosimendán en comparación con los controles (RR=0,82 [0,65-1,01], p para efecto=0,07, I2=0%). En el grupo de tratamiento con levosimendán, la PAP y el VSF se vieron reducidos de forma significativa, mientras que el IC, el IPC, la FE, la PAM y la SvO2 aumentaron de forma significativa. No se observaron diferencias en la puntuación SOFA, los días de ingreso en la UCI, la POAP ni la TFG.
ConclusionesEl levosimendán puede mejorar los parámetros hemodinámicos y la función cardíaca en comparación con un grupo de control, si bien no existen evidencias de beneficios en términos de supervivencia.
Cardiogenic shock complicates approximately 5% of myocardial infarctions. Consequent marked hypotension, reduced oxygen supply, and inadequate perfusion of various organs can result in multiple organ dysfunction. Despite utilization of an early revascularization strategy and advancing patients’ care, cardiogenic shock remains the leading cause of death in this population with high hospital mortality rate, approaching 50%.1,2
In cardiogenic shock complicating myocardial infarction early revascularization of the occluded vessel with a restoration of coronary cardiac blood flow preferably by means of PCI is the first line strategy. A supportive approach is to give mechanical support, such as Extracorporeal Membrane Oxygenation (ECMO).3 For inotropic support in patients with cardiogenic shock the drugs of choice is dobutamine. However, mortality of such patients in cardiogenic shock remains high. Further studies are needed to evaluate new therapeutic approaches to decrease mortality and morbidity of these patients.
Levosimendan is a relatively novel inotropic agent, which acts on cardiac troponin C, stabilizing the bound Ca2+, prolonging the interaction between actin and myosin, and thus enhances cardiac contractility.4 It is a calcium-sensitizer agent5 with vasodilatory properties,6 exerting beneficial effects particularly in cardiac surgery, a setting where it recently showed a survival benefit when compared with dobutamine.7
More recently, a number of clinical trials have now been completed. Therefore, the principal objective of this study was to critically review the literature to evaluate whether levosimendan compared to standard therapy, in patients with cardiogenic shock complicating myocardial infarction, is associated with improved clinical outcomes, in particular survival, and hemodynamics.
Materials and methodsSearch strategyAppropriate studies were independently searched in BioMedCentral, PubMed, EMBASE, Cochrane Central Register of clinical trials, and Chinese database (CNKI, WANGFANG DATA, and CQVIP) by 2 investigators. The full PubMed search strategy is available in the Appendix A. Supplementary data. No language restriction was enforced. The search was finalized on 1st May 2016. We decided to use a basic search strategy in order to make the strategy as sensitive as possible.
Abstracts from recent international conferences were searched for additional relevant studies. In addition, we use backward snowballing (i.e. scanning of references of retrieved articles and pertinent reviews). The search strategy aimed to include any controlled study with levosimendan administration in cardiogenic shock complicating myocardial infarction in adult humans.
Study selectionTwo authors reviewed all abstracts to identify potentially eligible controlled trials. If it was possible, full text articles were retrieved and reviewed to determine whether they met the eligibility criteria. If the complete paper was not available in the database, the corresponding author was contacted to get further material. Disagreements between reviewers were resolved by consensus.
The inclusion criteria was reports of controlled trials, comparing levosimendan to any other therapy for cardiogenic shock in adult human and reported at least one outcome of interest. The primary outcome was mortality, whereas secondary outcomes were length of ICU stay, Sequential Organ Failure Assessment (SOFA) score, cardiac index (CI), cardiac powder index (CPI), ejection fraction (EF), end systolic volume (ESV), mean blood pressure (MBP), pulmonary atrial pressure (PAP), mixed venous oxygen saturation (SvO2), glomerular filtration rate (GFR), and pulmonary artery occlusion pressure (PAOP). The time points of the collection of these variables should follow what reported by authors. There were no restrictions on time or dose of administration. The exclusion criteria were: duplicate publications, pediatric studies, and non-intravenous administration of levosimendan.
Data abstraction and study characteristicsTwo investigators abstracted baseline, procedural, and outcome data by using a data-recording table developed for this purpose. Data collected included: patient baseline characteristics, study design, sample size, clinical setting, study definition of cardiogenic shock, details of levosimendan and control regimens, and clinical outcomes.
Internal validity and risk of bias assessmentThe internal validity of each randomized controlled trial (RCT) was critically assessed for bias as reported by the Cochrane Collaboration methods.8 Each report was evaluated for risk of bias associated with the random sequence generation method, allocation concealment, blinding of participants and personnel, completeness of outcome data, free of selective reporting, and other bias. The overall risk of bias was presented as low, unclear, or high.
The internal validity of each non-randomized controlled trial (nRCT) was critically assessed for bias as reported by the Newcastle–Ottawa Scale Risk assessment for case-control studies.9 Each article was evaluated for risk of bias associated with selection, comparability, and exposure. We rated the risk of bias by applying a rating of star number to determine whether adequate measures were taken to protect against each potential source of bias in each study. The overall risk of bias was presented as star number.
Data analysis and synthesisTo analyze the binary outcome, we calculated risk ratio (RR) and 95% confidence interval. Mean difference (MD) and 95% confidence interval were computed for continuous variables. Furthermore, we compute numbers needed to be treated. Heterogeneity was measured using the Cochrane Q test, quantified with I2 statistic (I2>25% was defined as threshold indicating significant heterogeneity), and Tau-square (Tau2). The primary analysis was conducted by means of the Peto fixed effects method when I2<25% and with the random effects model when I2>25%. Publication bias was evaluated by visually inspecting funnel plot of the primary outcome.
Statistical significance was set at the 2-tailed 0.05 levels for hypothesis testing. Data analysis was performed using Review Manager (RevMan, version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014).
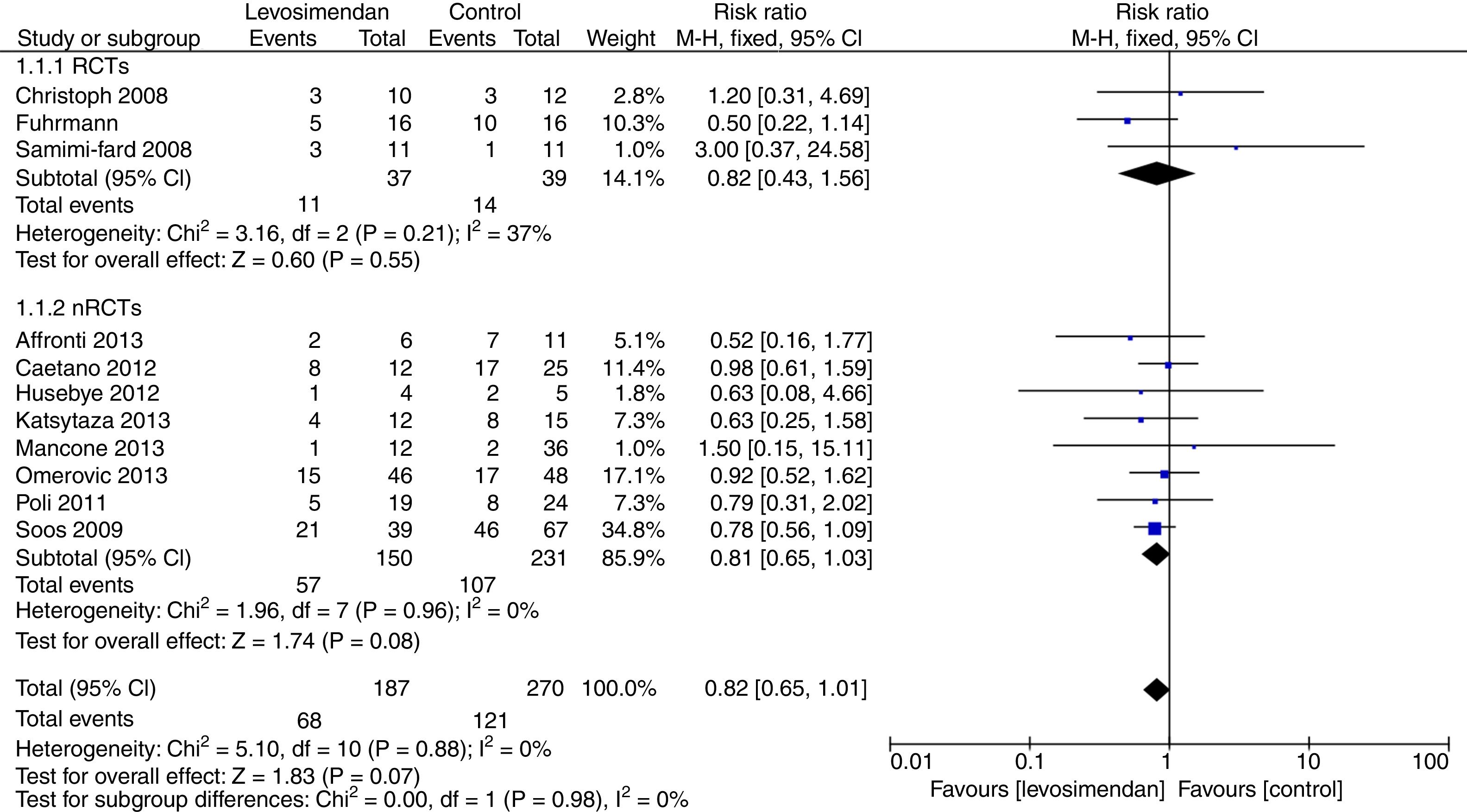
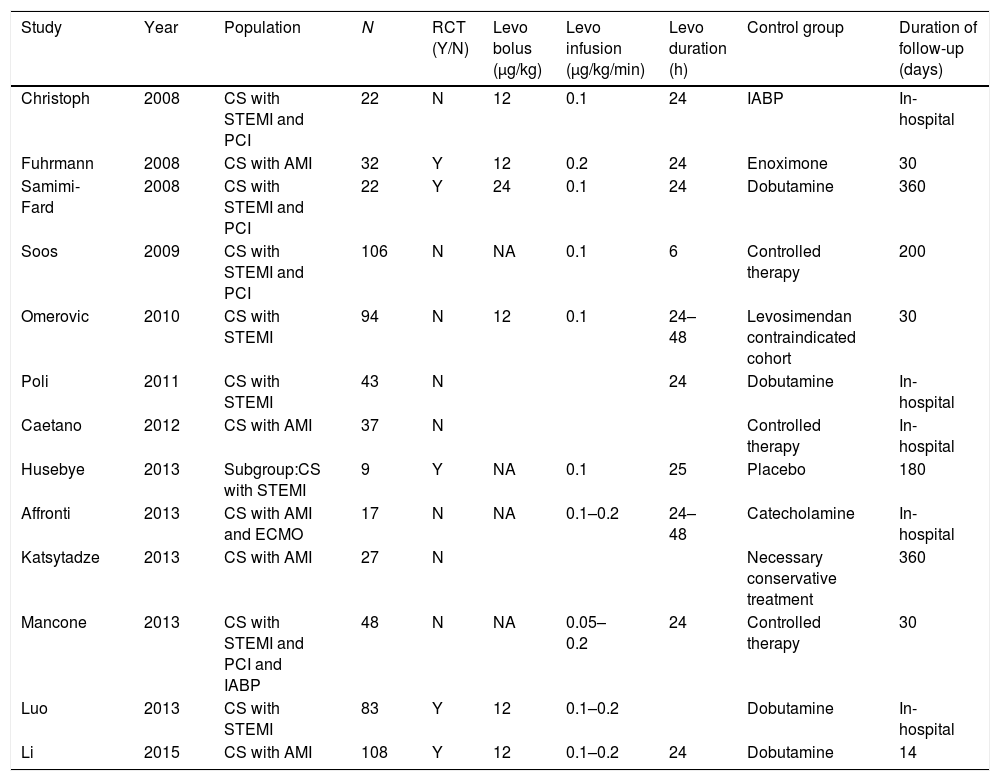
ResultsLiterature searchThere were 1137 reports identified by the search, 43 full or abstract articles were retrieved for in depth review (Appendix A. Supplementary data). Finally, 13 studies enrolling 648 participants fulfilled all eligibility criteria. There are 5 RCTs with 254 patients10–14 and 8 nRCTs with 394 patients.15–22 The trial characteristics are shown in Table 1 and in Appendix A. Supplementary data. There were 11 studies in English,10–12,15–22 two in Chinese.13,14
Characteristics of the studies included in the meta-analysis.
| Study | Year | Population | N | RCT (Y/N) | Levo bolus (μg/kg) | Levo infusion (μg/kg/min) | Levo duration (h) | Control group | Duration of follow-up (days) |
|---|---|---|---|---|---|---|---|---|---|
| Christoph | 2008 | CS with STEMI and PCI | 22 | N | 12 | 0.1 | 24 | IABP | In-hospital |
| Fuhrmann | 2008 | CS with AMI | 32 | Y | 12 | 0.2 | 24 | Enoximone | 30 |
| Samimi-Fard | 2008 | CS with STEMI and PCI | 22 | Y | 24 | 0.1 | 24 | Dobutamine | 360 |
| Soos | 2009 | CS with STEMI and PCI | 106 | N | NA | 0.1 | 6 | Controlled therapy | 200 |
| Omerovic | 2010 | CS with STEMI | 94 | N | 12 | 0.1 | 24–48 | Levosimendan contraindicated cohort | 30 |
| Poli | 2011 | CS with STEMI | 43 | N | 24 | Dobutamine | In-hospital | ||
| Caetano | 2012 | CS with AMI | 37 | N | Controlled therapy | In-hospital | |||
| Husebye | 2013 | Subgroup:CS with STEMI | 9 | Y | NA | 0.1 | 25 | Placebo | 180 |
| Affronti | 2013 | CS with AMI and ECMO | 17 | N | NA | 0.1–0.2 | 24–48 | Catecholamine | In-hospital |
| Katsytadze | 2013 | CS with AMI | 27 | N | Necessary conservative treatment | 360 | |||
| Mancone | 2013 | CS with STEMI and PCI and IABP | 48 | N | NA | 0.05–0.2 | 24 | Controlled therapy | 30 |
| Luo | 2013 | CS with STEMI | 83 | Y | 12 | 0.1–0.2 | Dobutamine | In-hospital | |
| Li | 2015 | CS with AMI | 108 | Y | 12 | 0.1–0.2 | 24 | Dobutamine | 14 |
CS, cardiogenic shock; STEMI, ST elevation myocardial infarction; PCI, percutaneous coronary intervention; AMI, acute myocardial infarction; ECMO, extracorporeal membrane oxygenation; IABP, intra-aortic balloon pump; Levo, levosimendan.
Study quality and risk of bias are reported in the Appendix A. Supplementary data. Two RCTs10,12 were rated as high risk of bias, 2 RCTs13,14 at unclear risk, and 1 RCT11 at low risk of bias, according to Cochrane Collaboration methods. Six nRCTs were rated as medium risk of bias,16–20,22 2 nRCTs15,21 were rated as high risk bias, according to Newcastle–Ottawa Scale.
Levosimendan in patients with cardiogenic shock: mortalityThe use of levosimendan in patients with cardiogenic shock was associated with a non significant reduction in mortality at the longest follow-up available (68/187 [36%] in the levosimendan group and 121/270 [53%] in the control group, RR 0.82[0.65,1.01], P for effect=0.07, P for heterogeneity=0.88, I2=0%, Tau2 0.00, numbers needed to treat=11; Appendix A. Supplementary data), with 11 studies included. No publication bias was present (Appendix A. Supplementary data).
When including only RCTs, there was no statistically significant reduction in mortality at the longest follow-up available in patients with cardiogenic shock (11/37 [29.7%] in the levosimendan group and 14/39[35.9%] in the control group, RR 0.82 [0.43,1.56], P for effect=0.55, P for heterogeneity=0.21, I2=37%, Tau2 0.00, numbers needed to treat=3 Appendix A. Supplementary data); with 3 studies included.
Even when including only nRCTs, there was no statistically significant reduction in mortality at the longest follow-up available in patients with cardiogenic shock (57/150 [38%] in the levosimendan group and 107/231[45.0%] in the control group, RR 0.81 [0.65,1.03], P for effect=0.08, P for heterogeneity=0.96, I2=0%, Tau2 0.00, numbers needed to treat=8, Appendix A. Supplementary data); with 8 studies included (Fig. 1).
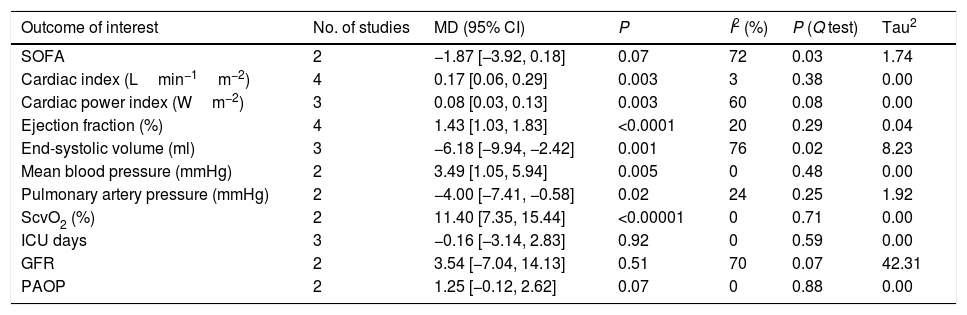
Levosimendan in patients with cardiogenic shock: SOFATwo studies10,17 reported SOFA that was not significant difference between levosimendan group and control group (MD −1.87[−3.92, 0.18], P for effect=0.07, P for heterogeneity=0.03, I2=72%, Tau2 1.74; Table 2 and Appendix A. Supplementary data).
Secondary end points.
| Outcome of interest | No. of studies | MD (95% CI) | P | I2 (%) | P (Q test) | Tau2 |
|---|---|---|---|---|---|---|
| SOFA | 2 | −1.87 [−3.92, 0.18] | 0.07 | 72 | 0.03 | 1.74 |
| Cardiac index (Lmin−1m−2) | 4 | 0.17 [0.06, 0.29] | 0.003 | 3 | 0.38 | 0.00 |
| Cardiac power index (Wm−2) | 3 | 0.08 [0.03, 0.13] | 0.003 | 60 | 0.08 | 0.00 |
| Ejection fraction (%) | 4 | 1.43 [1.03, 1.83] | <0.0001 | 20 | 0.29 | 0.04 |
| End-systolic volume (ml) | 3 | −6.18 [−9.94, −2.42] | 0.001 | 76 | 0.02 | 8.23 |
| Mean blood pressure (mmHg) | 2 | 3.49 [1.05, 5.94] | 0.005 | 0 | 0.48 | 0.00 |
| Pulmonary artery pressure (mmHg) | 2 | −4.00 [−7.41, −0.58] | 0.02 | 24 | 0.25 | 1.92 |
| ScvO2 (%) | 2 | 11.40 [7.35, 15.44] | <0.00001 | 0 | 0.71 | 0.00 |
| ICU days | 3 | −0.16 [−3.14, 2.83] | 0.92 | 0 | 0.59 | 0.00 |
| GFR | 2 | 3.54 [−7.04, 14.13] | 0.51 | 70 | 0.07 | 42.31 |
| PAOP | 2 | 1.25 [−0.12, 2.62] | 0.07 | 0 | 0.88 | 0.00 |
Four studies10,12,17,21 reported Cardiac Index that was significantly higher in the levosimendan group when compared with the control group (MD 0.17[0.06, 0.29], P for effect=0.003, P for heterogeneity=0.38, I2=3%, Tau2 0.00; Appendix A. Supplementary data).
Three studies10,12,17 reported Cardiac Power Index (CI*MAP*0.0022) that was significantly higher in the levosimendan group when compared with the control group (MD 0.08[0.03, 0.13], P for effect=0.003, P for heterogeneity=0.08, I2=60%, Tau2 0.00; Appendix A. Supplementary data).
Four studies12,14,21,22 reported Ejection Fraction that was significantly higher in the levosimendan group, when compared with the control group (MD 1.43[1.03, 1.83], P for effect<0.00001, P for heterogeneity=0.29, I2=20%, Tau2 1.33; Appendix A. Supplementary data).
Three studies13,21,22 reported End-Systolic Volume that was significantly lower in the levosimendan group when compared with the control group (MD −6.18[−9.94, −2.42], P for effect=0.001, P for heterogeneity=0.02, I2=76%, Tau2 8.23; Appendix A. Supplementary data).
Levosimendan in patients with cardiogenic shock: hemodynamicsTwo studies10,17 reported Mean Blood Pressure that was significantly higher in the levosimendan group when compared with the control group (MD 3.49[1.05, 5.94], P for effect=0.005, P for heterogeneity=0.60, I2=0%, Tau2 0.00; Appendix A. Supplementary data).
Two studies10,22 reported Pulmonary Atrial Pressure that was significantly lower in the levosimendan group when compared with the control group (MD −4.00[−7.41, −0.58], P for effect=0.02, P for heterogeneity=0.25, I2=24%, Tau2 1.92; Appendix A. Supplementary data).
Two studies10,19 reported SvO2 that was significantly higher in the levosimendan group when compared with the control group (MD 11.40[7.35, 15.44], P for effect<0.00001, P for heterogeneity=0.71, I2=0%, Tau2 0.00; Appendix A. Supplementary data).
Two studies10,17 reported pulmonary artery occlusion pressure (PAOP) was not significant difference between levosimendan group and control group (MD 1.25[−0.12, 2.62], P for effect=0.07, P for heterogeneity=0.88, I2=0%, Tau2 0.00; Appendix A. Supplementary data).
DiscussionWe performed a systematic review and meta-analysis of controlled trials and nRCTs to evaluate the effect of levosimendan compared with standard therapies or placebo on survival and hemodynamic parameters in patients presenting cardiogenic shock complicating myocardial infarction. It revealed that levosimendan is associated with improved cardiac function and many hemodynamic parameters, but it was not associated with a significant reduction in mortality, even if a non-significant trend was present. Notably, this is an important meta-analysis performed on levosimendan administration in patients with cardiogenic shock complicating myocardial infarction.
Cardiogenic shock is very different from the Low Cardiac Output Syndrome (LCOS). In many studies and meta-analyses, levosimendan was proved more effective than standard therapies in the patients with LCOS.23–25 The most important difference between cardiogenic shock and LCOS is the reduction in cardiac pump performance with hypo-perfusion to vital organs. Maybe the effect of Levosimendan could benefit more the population of LCOS than cardiogenic shock.
Levosimendan is a calcium enhancer with calcium-sensitizing activity. C-AMP independent and ATP neutral induce the improvement in calcium sensitivity of cardiac muscle cell.25 In 2003, Delle-Karth and co-worker26 published the successful use of levosimendan in patients with cardiogenic shock for the first time. In our meta-analysis, Levosimendan may eliminate the SOFA scores, enhance the cardiac functions, improve cardiac ejection fraction and cardiac volume of systolic and diastolic stage, and improve the other hemodynamic parameters. The effects could come from the levosimendan mechanism. Levosimendan exhibits calcium-dependent binding to the N-terminal domain of cardiac troponin C (TnC) with a higher affinity at high calcium concentrations and lower affinity at low calcium concentrations.25 The positive inotropic effect is obtained without increasing intracellular calcium concentration or without a significant increase in myocardial oxygen demand, usually seen with other inotropes.27,28 This implies that less energy is utilized by the cardiomyocytes, because re-internalization of calcium increases ATP expenditure and accounts for 30% of energy consumed by the cardiomyocyte during the contraction-relaxation cycle. A comparison of levosimendan and milrinone29 showed that both intensify cardiac contraction, but milrinone increased oxygen consumption, levosimendan did not. In other studies, levosimendan was also shown to be superior to dobutamine in term of myocardial efficiency.30,31
Again, we found that Levosimendan may improve the hemodynamic parameters. Several clinical observations reveal that levosimendan improve hemodynamics even in patients with cardiogenic shock if it is combined with catecholamines to maintain adequate perfusion pressure.32,33
Levosimendan was associated with a non-significant improve in survival. This may suggest that use of levosimendan in patients with cardiogenic shock complicating myocardial infarction does not offer a mortality benefit or, more probably, that too few patients have been randomized so far to reach powered conclusions on mortality. On the other hand, we also found that levosimendan can improve the hemodynamic parameters. Maybe the pooled analysis could have been insufficiently powered to detect a clinically relevant reduction in mortality in a general population of patients with cardiogenic shock. Perhaps, further larger high-quality RCTs are warranted to reach conclusions on the topic.
This meta-analysis has strengths and limitations. The methodological quality of the studies included in this meta-analysis was not optimal, with only one trial presenting low risk of bias.11 Not all trials reported all hemodynamic parameters, so these estimations are drawn from small numbers of measurements and should therefore be interpreted with caution. Unfortunately, there are only 5 randomized trials analyzing the effect of levosimendan in patients with cardiogenic shock. Ideally, a large randomized controlled trial on the lookout for the eventual beneficial effects of levosimendan in cardiogenic shock setting should be performed.
ConclusionIn summary, this systematic review of 13 clinical trials found that there was no evidence of survival benefit when levosimendan was compared to control therapies. On the other hand, levosimendan was associated with an improvement in hemodynamics and cardiac function. Further high quality studies on levosimendan for patients with cardiogenic shock complicating myocardial infarction are needed.
Authors’ contributionsStudy design: FM; study conduct: FM, CH; data analysis: FM, CH; data interpretation: FM, CH, WZ; writing and revising paper: FM, CH, WZ. All authors read and approved the final manuscript.
FundingThere was no funding source for this study.
Conflict of interestsThe authors did not have any conflict of interests.