Massive obstetric hemorrhage is a major cause of maternal mortality and morbidity worldwide. It is defined (among others) as the loss of >2500ml of blood, and is associated to a need for admission to critical care and/or hysterectomy. The relative hemodilution and high cardiac output found in normal pregnancy allows substantial bleeding before a drop in hemoglobin and/or hematocrit can be identified. Some comorbidities associated with pregnancy can contribute to the occurrence of catastrophic bleeding with consumption coagulopathy, which makes the situation even worse. Optimization, preparation, rational use of resources and protocolization of actions are often useful to improve outcomes in patients with postpartum hemorrhage. Using massive obstetric hemorrhage protocols is useful for facilitating rapid transfusion if needed, and can also be cost-effective. If hypofibrinogenemia during the bleeding episode is identified, early fibrinogen administration can be very useful. Other coagulation factors in addition to fibrinogen may be necessary during postpartum hemorrhage replacement measures in order to effectively correct coagulopathy. A hysterectomy is recommended if the medical and surgical measures prove ineffective.
La hemorragia masiva obstétrica es una de las causas principales de morbimortalidad materna en el mundo. Entre otras definiciones, se conoce como la pérdida>2.500ml de sangre y se asocia a ingreso en unidades de pacientes críticos y a histerectomía. Los cambios fisiológicos del embarazo permiten una hemorragia cuantiosa antes de objetivar una caída de la hemoglobina y/o el hematocrito. Dentro de los cambios fisiológicos del embarazo, existe una hipercoagulabilidad asociada a la gestante. Algunas comorbilidades asociadas al embarazo pueden contribuir a la aparición de una hemorragia catastrófica con una coagulopatía de consumo, que hace la situación aún más grave. La optimización, la preparación, el uso racional de recursos y la protocolización de actuaciones son útiles para mejorar los resultados en estas pacientes. El uso de protocolos basados en point of care con test viscoelásticos está demostrando utilidad. Si se produce una hipofibrinogenemia durante la hemorragia, la administración precoz de fibrinógeno puede ser muy útil. Para corregir eficazmente la coagulopatía pueden ser necesarios otros factores de la coagulación, además de fibrinógeno, durante la reposición en la hemorragia posparto. Se recomienda la realización de una histerectomía si las medidas médicas y quirúrgicas se han mostrado ineficaces.
Massive obstetric hemorrhage (MOH) is one of the leading causes of maternal morbidity–mortality in the world, particularly in developing countries–though in the industrialized world it is a growing cause for concern. Uterine atony is an increasingly frequent cause of MOH. This fact, and the growing number of cesarean sections, implicated in an increase in the number of cases of placenta accreta or abnormal placental attachment, have caused the incidence of MOH to increase. Furthermore, some cases of MOH are characterized by very severe coagulopathy and require adequate and intensive blood product replacement measures.1–3
Despite increasingly improved knowledge of MOH, research in this field has fundamentally centered on patients with massive hemorrhage associated to trauma – few studies having been focused on postpartum hemorrhage. However, obstetric patients differ markedly from trauma patients. In effect, the latter are often males; pregnancy is characterized by a series of physiological changes; and the mechanisms underlying hemorrhage in the two scenarios are completely different. These distinct features imply that the approach to management also may be different.
Recently, several clinical guides have been published on massive hemorrhage, with special emphasis on MOH.4–6
The present study deals with MOH in particular, beginning with its definition and reviewing the physiological and hemostatic changes in the pregnant patient, with a view to better understanding the physiopathology of MOH. With regard to treatment, we will describe the medical measures, the role of fibrinogen, and the transfusion indications involving protocols based on experience or guided by viscoelastic tests. Lastly, a series of recommendations will be made, with a series of key points, designed to help the reader to summarize and systematize the management of MOH.
Definition of obstetric hemorrhageHemorrhage is physiological following delivery. However, when bleeding exceeds a certain magnitude, it is considered pathological. It is difficult to clearly define obstetric hemorrhage, and many definitions have been proposed (Table 1).7–10
Summary of the main definitions of obstetric hemorrhage.
| Clinical guides | Definition |
|---|---|
| Australian, 20088 | Blood loss>500ml after delivery and 750ml after cesarean section |
| Austrian, 20084 | Blood loss 500–1000ml and signs of hypovolemic shock or bleeding>1000ml |
| German, 20084 | Blood loss>500ml after delivery Severe: loss>1000ml in 24h |
| RCOG, United Kingdom, 200910 | Primary: estimated loss>500–1000ml without signs of shock Severe: estimated loss>1000ml or signs of shock |
| WHO9 | Loss>500ml in 24h after delivery Severe: loss>1000ml in 24h |
RCOG, Royal College of Obstetricians and Gynaecologists; WHO, World Health Organization.
The quantification of hemorrhage is particularly difficult during delivery and/or cesarean section, since blood becomes mixed with other fluids. Furthermore, in the event of postpartum atony, a large amount of blood may be retained within the uterus, regardless of whether delivery has been normal (vaginal) or through cesarean section.1,2
The classical clinical signs (tachycardia and hypotension) are misleading in pregnancy, due to the notorious increase in plasmatic volume, and might not manifest until bleeding becomes very abundant.
The relative hemodilution and increased cardiac output inherent to normal pregnancy allow important blood loss to occur before a drop in hemoglobin concentration and/or hematocrit is identified.1,2
Hemorrhage is considered abnormal when over 500ml after vaginal delivery and over 1000ml after cesarean section. These volumes are exceeded in 1:20 deliveries or cesarean sections, respectively.11
Massive obstetric hemorrhage is defined as the loss of over 2500ml of blood, and is associated to significant morbidity; the need for admission to intensive care; and the indication of obstetric hysterectomy. Other definitions include: a drop in hemoglobin concentration of ≥4g/dl; the need for transfusion of ≥5 red cell concentrate units (RC); or the need to treat coagulopathy or perform invasive management procedures.12–14
The MOH rate is 6:10,000 deliveries, and the associated mortality rate is 1:1200 cases of MOH. The overall mortality rate due to obstetric hemorrhage is 0.39 per 100,000 maternities, and MOH is currently the third most frequent direct cause of maternal mortality in the United Kingdom.11
Specific problems of the obstetric patientPhysiological changes in pregnancyThe 20–30% increase in red cell mass, together with the 50% increase in plasmatic volume, cause pregnant women to present physiological dilutional anemia.
Pregnancy is characterized by inherent hypercoagulability, with an increase in the plasma concentration of almost all the coagulation factors (fibrinogen and factors vii, viii and ix), while the fibrinolytic system shows a decrease in activity. Plasminogen is seen to be increased, though its activity decreases due to the increase in plasminogen activator inhibitor type 2 (Table 2). Likewise, pregnancy is characterized by physiological hyperfibrinogenemia.
Physiological hematological changes in pregnancy.
| Increased factors | I, VII, IX, XII | ↑↑↑ |
| Unaltered factors | II, V | = = = |
| Decreased factors | XI, XIII | ↓↓↓ |
| Other parameters | PT ↑ 20%, aPTT ↑ 20%, platelets ↓, fibrinopeptide A↑ AT III ↓ PDF ↑ PAI 2 ↑ Plasminogen ↑ Proteins C and S ↓ TEG®/ROTEM®: hypercoagulable Red cell mass (20%) ↑ Plasmatic volume (50%) ↑↑ |
aPTT, activated partial thromboplastin time; AT III, antithrombin iii;PAI 2, plasminogen activator inhibitor type 2; TEG®/ROTEM®, viscoelastic tests (thromboelastography/thromboelastometry); PT, prothrombin time.
The natural anticoagulants, such as protein S, are seen to decrease–thereby contributing to a prothrombotic state, with an increase in fibrinolysis, particularly in the uterus, at the time of placental separation.
Pregnancy is associated to physiological thrombopenia, with no associated increased bleeding tendency.15
These changes result in a shortening of prothrombin time (PT) and activated partial thromboplastin time (aPTT), as well as an increase in thromboelastographic parameters: maximum clot firmness and maximum amplitude.16,17
Certain comorbidities associated to pregnancy can contribute to the appearance of catastrophic hemorrhage with consumption coagulopathy or disseminated intravascular coagulation (DIC).3
Monitoring of hemostasis and physiopathology of coagulopathy (Table 3)Routine coagulation tests are the most commonly used hemostasis monitoring tools in MOH. However, these tests are very slow in the context of a situation as dynamic as MOH. Furthermore, their sensitivity (PT, aPTT) might not be the most adequate. If the plasma fibrinogen level is used, it should be assessed with the Clauss method16–18 (Table 3).
Available viscoelastic tests.
| Test | Information | |
|---|---|---|
| ROTEM® | INTEM EXTEM FIBTEM APTEM HEPTEM | Coagulation factors, fibrin polymerization. Sensitive to heparin excess Coagulation factors, fibrin polymerization and fibrinolysis Assesses contribution of fibrinogen to clot firmness Assesses fibrinolysis Assesses correction of heparin excess |
| TEG® | Kaolin Kaolin heparinase Functional fibrinogen | Coagulation factors, fibrin polymerization and fibrinolysis Assesses correction of heparin excess Assesses contribution of fibrinogen to clot firmness |
The use of point of care systems based on thromboelastography (TEG, Haemonetics, Braintree, MA, USA) or thromboelastometry (ROTEM, TEM GmbH, Munich, Germany) is presently infrequent in delivery rooms. Viscoelastic tests, TEG/ROTEM, assess the viscoelastic properties of coagulation globally (cellular model of coagulation). The results are displayed graphically, allowing evaluation of clot formation and lysis in less than 10min. These tests can be performed at the patient bedside (point of care) and offer a number of advantages with respect to conventional coagulation tests: the results are obtained quickly, favoring early clinical decision making, and moreover global assessment of coagulation can be made in a whole blood sample. This in turn facilitates intensive replacement measures in MOH. Obstetric patients often present early and severe coagulation disorders that must be dealt with on an individualized basis. In this regard, TEG/ROTEM allows the individualized administration of blood components (fresh plasma and platelets) and of coagulation factor concentrates where necessary and in adequate amounts.6
The type, severity and incidence of coagulopathy differ according to the etiology of bleeding. In the case of atony and tearing of the genital canal, coagulopathy is predominantly dilutional. In contrast, if bleeding is due to placental detachment (abruptio placentae), consumption coagulopathy quickly develops, characterized by the rapid generation of hypofibrinogenemia and thrombopenia even with relatively limited initial blood losses.15,16
Factors consumption does not always meet the criteria of consumption coagulopathy. Genuine consumption coagulopathy is seen if amniotic fluid embolization, in some cases of severe preeclampsia or HELLP syndrome, and in severe placental detachment (abruptio placentae). These patients can quickly reach critical plasma fibrinogen levels. The local activation of coagulation (in the placental bed) and of the fibrinolytic system also contributes to the rapid development of consumption coagulopathy.3,15
The coagulation changes observed in pregnancy with the use of ROTEM include the identification of hypercoagulability,19 which can be confirmed with both TEG and ROTEM.20,21 This situation results in shorter coagulation times (CT in ROTEM or r in TEG) and in increased clot firmness values (maximum clot firmness in ROTEM and maximum amplitude in TEG). The thresholds for starting treatment therefore may be different from those applicable in other critical patients.16,22,23
A good correlation is observed between the standard coagulation test values and the ROTEM values on evaluating both variables in the immediate postpartum period.22,24,25
Likewise, correlations have been observed between ROTEM FIBTEM and fibrinogen concentration.22
It seems that ROTEM FIBTEM A5 (available in 10min) can be used in a way equivalent to the measurement of fibrinogen with the Clauss method, though it does not measure the same parameters. Roughly speaking, a FIBTEM of 15mm is equivalent to a fibrinogen value (Clauss) of about 3g/l; in turn, a value of 10mm is equivalent to 2g/l, while a value of 6mm is equivalent to 1g/l of fibrinogen.26
No large studies have been published to date on ROTEM and MOH, though if such studies were available, they could guide replacement therapy quickly and directly, and help to determine whether hemorrhage is being aggravated by altered hemostasis.
Medical treatmentIn the same way as in other situations of massive hemorrhage, the correct identification of MOH is crucial, since delays in establishing the diagnosis are accompanied by metabolic acidosis, hypothermia, coagulopathy and anemia–a combination that can prove fatal. Firm evidence supports the recommendation to correct these factors in massive hemorrhage.5
As regards communication and teamwork, close monitoring and precise documentation of the observations and times is required throughout the duration of the MOH episode. It is important to inform other team members of well-founded suspicions on an early basis.
The management of MOH begins with general and first line measures: manual and pharmacological interventions that must be introduced early and firmly, in under 30min.
General measures and resuscitation with intravenous fluidsThe correction of hypovolemia by means of the intravenous administration of crystalloids and/or colloids is a priority measure in all cases of acute hemorrhage.
Once the estimated blood losses have exceeded 1000ml and bleeding is continuous, it is advisable to open two large-caliber peripheral venous lines and start the administration of warmed colloids. Likewise, a sample should be sent to the blood bank for group typing and screening for irregular antibodies.
Resuscitation with intravenous fluids should begin quickly, without relying on a simple hemoglobin test result, which only serves to inform us of where the starting point happens to be.
The best volume replacement strategy can be the subject of much discussion.4
The maximum infusion volume should be limited, without exceeding 3.5l (up to 2l of crystalloids warmed as quickly as possible)–extendable to another 1500ml while in wait of the arrival of compatible blood.27 It must be taken into account that excessive fluid administration inexorably leads to dilutional coagulopathy.
The most widely used crystalloids are 0.9% isotonic saline solution, Ringer solution and other “balanced” solutions such as Hartmann solution (Ringer lactate). Generally, only 25% of the administered volume remains within the intravascular compartment. These solutions are inexpensive, do not alter hemostasis or renal function, and there is extensive experience with their use in clinical practice.
Colloids have a greater impact upon intravascular volume, but can inhibit platelet aggregation and interfere with correct measurement of the fibrinogen levels.28 The available colloids are hydroxyethyl starches, gelatins and human albumin. The infusion of 5% albumin produces plasmatic expansion equivalent to 75% of the infused volume. Due to their low molecular weight, gelatins have a short intravascular half-life (2–3h), and their expansive capacity is limited (70–80%). The hydroxyethyl starches administered at a concentration of 6% have a longer intravascular half-life (6–8h) and a greater expansive capacity (80–120%). The hydroxyethyl starches are currently the colloids most widely used for volume expansion purposes.6
In any case, when large volumes of fluids are administered via the intravenous route, it is advisable to warm them first.29
Identification of the cause of bleeding is also important, since it can exert a fundamental influence upon the patient management strategy. The “rule of 4 Ts” is easy to memorize and is widely used in MOH (Tone, Trauma, Tissue, Thrombin).30
First line measuresUterine atony is the most frequent cause of MOH, and the most widely used first line measures are: extraction of retained placenta fragments, uterine massage and bimanual compression. Optimization, preparation, rational use of resources and protocolization of interventions usually contribute to improve the outcomes in patients with MOH.30
Oxytocin is the most commonly used drug in MOH. The recommended dose varies from one center to another, though it must be underscored that while useful for treating uterine atony, administration of this drug–particularly as a bolus dose–is associated to vasodilatation, increased cardiac output, tachycardia and arterial hypotension. There have been occasional reports of myocardial ischemia associated to oxytocin use. Rapid administration of the drug should be avoided, since side effects may occur–particularly severe hypotension. If uterine tone is inadequate, we can administer 10–20 additional oxytocin units to an infusion of 1000ml of saline solution.31
Second line measuresErgot alkaloids are used when oxytocin proves ineffective (second line treatment). In this regard, 0.2mg of methylergonovine via the intramuscular route induces tetanic uterine contraction. These drugs cause intense vasoconstriction secondary to deep adrenergic stimulation. They are contraindicated in patients with hypertension, preeclampsia, ischemic heart disease or pulmonary hypertension.32
If methylergonovine does not prove effective or is contraindicated, the next drug on the list is prostaglandin F2α or carbaprost. This drug is injected via the intramuscular route (250mg), with repetition of the dose every 15–30min, until reaching a maximum of 2g. It is contraindicated in asthmatic women, since bronchospasm may occur.32
The second line treatments are indicated in the event of persistent hemorrhage. Hysterectomy must be viewed as the last option, and should be reserved for extreme cases of incoercible MOH that proves refractory to other management measures.13
Fibrinogen and postpartum hemorrhageAs has been commented, the determination of plasma fibrinogen using the Clauss method or FIBTEM in ROTEM® or Functional Fibrinogen in TEG® is recommended for diagnostic purposes, or when clinical management decisions must be made in the context of massive hemorrhage (grade of recommendation 1C).6
Recently, studies have been made of the changes in maternal coagulation profile in MOH. Low fibrinogen levels prior to delivery have been identified as an important risk factor for the development of MOH.23,26 Consumption coagulopathy characteristically accompanies different comorbidities in obstetrics (placental detachment, amniotic fluid embolization, dead fetus retention), though it is not so often correlated to other more common disorders such as uterine atony.32
Blood product replacement in MOH should place special emphasis on the early quantitation of plasma fibrinogen levels, with the rapid adoption of measures in response to low fibrinogen levels. In patients with MOH, plasma fibrinogen measurement has been identified as the parameter most closely correlated to the risk of massive postpartum hemorrhage and concomitant coagulopathy.4,23,26,33
Maternal fibrinogen levels have been independently associated to the severity of bleeding. If the fibrinogen concentration drops to under 2g/l at the onset of hemorrhage, the positive predictive value of this parameter in predicting MOH is 100%.4,23,33
Likewise, MOH increases the risk of thromboembolic phenomena during the postpartum period. Prophylactic measures against thromboembolic disease are therefore advised as soon as MOH ceases.18
The preventive administration of fibrinogen in cases of MOH has not been shown to be effective versus placebo.34 However, in this randomized clinical trial (RCT), fibrinogen was administered late, once important blood loss had occurred (>1000ml), and in the form of fixed doses. Further studies are needed to clarify its role in this context.
Obstetric hemorrhage and transfusionAlthough drug treatments and transfusion therapy are the principal tools in the management of MOH, the level of supporting evidence is low for most of these procedures. As a result, there may be bias in recommending certain actions. More quality research is essential in this field in order to be able to recommend safe measures and therapies for obstetric patients with MOH.35–39
The transfusion rate in obstetric patients is relatively low in developed countries (0.9–2.3%), though it has increased in recent years. Transfusion is an important indicator of obstetric morbidity, and should be a cause for concern among the administrations, as it has already become in the United States.40,41
The incidence of massive transfusion (10 or more RC units) is only 6 out of every 10,000 deliveries. The most frequent reason for massive transfusion is placentation anomalies (27%). This situation is worrisome, since the hysterectomy rate has increased in the United States in recent years.3
Rational use of the blood bank resources is mandatory. All Units attending deliveries should have several group O Rh-negative RC bags available (from 2 to 4) on an immediate basis.
Routine blood grouping and antibody screening is to be based on an individualized evaluation of risk: maternal history, possibility of complications (placenta accreta, placenta previa, previous cesarean sections), and the local policies applied in each hospital. A routine request for screening is not justified or necessary in normal and uncomplicated obstetric cases before cesarean section or delivery. Some studies on systematic blood grouping and screening in cesarean sections have found that only 1.7–3.3% of the patients required transfusion. Furthermore, over 60% of the cases of bleeding had no risk factors for transfusion. A full 98% of the patients with blood grouping and screening did not require transfusion. It is advisable first to stratify the risk and then, depending on the results, request the study in a standardized manner in each hospital.41
This is the example of Stanford Hospital (USA)3:
- (a)
In patients with low transfusion risk: only ABO and Rh testing.
- (b)
In patients with moderate transfusion risk: group typing and screening.
- (c)
In patients with high transfusion risk: screening and cross-matching.
In this hospital, the above strategy reduced testing by 55%, thereby contributing to a more rational use of resources.
- -
Group typing is performed in all patients upon admission. No additional studies are made in the case of low risk deliveries.
- -
In the event of excessive bleeding during delivery, an additional sample is collected for screening.
- -
A sample for screening is obtained in all cesarean sections.
- -
Cross-matching is requested in the case of elective high-risk cesarean sections.
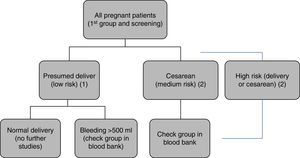
Protocol of Hospital Universitario La Paz for blood bank sample requests. All patients undergo blood sampling upon admission to hospital, with blood group typing and irregular antibody screening. (1) In presumed low-risk deliveries: no further studies are made. (2) In the case of hemorrhage>500ml, elective or emergency cesarean section, or presumed high-risk delivery (twins, anterior cesarean section, placentation anomalies, etc.), a second sample is sent to the blood bank for group confirmation, thereby facilitating the availability of compatible blood if needed.
The process for quickly obtaining RC in MOH can consume time and resources needed for other activities. The development of a MOH protocol facilitates the request and transport of RC. This aspect should be contemplated by the protocol algorithm of each hospital center.1,2,42,43
The use of MOH protocols has been shown to be useful for facilitating rapid transfusion of a sufficient volume of blood products, and such protocols are also cost-effective (lesser general use of products). Massive obstetric hemorrhage protocols moreover improve the communication lines referred to the request for transfusion and transport from the blood bank to the place where the blood is needed. Electronic or verbal instructions are required to activate the protocol, and the blood bank must be able to respond to such instructions within 5–10min.1,2,44
The composition of the MOH packets in the protocol varies. In general, they consist of 6–10 RC units (group O Rh-negative, without cross-matching), four units of AB plasma, and an apheresis platelets unit. If the results of the screening process are known, the blood bank may send isogroup RC units and A plasma instead of AB fluid, which is the first option in the emergency care setting. The plasma and platelets are to be administered early to correct coagulopathy and thrombopenia, which are so often seen in MOH. The correct RC/plasma ratio in the context of MOH is not clear, but the massive hemorrhage protocol packets are designed to simulate whole blood as closely as possible–thereby minimizing the impact of dilutional coagulopathy and hypovolemia.3,44
The current clinical guides4–6 clearly recommend the development of protocols for the management of MOH, though the level of evidence is modest. Nevertheless, the predefined ratios for the administration of blood products have not demonstrated their usefulness in this context. Likewise, the administration of factor concentrates lacks clear and defined indications.4
In the United States, 90–95% of all Units have a MOH protocol, though the corresponding availability and utilization rates are not known.44
In our center:
- -
The protocol is activated when the estimated blood loss exceeds 2000ml, or when despite smaller losses the patient is found to be hemodynamically unstable or presents altered consciousness. The anesthetist immediately requests help and is in charge of activating the protocol by means of a call to the blood bank.
- -
The blood bank immediately prepares a portable refrigerated box with 10 RC units, 10 fresh frozen plasma (FFP) units and two pools of platelets.
- -
The administration of fibrinogen concentrate starts once the protocol has been activated (4g via the intravenous route on an immediate and empirical basis). This product is immediately available in the delivery room (i.e., it does not have to be requested from the blood bank).
- -
If bleeding is not particularly serious, and particularly if it is not of very rapid onset, the request addressed to the blood bank follows the normal route (electronic or in paper format), which implies a longer response time on the part of the blood bank.
Intraoperative salvage involves the collection of blood from the surgical field, followed by washing and filtration, and reinfusion to the patient. Its use reduces the need for allogenic transfusions and their associated risks. This procedure moreover may be an acceptable option for people who reject transfusions. Bleeding must be considerable in order to collect a significant amount of blood amenable to reinfusion, and it is not always possible to foresee this when the intraoperative salvaging system is called for.45,46
The intraoperative recovery of blood in obstetric practice is now relatively safe; as a result, concerns about its use are being overcome (contamination with fetal components, activation of factors, etc.). Some international organizations such as the National Institute of Clinical Excellence, in the United Kingdom,10 recommend its use in MOH. The European guides on massive perioperative bleeding establish the following recommendations in this regard: “Perioperative recovery of blood in obstetrics is well tolerated, taking into account the necessary precautions referred to Rh isoimmunization” (grade of recommendation C). “We suggest that perioperative blood salvage during cesarean section may reduce the need for homologous blood and shorten hospital stay” (grade of recommendation 2B).4
A cost-effectiveness analysis of these salvage systems is needed. Intraoperative recovery of blood in MOH is only cost-effective in cesarean section with a high probability of bleeding (with the reinfusion of 2 or 3 RC units). Consideration is required not only of the costs of transfusion but also of the expendable materials, technical resources, time, etc. On the other hand, the current risks of allogenic transfusion must be added to the balance.45,46
Blood salvage systems have also recently been successfully used in vaginal deliveries.47
Selective arterial embolizationIf initial management proves ineffective, therapy must move on to second or third line treatment, with the activation of more resources and personnel. These measures include certain maneuvers or surgical options (intrauterine balloon, uterine contention sutures, etc.), in an attempt to avoid hysterectomy. Such maneuvers are more effective when applied on an early basis, and their effectiveness in turn decreases with increasing blood loss.1
If the abovementioned measures fail to stop the bleeding, we can resort to selective arterial embolization, pelvic devascularization or hysterectomy.2
In recent years, arterial embolization has become standard treatment for avoiding hysterectomy and preserving patient fertility. The success rate of this technique is over 80%, with a complications rate of under 10%. In some high risk cases, with placenta accreta or percreta, prophylactic arterial balloons have been placed in the common iliac or internal iliac arteries, with the aim of using them after delivery in necessary, and gain time.14
Deciding replacement measures (Fig. 2)Although the conventional coagulation tests are scantly reliable in guiding management in patients with massive postpartum hemorrhage,4 it must be remembered that viscoelastic testing is not available in many delivery rooms (Fig. 2).
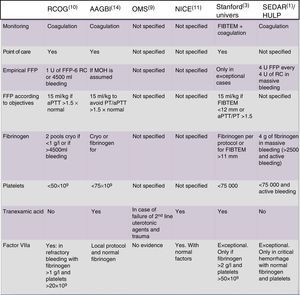
Recommendations of different international scientific societies on massive obstetric hemorrhage. AAGBI, Association of Anaesthetists of Great Britain and Ireland; aPTT, activated partial thromboplastin time; RC, red cell concentrate; FIBTEM, thromboelastometry measure (clot firmness and fibrinogen); MOH, massive obstetric hemorrhage; HULP, Hospital Universitario La Paz (Madrid, Spain); NICE, National Institute of Clinical Excellence (United Kingdom); WHO, World Health Organization; FFP, fresh frozen plasma; RCOG, Royal College of Obstetricians and Gynaecologists (United Kingdom); Stanford Univers, Stanford University (USA); SEDAR, Sociedad Española de Anestesia y Resuscitación (Spain); PT, prothrombin time.
Most of the guides and protocols currently used in reference to MOH are derived from the polytraumatized patient setting. Although there are no objective data warranting their use, some authors explicitly recommend application of the same general rules.18 In the obstetric population, an alteration of the conventional times (PT, aPTT) usually indicates altered hemostasis, and rapid and firm intervention may be required. An Italian clinical guide advises a PT and/or aPTT time >1.5 times beyond the normal limit as trigger reference for the administration of FFP.48 This same guide recommends the empirical administration of FFP if testing cannot be performed within a reasonable time limit–though some articles underscore that excessive FFP doses are often used.49
The British Society of Haematology guide on critical hemorrhage establishes the following recommendation: serial conventional hemostatic testing, including platelet count, prothrombin activity, aPTT and fibrinogen before and after patient resuscitation with fluids and blood products, should be requested regularly every 30–60min, depending on the severity of bleeding, with the purpose of serving as a guide and ensuring the correct use of hemostatic agents and blood components (grade of recommendation 1C).18
Furthermore, the plasma fibrinogen concentrations decrease in most patients with MOH, despite the excessive administration of FFP, thus pointing to the need for other alternatives.4
The current studies indicate that a plasma fibrinogen level of 1g/l is too low to secure adequate hemostasis in MOH, and that a threshold level of 2g/l would be more adequate in these patients. Some guides include specific recommendations on MOH, particularly as regards the use of tranexamic acid (TXA) and fibrinogen.
Fig. 2 offers a comparison of the recommendations of the main societies.1,3,7–10,14
Fresh frozen plasmaIn the same way as with other products, the rationale for the use of fresh frozen plasma (FFP) is based on its utilization in polytraumatized patients. A great variety of protocols have been developed, some using a fixed 1:1 ratio between RC and FFP, while others furthermore recommend the addition of a pool of platelets, likewise in 1:1:1 proportion. These products are generally used in the form of a “shock pack” once the MOH protocol has been activated. The reason for using these products is to try to maintain thrombin and fibrinogen generation through the replacement of factors as soon as possible, usually without having laboratory test results confirming the exact amounts to be replaced. The inconvenience of these packs without monitoring is that most women will be receiving products with a poor fibrinogen content–possibly lower than their circulating fibrinogen levels at that time. Donor plasma comes from non-pregnant donors and has a fibrinogen content of 2g/l. This concentration will result in a decrease in fibrinogen levels and in factor VIII and Von Willebrand factor concentrations following administration, as a consequence of the dilution.50,51
The current guides make no distinction with respect to the etiology of MOH; consequently, early and empirical plasma replacement measures would be warranted if it is suspected that important consumption of factors will take place (placental detachment or amniotic fluid embolization), or if important blood losses are expected (uterine rupture, placenta accreta). In contrast, in the event of uterine atony or tearing of the canal, early hemostatic alterations are not expected, and the empirical use of plasma therefore would not be justified. The administration of FFP at predefined ratios may be useful in trauma and possibly also in MOH, though the grade of recommendation in the case of MOH is only 2C.4
If the aPTT/PT ratio is taken as plasma transfusion trigger, hemostatic alteration already may be very great once the ratio reaches 1.59. If we wait for this value to be reached, it probably will be too late to start plasma infusion in MOH.14
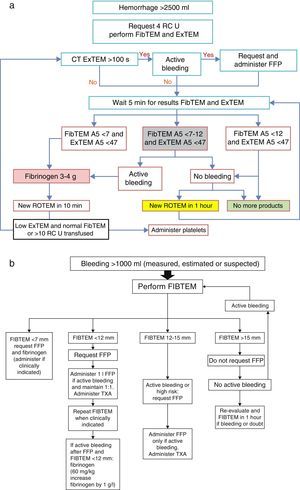
An algorithm (Fig. 3) has recently been published in relation to the infusion of FFP, based on the FIBTEM A5 (measure of functional fibrinogen after 5min), in severe hemorrhage (>2500ml and active bleeding). The algorithm recommends administration of fibrinogen concentrate immediately in the event of FIBTEM A5 <7mm, which corresponds to a very low plasma fibrinogen level for a pregnant woman (in place of the shock pack of FFP+platelets), and if bleeding is very intense, the consideration of fibrinogen administration is advised in the event of FIBTEM A5<12mm. With this protocol, the authors have demonstrated a decrease in the use of RC, FFP and platelets, with fewer complications such as circulatory overload associated to transfusion.16
(a) Protocol referred to massive obstetric hemorrhage>2500ml; (b) protocol referred to massive obstetric hemorrhage>1000ml; both guided by ROTEM. RC, red cell concentrate; EXTEM A5, thromboelastometry measure (extrinsic pathway) after 5min in mm; FIBTEM A5, thromboelastometry measure (clot firmness and fibrinogen) after 5min in mm; FFP, fresh frozen plasma; ROTEM, thromboelastometry; TXA, tranexamic acid.
Source: Collis and Collins.14
Future studies may further strengthen the use of point of care therapy. In this study, once bleeding has ceased, further hemostatic products should not be administered, regardless of the ROTEM result. The use of shock packs causes patient over-transfusion, and consequently no benefit at all can be expected in many cases.16
PlateletsThe guides recommend keeping the platelet count above 50×109l−1 during active hemorrhage. This means that infusion perhaps should be started once the count reaches 75×109l−1. A platelet count of under 75×109l−1 is very infrequent in pregnancy (except in situations of abruptio placentae, amniotic fluid embolization, severe preeclampsia or immune thrombopenia). Therefore, the 1:1:1 shock pack strategy will result in platelet over-transfusion, and this does not seem to be justified in all cases.14,15,50,51
Tranexamic acidThe administration of tranexamic acid (TXA) has a high grade of recommendation (1B) and is advised by a number of societies.5 The use of TXA has been shown to reduce the amount of bleeding and the need for transfusion in non-obstetric massive hemorrhage. Its administration in MOH has not been fully established to date, though it is increasingly used in daily practice, in view of the favorable results obtained in major surgery and in trauma. The current recommendations are summarized in Fig. 1.52,53
To date there are only small studies warranting the use of TXA in MOH. The WOMAN trial is a prospective, double-blind multicenter study that has been designed to investigate the use of TXA in early MOH. The trial will include 20,000 women on a randomized basis, with the administration of TXA or placebo following a postpartum blood loss of 500ml. The primary objective of the study is to evaluate the mortality and hysterectomy rates and determine whether these vary according to whether TXA is used or not. The trial will help clarify the usefulness of TXA in reference to the progression of hemorrhage.54
Cryoprecipitates and fibrinogen concentrateCryoprecipitates are not available in many Spanish hospitals. They have been used to maintain fibrinogen levels above 1–1.5g/l in those cases where the administration of FFP proves insufficient. A cryoprecipitate pool can increase plasma fibrinogen by 0.5g/l, though this is dependent upon consumption of the latter. In any case, the dose also depends on the plasma fibrinogen level we intend to reach.3,15 In addition to fibrinogen, the cryoprecipitate contains a high concentration of factor viii, Von Willebrand factor and factor xiii.14
Fibrinogen concentrates have been used to correct hypofibrinogenemia during MOH, though this application is not contemplated among the indications specified in the Summary of Product Characteristics in many countries. The existing literature is mainly based on clinical cases, case series and uncontrolled studies.2,55
Approximately 60mg/kg are needed to raise the level of fibrinogen by 1g/l, though in the event of additional consumption or dilution, the increments obtained can be lower.56
A metaanalysis published in 2012, with the inclusion of 6 randomized clinical trials involving no obstetric patients, concluded that the administration of cryoprecipitates reduces bleeding volume and the need for transfusions, but not mortality.57
Prothrombin complex concentrateProthrombin complex concentrate contains coagulation factors ii, vii, ix and x,and in some cases is used on an off-label basis in MOH. A study is currently being carried out on the use of prothrombin complex concentrate and fibrinogen during MOH.58
Prothrombin complex concentrate can be associated to thrombotic phenomena in non-pregnant patients; its use therefore must be well justified (risk-benefit balance), and should always be carried out after consulting the hematologist.14
Recombinant factor VIIThere has been great interest for a number of years in the use of recombinant factor vii for the treatment of life-threatening MOH, or for avoiding the need for hysterectomy, though the manufacturer of this product does not recommend it for this use.51,59
In the United Kingdom, the Royal College of Obstetricians and Gynecologists recommends the use of recombinant factor vii in MOH provided plasma fibrinogen is >1g/l and the platelet count >20×109l−1. The National Institute of Clinical Excellence moreover adds that the coagulation values should be normal before considering the use of factor vii.51
A Cochrane review has associated the use of factor vii to the appearance of arterial and venous thrombotic phenomena, and recommends limiting its use to the controlled clinical trial setting.60
Practical management of MOH- -
Careful quantification of blood loss is required (measurement, weight of dressings, pads, etc.).
- -
The implication of all human and material resources at the right moment is crucial in MOH, with appropriate and coordinated leadership in the delivery rooms and Units where patients susceptible to develop MOH may be located.
- -
Most cases of bleeding can be controlled without the need for transfusion, though in the event of MOH, transfusion is the norm.
- -
Once the MOH protocol has been activated, samples are to be collected for FIBTEM, with the measurement of hemoglobin, hematocrit and coagulation tests, according to the availability in each hospital center.
According to Collis and Collins14 (Fig. 3b):
- -
If FIBTEM>15mm (fibrinogen 3g/l), the rest of the factors will be normal, and FFP or cryoprecipitates probably will not be needed. Attention should focus mainly on monitoring and on the patient cardiovascular condition. Since most cases of non-massive postpartum hemorrhage are due to atony and trauma of the birth canal, which is not associated to great consumption of coagulation factors with early coagulation disorders or hypofibrinogenemia, early obstetric intervention generally suffices to control the situation without having to use coagulation products.14,32
- -
In the event of important blood loss (>1000ml) where active bleeding has ceased and FIBTEM is >15mm or between 12 and 15mm, the administration of FFP is not advised. In these cases it is not essential to move the patient to the operating or delivery room (if she is not already there) for revision of the canal or uterine cavity under anesthesia.14
- -
In the event of FIBTEM <12mm with persistent bleeding, the patient is to be urgently moved to the operating or delivery room (if she is not already there). If in addition the PT/aPTT ratios are altered, we administer 15ml/kg of FFP, and according to many authors fibrinogen concentrate should be used on an early basis, not only when plasma administration has failed to correct the bleeding. If the PT/aPTT ratio is >1.5, according to the Collis algorithm, we administer a larger FFP dose, after consulting the hematologist.14
Many authors, in line with the existing evidence (grade of recommendation 1B), advocate the use of 1g of TXA if hemorrhage cannot be controlled by the initial obstetric measures–particularly in the presence of hemostatic alterations.18,52
The role of simulation in MOHIn view of the great impact of MOH upon maternal mortality, the Confidential Enquiry into Maternal and Child Health recommends the use of simulation as a teaching and training tool for the implicated professionals.11
Simulations have been carried out for the quantification of hemorrhage, the identification of potential medication errors or the improvement of times to correct resolution of the MOH episode. One of the most recurrent issues in this scenario is the underestimation of bleeding by the participants. This aspect could be improved through periodic courses designed to ensure earlier intervention in real bleeding (particularly severe hemorrhage), since the greater the actual blood loss, the less accurate the estimated blood loss. However, when blood loss is small, the tendency is to overestimate the loss, and this also involves certain risks.61,62
Certain obstetric maneuvers can be practiced with simulators in order to acquire the necessary skills for adequate application in the real life scenario, in the same way as chest compression efficacy is tested in relation to cardiopulmonary resuscitation maneuvering.62
In sum, training in obstetric emergencies and particularly in MOH is a very potent tool that can improve the patient outcomes. However, it is only a tool–not a solution in itself. Simulation should be imparted by experts in the field, with the implication of different team members, in order to improve effectiveness and secure benefits for all.
Key points and recommendations- -
The early identification of MOH is crucial in order to avoid metabolic acidosis, hypothermia, coagulopathy and anemia–a combination that can prove fatal.
- -
Communication and teamwork: Close monitoring is required for the duration of MOH, with careful recording of the observations. It is important to inform other team members of well-founded suspicions of MOH on an early basis.
- -
Resuscitation with intravenous fluids should begin quickly, without relying on a simple hemoglobin test result, which only serves to inform us of where the starting point happens to be. Hypotension is always a late sign, and when it appears, immediate intervention is required.
- -
If our first efforts prove unsuccessful, more expert advice should be sought in order to make the right decisions (including hysterectomy) at the right moment.
- -
Uterotonic agents may salvage MOH, but can be hazardous if not used with caution. Carbetocin has not been shown to be better than oxytocin in uterine atony, and its current use in MOH is off-label. Misoprostol should only be used in concordance with the written recommendations and protocols. Methylergonovine is dangerous in the case of hypertensive patients, individuals with cardiovascular disease, and in certain ethnic groups, since it can cause vascular spasm.
- -
Adequate understanding and identification of the cause underlying the bleeding problem is required, establishing a correct diagnosis and not only prescribing symptomatic treatment.
- -
All Units that deal with deliveries should have a certain amount of type O Rh-negative blood immediately available in case of need (2–4 RC units).
- -
If hypofibrinogenemia is identified during hemorrhage, the early administration of fibrinogen may be very useful, though the precise indications of fibrinogen supplementing have not been fully established. Other coagulation factors, in addition to fibrinogen, may be needed to secure minimum thrombin formation levels.
- -
A hysterectomy is recommended only if the medical and surgical measures have been found to be ineffective.
- -
The personalization of management according to the concrete diagnosis is recommended, with continuous re-evaluation of the patient (since the situation in such cases is very dynamic), instead of insisting on ineffective or inappropriate treatment.
Emilia Guasch has received payment for conferences organized by CSL Behring, and has collaborated in the drafting of documents sponsored by that company. Fernando Gilsanz declares that he has no conflicts of interest.
Please cite this article as: Guasch E, Gilsanz F. Hemorragia masiva obstétrica: enfoque terapéutico actual. Med Intensiva. 2016;40:298–310.