Mortality in Acute Respiratory Distress Syndrome (ARDS) is decreasing, although its prognosis after hospital discharge and the prognostic accuracy of Berlin's new ARDS stratification are uncertain.
MethodsWe did a restrospective analysis of hospital and 6 month mortality of patients with ARDS admitted to the Intensive Care Unit of a University Hospital in Buenos Aires, between January 2008 and June 2011. ARDS was defined by PaO2/FiO2 lower than 200mmHg under ventilation with at least 10cmH2O of PEEP and a FiO2 higher or equal than 0.5 and the presence of bilateral infiltrates in chest radiography, in the absence of cardiogenic acute pulmonary edema, during the first 72h of mechanical ventilation. Mortality associated risk factors, the use of rescue therapies and Berlin's stratification for moderate and severe ARDS patients were considered.
ResultsNinety eight patients were included; mean age was 59±19 years old, 42.9% had mayor co-morbidities; APACHE II at admission was 22±7; SOFA at day 1 was 8±3. Prone position ventilation was applied in 20.4% and rescue measures in 12.2% (12 patients with nitric oxide and 1 with extracorporeal membrane oxygenation). Hospital and 6 months mortality were 37.7 and 43.8% respectively. After logistic regression analysis, only age, the presence of septic shock at admission, Ppl>30cmH2O, and major co-morbidities were independently associated with hospital outcome. There was no difference between moderate and severe groups (41.2 and 36.8% respectively; p=0.25).
ConclusionIn this cohort, including patients with severe hypoxemia and high percentage of mayor co-morbidities, ARDS associated mortality was lower than some previous studies. There was no increase in mortality after hospital discharge. There was no difference in mortality between moderate and severe groups according to Berlin's definition.
La mortalidad del distress respiratorio agudo está disminuyendo, aunque hay poca evidencia sobre su pronóstico después del egreso hospitalario y la adecuada estratificación pronóstica con la nueva clasificación de Berlín.
MétodosSe analizó retrospectivamente la mortalidad de pacientes con SDRA admitidos en la Unidad de Cuidados Críticos de1 Hospital Universitario de la ciudad de Buenos Aires, desde el 1 de 2008 hasta el 6 de 2011. Se definió SDRA por hipoxemia con PaO2/FiO2≤200mmHg con al menos 10 cmH2O de PEEP y FiO2≥0,5 e infiltrados bilaterales en la radiografía de tórax en ausencia de edema agudo de pulmón cardiogénico en las primeras 72h de ventilación mecánica. Se registraron la mortalidad hospitalaria y a 6 meses, los factores asociados a mortalidad, la utilización de terapias de rescate, y la validez de la clasificación de Berlín para casos moderados y graves.
ResultadosSe incluyeron 98 pacientes; edad 59±19 años; 42,9% con comorbilidades mayores; APACHEII 22±7; SOFA (día 1) 8±3. La VM en posición prono se aplicó en 20,4% y en 12,2% rescates especiales (12 óxido nítrico y 1 ECMO). La mortalidad hospitalaria y a 6 meses fue de 37,7 y 43,8% respectivamente. Los factores asociados a mortalidad fueron: edad, shock séptico en las primeras 72 h, presión plateau (Ppl) >30cmH2O durante las primeras 72 h y la presencia de comorbilidades preexistentes. No hubo diferencia de mortalidad entre los grupos moderado y grave (41,2 vs. 36,8%; p=0,25).
ConclusionesEn este estudio que incluyó pacientes con hipoxemia más grave y alto porcentaje con comorbilidades mayores, la mortalidad fue menor que en algunos estudios previos; no hubo incremento en la mortalidad después del egreso hospitalario. La clasificación de Berlín no diferenció el pronóstico entre los casos moderados y graves.
Despite improved knowledge of the physiopathology of acute respiratory distress syndrome (ARDS) and technological advances, controversy remains as to whether there has been a resulting decrease in patient mortality. In this regard, recent epidemiological studies describe a high in-hospital mortality rate despite the introduction of protective mechanical ventilation (MV) strategies.1,2 However, in patients with important hypoxemia, the use of higher positive end-expiratory pressure (PEEP) levels could reduce mortality associated to refractory hypoxemia, the need for recue therapeutic measures, and the days on MV.3–5 Likewise, and as indicated by a recent trial, MV in the prone position appears to result in significantly improved survival.6 However, randomized clinical trials tend to exclude seriously ill patients and individuals with a poorer prognosis.7 It therefore would be very interesting to obtain prognostic information from studies conducted outside the specific context of clinical trials, and involving protective MV strategies with low tidal volumes (Vt) and higher PEEP levels.8
A recently proposed definition of ARDS has included a minimum PEEP level for considering oxygen alteration, and has classified severity according to the PaO2/FiO2 ratio.9 Since its publication, some authors have questioned the clinical usefulness of this stratification, since the PEEP value at the time of diagnosis does not appear to have prognostic relevance,10,11 at least not without adapting PaO2/FiO2 to standardized PEEP and FiO2 levels.
The present study describes mortality among the patients with ARDS in our Intensive Care Unit (ICU), including only those individuals with moderate to severe ARDS and persistent hypoxemia after 24h of MV, with adjusted PEEP and FiO2 levels. Likewise, it defines the conditions associated to mortality, and determines whether the Berlin classification allows prognostic stratification of our patients.
Material and methodsPatientsA review was made of the case histories of all the patients admitted to our ICU between January 2008 and June 2011. We initially selected patients over 18 years of age with a diagnosis of ARDS according to the criteria of the American-European consensus of 1994 (AECC).12 We only considered patients presenting hypoxemia with PaO2/FiO2≤200mmHg on MV with PEEP levels ≥10cmH2O and with FiO2≥0.5 within the first 72h of MV, and with chest X-rays revealing bilateral infiltrates in four quadrants in the absence of cardiogenic acute lung edema. The decision to include only patients with hypoxemia under concrete PEEP and FiO2 levels was based on a previous study in which a PaO2/FiO2 ratio of ≤200mmHg with at least 10cmH2O of PEEP clearly differentiated patient prognosis.
Data acquisitionThe data were collected retrospectively from the case histories of the patients admitted to the ICU of our University Hospital over a period of 42 consecutive months. We recorded demographic and etiological data, the APACHE II score upon admission, major comorbidities (active cancer disease, solid organ transplants, hematopoietic cell transplants and other immune depression states), gas exchange (pH, pCO2, pO2, PaO2/FiO2), MV parameters (peak pressure, plateau pressure [Ppl], total PEEP, working pressure [Ppl minus total PEEP], exhaled Vt, Vt per kg ideal body weight [Vt/kg], static compliance of the respiratory system) and the SOFA score for assessing organ dysfunction.13 In our Unit, all patients are measured upon admission with a metric tape, recording height and ideal body weight both in the case history and in the daily monitoring table for patients on MV.
The data referred to MV and organ dysfunction were recorded on days 1, 3, 7 and 10 of MV. In-hospital mortality and mortality after 6 months were documented.
Refractory hypoxemia and hypercapnia were defined as the persistence of PaO2/FiO2≤80mmHg with FiO2≥0.9 during at least 1h, and pH≤7.20 with PaCO2≥80mmHg, respectively, after optimizing MV with recruitment maneuvers, reducing Vt to 4–6ml/kg ideal body weight, elevating the respiratory frequency (RF) (limited by the generation of auto-PEEP or intrinsic PEEP) and adopting patient pronation for 12h in the selected individuals. All these data were collected from the abovementioned MV monitoring table, used in all patients subjected to MV in our Unit. This table is updated every 8h from the start of MV until patient extubation or death. Mechanical ventilation in the prone position was used in patients presenting PaO2/FiO2≤100mmHg with at least 15cmH2O of PEEP, and following recruitment maneuvering.
All patients were ventilated in flow assisted/controlled mode with a constant flow curve. Sedoanalgesia was administered in the form of a continuous infusion of midazolam or propofol plus fentanyl, and occasionally using muscle relaxants according to the criterion of the attending physician.
Due to the retrospective nature of the study, the fact that the data were obtained from the normal patient records, and the observation of patient confidentiality, the hospital Ethics Committee approved conduction of the study without requiring the obtainment of informed consent.
Statistical analysisContinuous variables were reported as the mean and standard deviation (SD) or median with the interquartile range, as applicable. Continuous variables and proportions were compared using the Student t-test and chi-squared test, respectively. A logistic regression model was developed, entering those variables that proved significant in the univariate analysis between survivors and deceased individuals, and considering mortality as final outcome or endpoint.
A forward stepwise model was developed. Statistical significance was considered for p≤0.05 in all cases.
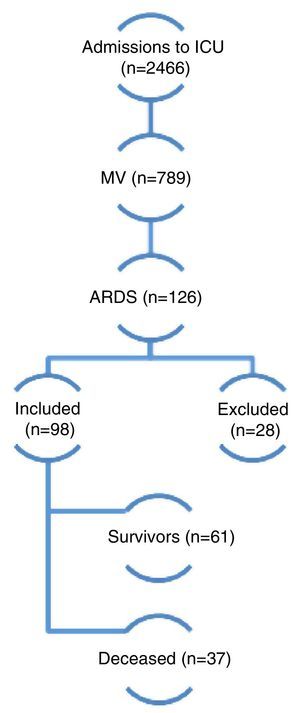
ResultsDuring the study period a total of 2466 patients were admitted to the ICU and 789 required MV. Of these, 98 (12.4%) met the inclusion criteria and were finally included in the analysis (Fig. 1). Follow-up during 6 months proved possible in 52 of 61 patients discharged alive (85.2%). The demographic and physiological data are reported in Table 1. The mean age was 59 years; 57% were males; the APACHE II score upon admission was 22±7 points; and the SOFA score on day 1 was 8±3 points.
A total of 2466 patients were admitted to the ICU over 42 consecutive months from January 2008 to June 2011. Of these, 789 required MV, and 126 presented ARDS according to the criteria of the American-European consensus of 1994 (AECC). Among the latter subjects, 28 were excluded due to improvement in under 24h of adjusting MV with adequate PEEP and FiO2 levels. A total of 98 patients were finally included in the study. Of these, 37 died and 61 survived (overall mortality rate 37.7%). MV: mechanical ventilation; ARDS: acute respiratory distress syndrome.
Patient demographic data.
| Total population (n=98) | Survivors (n=61) | Deceased (n=37) | p-value | |
|---|---|---|---|---|
| Age (years) | 59 (19) | 55 (18) | 66 (17) | 0.01 |
| Ideal body weight (kg) | 60 (10) | 59.1 (10.7) | 63.2 (8.6) | 0.11 |
| APACHE II score | 22 (7) | 21 (7) | 23 (6) | 0.22 |
| SOFA score (day 1) | 8 (3) | 7 (3) | 9 (3) | 0.01 |
| Major comorbiditiesa(%) | 42 (42.9) | 15 (24.5) | 27 (73) | 0.01 |
| Septic shock (%) | 52 (53) | 27 (44.2) | 25 (67.5) | 0.03 |
| Primary injury (%) | 59 (60.2) | 37 (60.6) | 22 (59.4) | 0.82 |
| Secondary injury (%) | 39 (39.8) | 24 (39.3) | 15 (40.5) | 0.73 |
| Reason for admission to ICU (%) | ||||
| Pneumonia | 50 (51) | 30 (49.2) | 20 (54) | |
| Extrapulmonary sepsis | 24 (24.5) | 13 (21.3) | 11 (29.8) | |
| Chest surgery | 7 (7.1) | 4 (6.5) | 3 (8.1) | |
| Trauma | 11 (11.2) | 10 (16.4) | 1 (2.7) | |
| Burns | 3 (3.1) | 2 (3.3) | 1 (2.7) | |
| Othersb | 3 (3.1) | 2 (3.3) | 1 (2.7) | |
APACHE II: Acute Physiology, Age and Chronic Health Evaluation; SOFA: Sepsis-related Organ Failure Assessment.
Data expressed as mean (standard deviation), or number of patients (percentage).
The most frequent comorbidity was active, advanced-stage solid organ cancer, present in 24 patients (24.5%). Fourteen patients (14.3%) suffered other immune depressive conditions (5 solid organ transplants, 4 cases of acute leukemia with hematopoietic cell transplantation, 2 with immunosuppressive treatment due to systemic lupus erythematosus, one with immunosuppressive treatment due to Wegener's disease, one with chronic corticosteroid use, one with CHILD class C cirrhosis due to hepatitis B infection). The main diagnoses upon admission were pneumonia (51%), extrapulmonary sepsis (24.5%), polytraumatism (11.2%) and chest surgery (7.1%).
The PEEP and Vt/kg levels used in the first 48h of MV were 13.3 (±2.9)cmH2O and 6.8 (±1)ml/kg, respectively. The Ppl value was 27.4 (±4)cmH2O (Table 2). The PaO2/FiO2 ratio on day 1 of MV was 145 (±40)mmHg. In 12 cases (12.2%) rescue treatment measures were adopted due to refractory hypoxemia or hypercapnia: nitric oxide (NO) in 12 patients, with extracorporeal membrane oxygenation (ECMO) in one of them. Ventilation in the prone position was carried out in 20 patients (20.4%). Although this was not a randomized or prospective study, and no comparative analysis therefore could be made, the mortality rate among the global patients who received alternative treatments was 31%, which is lower than the global mortality rate for our series, despite the fact that these were patients with greater ventilatory and oxygenation problems.
Mechanical ventilation and gas exchange data.
| Total population (n=98) | Survivors (n=61) | Deceased (n=37) | p-value | |
|---|---|---|---|---|
| Total PEEP (cmH2O) | 13.3 (3.2) | 12.2 (2.9) | 13.5 (2.8) | 0.7 |
| Tidal volume (ml) | 411 (68) | 407 (66) | 417 (70) | 0.8 |
| Tidal volume (ml/kg/ideal body weight) | 6.8 (1) | 6.8 (1.1) | 6.8 (0.7) | 0.7 |
| Ppl (cmH2O) | 27.4 (4) | 26.8 (4) | 28.5 (4) | 0.9 |
| Csr (ml/cmH2O) | 30 (9) | 31 (8) | 29 (10) | 0.4 |
| PaO2/FiO2 (mmHg) | 145 (40) | 147 (40) | 142 (39) | 0.5 |
| pH | 7.28 (0.08) | 7.29 (0.06) | 7.26 (0.09) | 0.04 |
| PaCO2 (mmHg) | 47 (9) | 47 (9) | 48 (11) | 0.3 |
All data were recorded on day 1 of mechanical ventilation.
Csr: static compliance of the respiratory system; FiO2: fraction of inspired oxygen; PaO2: partial pressure of oxygen in arterial blood; PaCO2: partial pressure of carbon dioxide in arterial blood; PEEP: positive end-expiratory pressure; Ppl: end-inspiratory plateau pressure.
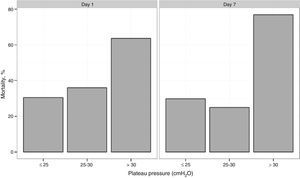
The in-hospital and 6 months mortality rate was 37.7% (37 of 98 patients) and 43.8% (39 of 89 patients), respectively. The duration of in-ICU and in-hospital stay was 21 (range 14–39) and 34 (range 21–52) days, respectively. Mortality was higher among the patients with comorbidities (60.5% vs 23.3%, p≤0.01). All the patients with acute leukemia and hematopoietic cell transplantation died. The mortality rates in the patients with PaO2/FiO2 >100mmHg or ≤100mmHg showed no significant differences (41.2% vs 36.8%; p=0.25). In 27 patients, Ppl was >30cmH2O for at least 24h during the first 48h. The mortality rate in this group was 63% versus 28% in the cases with Ppl≤30cmH2O (p=0.002). On grouping the patients into tertiles according to increasing Ppl ranges (≤25, >25–≤30 and >30cmH2O), the mortality rate was seen to increase progressively (22.9%, 41.9% and 55%, respectively; p=0.046) (Fig. 2).
The working pressure was 14.6 (±3.7)cmH2O in the patients who survived and 16.2 cmH2O (±3.6) in those who died (p=0.04).
Other factors associated to increased mortality were the presence of septic shock (67.5% vs 25.5%; p=0.03) and the SOFA score of day 1 (8.9 [±3.1] vs 7.4 [±2.7] points; p=0.01).
The logistic regression model for predicting in-hospital mortality included patient age, the SOFA score, working pressure (day 1), pH (day 1), Ppl>30cmH2O for at least 24h during the first 48h, the presence of major comorbidities and septic shock. Only age, the presence of septic shock, Ppl>30cmH2O and major comorbidities were found to be independently associated to increased mortality (Table 3).
DiscussionIn this study of patients with ARDS we found the mortality rate to be lower than reported in previous studies, even on considering the more seriously ill cases8,14–18 (Table 4). The factors associated to mortality were the presence of major comorbidities, age, septic shock and MV with Ppl>30cmH2O. In contrast to the proposal of the Berlin classification of ARDS, where the prognosis is stratified according to impairment of the PaO2/FiO2 ratio, we observed no significant differences in mortality between patients with moderate ARDS (PaO2/FiO2>100mmHg and ≤200mmHg) and severe ARDS (PaO2/FiO2≤100mmHg).9
Comparison of the principal demographic and ventilatory variables and of mortality between the different published series and our population.
| ALIVE (2003) | VENTILA (2004) | KCLIP (2005) | FINNALI (2009) | ALIEN (2011) | Villar et al. (2007) | CEMIC (2011) | |
|---|---|---|---|---|---|---|---|
| n=401 | n=198 | n=828 | n=68 | n=255 | n=99 | n=98 | |
| Inclusion criterion | AECC (≤200) | AECC (≤200) | AECC (≤200) | AECC (≤300) | AECC (≤200) | AECC (≤200)+PEEP≥10 and FiO2≥0.5 | AECC (≤200)+PEEP≥10 and FiO2≥0.5 |
| Age | 55.4 (18) | 62 | 60.6 | 61 | 58 (41–73) | 56 (44–67) | 63 (47–72) |
| APACHE II | n/d | n/d | 26 (8) | n/d | 21.6 (5.9) | 20.8(6.9) | 22 (7) |
| PaO2/FiO2 | 119 (43) | n/d | n/d | 200 | 114 (40) | 155.8 (30) | 146 (40) |
| PEEP | 7.7 (3.5) | 5–12 | n/d | 8 | 9.3 (2.4) | 11.4 (3.1) | 13 (3) |
| Vt/kg | 8.3 (1.9) | 6–8 | n/d | 8.6 | 7.2 (1.1) | 7.5 (1.7) | 7 (1) |
| Mortality | 57.9% | 63% | 41.10% | 47% | 47.8% | 45.50% | 37.70% |
FiO2: fraction of inspired oxygen; PaO2: partial pressure of oxygen in arterial blood 1; PEEP: positive end-expiratory pressure; Vt/kg: tidal volume per kg ideal body weight.
There is controversy as to whether the introduction of protective MV has resulted in effectively decreased mortality in ARDS. A recent systematic review including studies carried out in the protective ventilation era recorded no significant differences in mortality between the years 1994 and 2006.2 However, two of the studies involving high PEEP levels (LOVS and Express) were not included in this analysis.3,4 Although these two studies revealed no improvement in survival, a metaanalysis that included both these trials and the ALVEOLI study19 detected an improved prognosis in the subgroup of patients with greater oxygenation problems and exposed to high PEEP levels.
In our population, the criterion for selecting PEEP initially included the level needed to obtain a Ppl of up to 28–30cmH2O (as used in the Express trial),3 and in the group of patients with more intense hypoxemia, PEEP was selected on the basis of the transpulmonary pressure (Ptp) with the aim of reaching and end-expiratory Ptp of between 0 and 5cmH2O.20 These selection methods surely resulted in the application of higher PEEP levels, which in addition to the strict selection of low Vt values, may have contributed to the decrease in mortality observed in a group of patients with mild ARDS. In this group the application of high PEEP levels may be deleterious.5
A previous study, involving stratification according to FiO2 and PEEP in the group of patients with PaO2/FiO2≤200mmHg and at least 10cmH2O of PEEP (i.e., similar to the criteria used in selecting our own patients), reported higher mortality8 – though statistical significance cannot be inferred, due in part to the size of the populations in the two studies. Of the different factors that could account for this difference, mention must be made of the utilization of PEEP levels lower than those used in our study (11.45±3.09 vs 13.3±2.9cmH2O), and the lesser use of rescue measures in refractory cases. In our population, special rescue therapies were applied in 12% of the patients, particularly ON inhalation, and ECMO in a single case.
Controversy remains regarding the role of rescue therapies in the management of ARDS. As an example, no improvement in patient survival has been demonstrated with the use of NO, though in this case the clinical trials were carried out before the introduction of protective MV strategies, and generally involved patients without severely impaired oxygenation.21 On the other hand, the utilization of ECMO has gained renewed impulse in recent years thanks to technological developments and the results of a clinical trial demonstrating benefit in terms of survival among selected patients.22–25
Another ventilation strategy that may have contributed to the improved survival observed could be the use of ventilation in the prone position, which was more widely employed than in some previous studies,26 reaching 20.4% of the population. To date, only some trials had evaluated the effect of ventilation in the prone position, with no observation of increased benefits. However, a recent study has recorded significant improvement in survival with ventilation in the prone position introduced early and with longer sessions, together with protective MV measures.6
Another aspect to be considered in the ventilation strategy is the general recommendation to maintain Ppl levels of <30cmH2O.27 It has been questioned whether this recommendation applies to all patients, taking into account that the true pressure that distends the lungs is Ptp.28 Nevertheless, a Ppl of >30cmH2O was independently associated to increased mortality. In this sense, some studies have demonstrated the presence of alveolar overdistension and the release of inflammatory mediators with Ppl levels even >25cmH2O.20,29 The subgroup of patients in which Ppl levels of >30cmH2O were allowed surely represents the cases most refractory to treatment despite the selection of high PEEP levels, strict reduction of Vt, and the application of recruitment maneuvering. The observation of increased mortality in the patients with Ppl>30cmH2O could warrant the earlier introduction of alternative therapies which through the extracorporeal removal of CO2 could allow even further lowering of the applied Vt to below 4ml/kg.30
The difference in mortality between the different cohorts could also be explained by other factors such as the patient screening criteria used, the clinical characteristics, the severity of ARDS, and comorbidities. The previous clinical characteristics of the patients are important in relation to the prognosis, particularly the presence of major comorbidities such as cancer or immune depression. In our study all the patients with hematopoietic cell transplantation and neutropenia who developed severe ARDS died. The mortality rate in patients with immune impairment or active malignancy remains high, since the cause giving rise to ARDS is usually difficult to revert. Furthermore, it is not uncommon for these patients to present several subsequent complications.31,32
Another factor associated to increased mortality was the concomitant presence of septic shock and its association to multiorgan failure, which could explain the poorer prognosis in comparison with other etiologies of ARDS.18,33 Lastly, older age was also associated to poorer survival. Although the usefulness of age as a prognostic marker in certain diseases has been questioned,34 in the case of ARDS the association has been previously established by a number of studies.18,35
A new definition of ARDS has recently been proposed: the Berlin definition, based on the PaO2/FiO2 ratio in patients ventilated with at least 5cmH2O of PEEP.9 Likewise, a prognostic stratification based on the PaO2/FiO2 ratio has been proposed. In our series, in the same way as in some recent studies published after the Berlin classification,10,11 we observed no significant differences in mortality between the groups of patients with PaO2/FiO2 above or below 100mmHg. The application of ventilation in the prone position in patients with greater gas exchange alterations, and the option of using special rescue measures, possibly may have contributed to reduce mortality in the more serious cases. On the other hand, the mortality associated to ARDS depends not only on the degree of lung injury but also on the patient basal conditions and the presence of other organ dysfunctions.
Although there were some missing data in the registry of mortality after 6 months in our study (9 patients were lost), only two of the patients discharged from hospital died in the course of the follow-up period, and both of them had pre-existing comorbidities. The epidemiological studies in general only report in-hospital mortality and/or mortality after 90 days.14–18 In series involving longer follow-up, pulmonary, cognitive, neuromuscular and psychological defects have been recorded as long as 5 years after the ARDS episode.36–39 In an epidemiological study with 109 survivors, the mortality rate after 12 months was reported as 11%, with most of the fatalities occurring in the first few months.37
Our study has several limitations. Firstly, its retrospective nature increases the risk of missing data. Nevertheless, we were able to compile full information on most of the patients, even after discharge. Secondly, as this was a single-center study, its external validity may be questioned. Likewise, other conditions that could have an impact upon survival, such as ventilator-associated pneumonia (VAP) or pneumothorax, were not contemplated. Lastly, although we evaluated mortality over the long term, no analysis was made of morbidity, chronic MV dependency or other disabilities.
If the introduction of protective MV is found to result in an improved patient prognosis beyond the context of controlled studies, multicenter epidemiological studies involving large patient samples would be needed for confirmation purposes.
In conclusion, our findings suggest that mortality associated to severe ARDS is less than reported in earlier years, and is independently correlated to patient age, septic shock, MV with Ppl>30cmH2O, and the existence of previous comorbidities. Mechanical ventilation using low Vt and high PEEP levels, together with ventilation in the prone position and the adoption of rescue measures in patients with refractory hypoxemia or hypercapnia may have contributed to this potential benefit in terms of survival. In those patients discharged from hospital, we observed no increase in mortality upon assessment after 6 months. Lastly, the Berlin classification did not allow stratification of the prognosis between cases of severe ARDS and moderate ARDS.
Conflicts of interestThe authors have received no financial support for the conduction of this study, and declare that they have no conflicts of interest.