Prognostic scales are needed in acute exacerbation of chronic heart failure to detect early mortality. The objective of this study is to create a prognostic scale (scale EAHFE-3D) to stratify the risk of death the very short term.
Patients and methodWe used the EAHFE database, a multipurpose, multicenter registry with prospective follow-up currently including 6597 patients with acute heart failure attended at 34 Spanish Emergency Departments from 2007 to 2014. The following variables were collected: demographic, personal history, data of acute episode and 3-day mortality. The derivation cohort included patients recruited during 2009 and 2011 EAHFE registry spots (n=3640). The classifying variable was all-cause 3-day mortality. A prognostic scale (3D-EAHFE scale) with the results of the multivariate analysis based on the weight of the OR was created. The 3D-EAHFE scale was validated using the cohort of patients included in 2014 spot (n=2957).
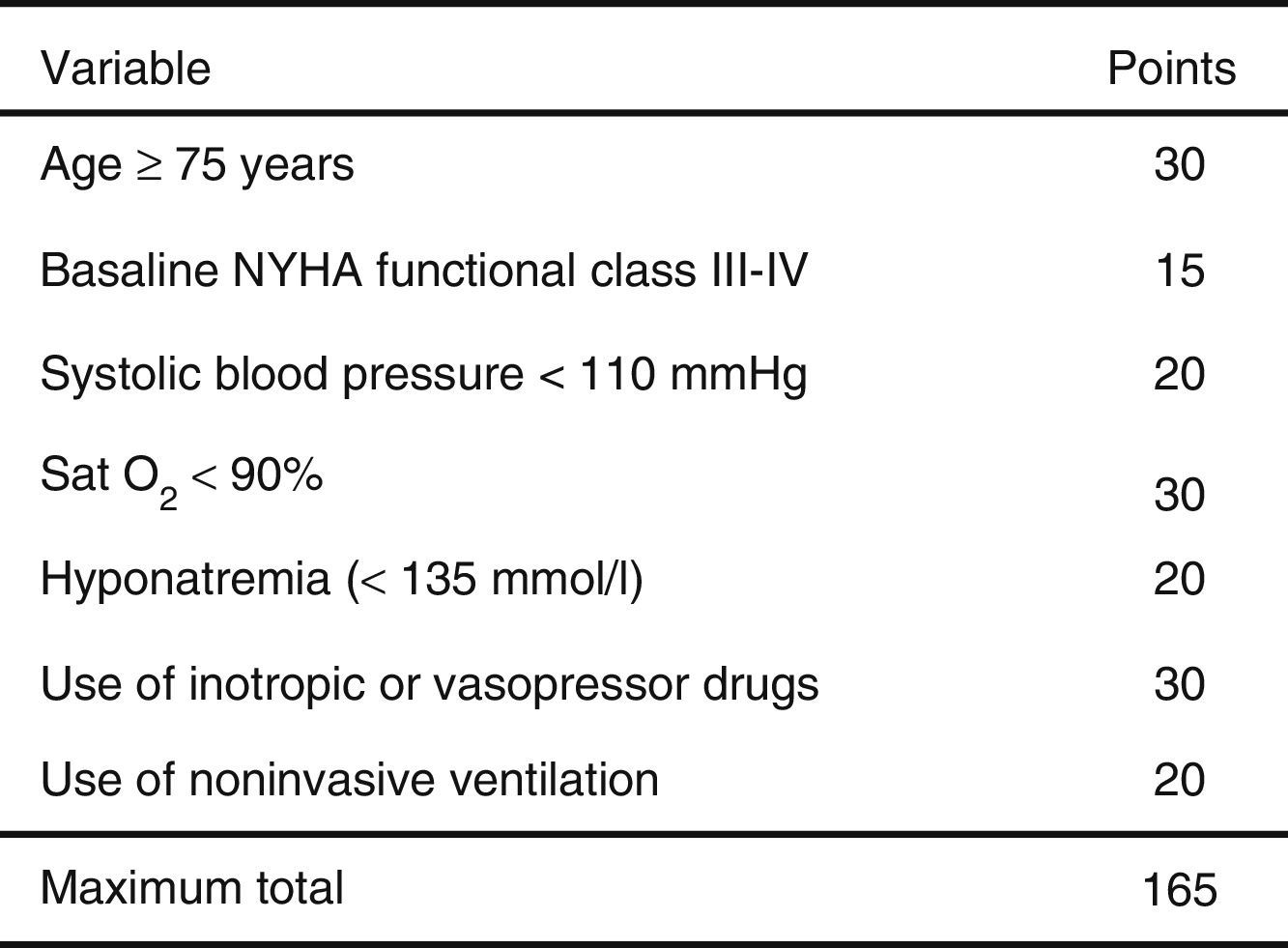
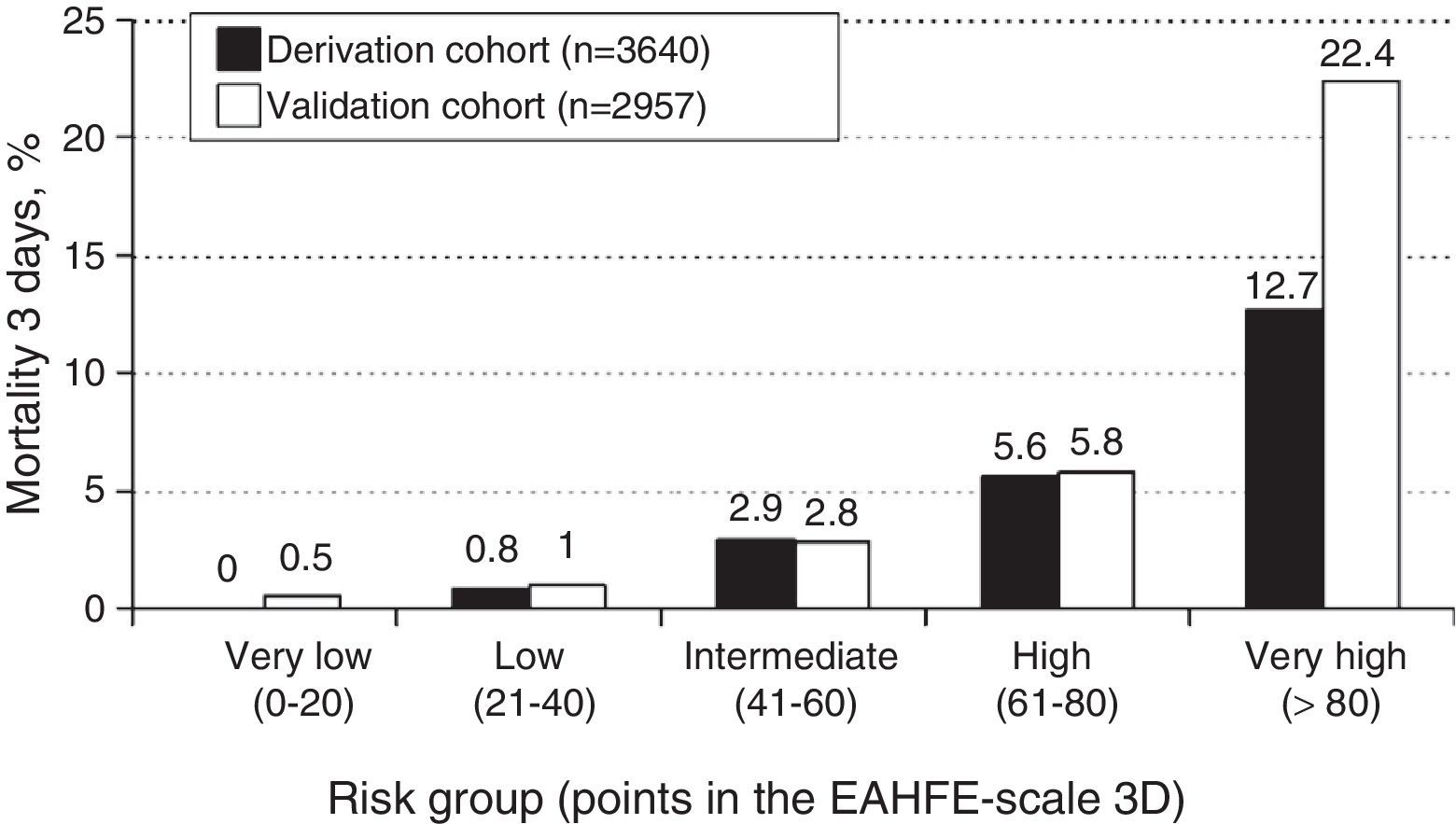
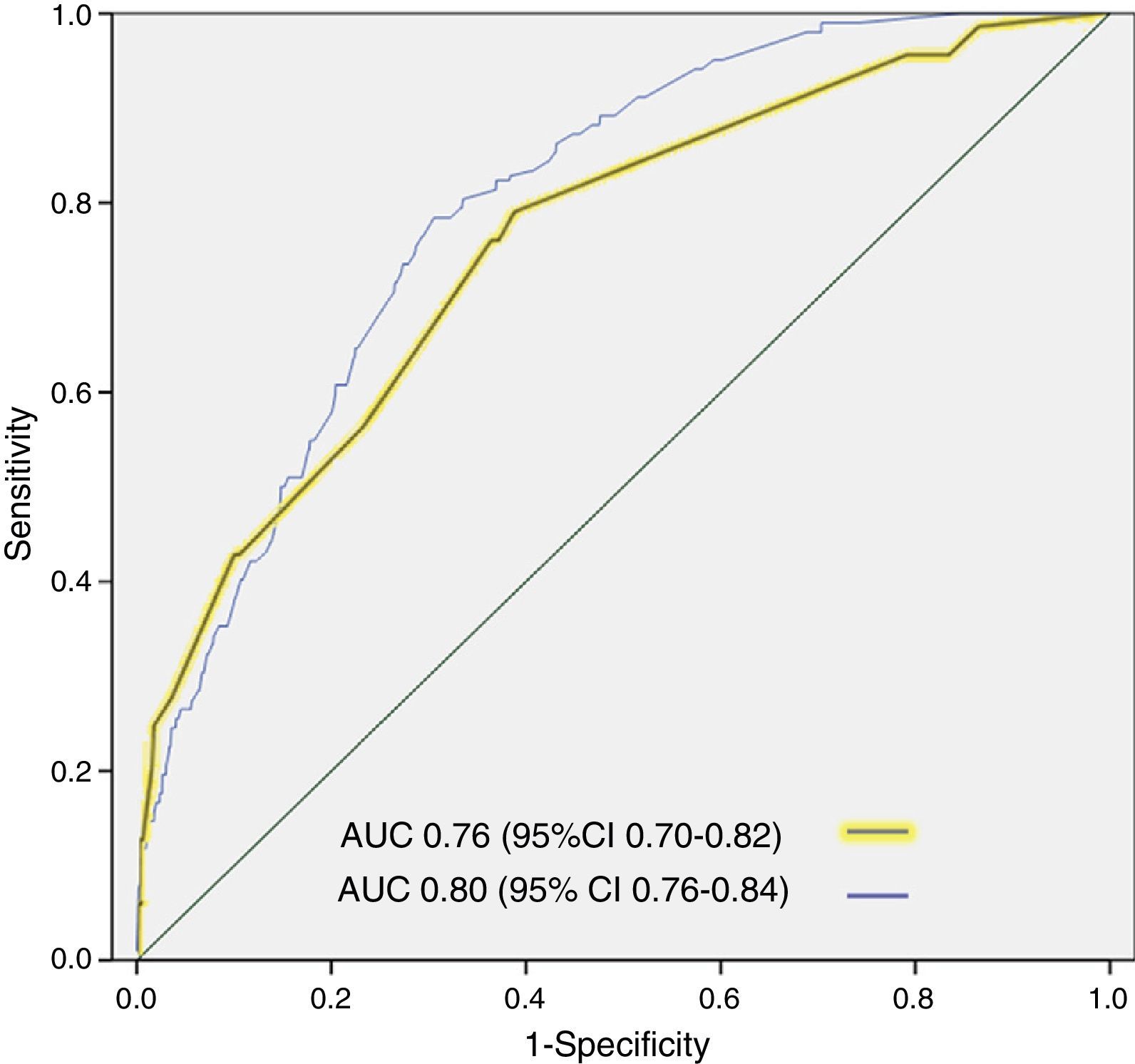
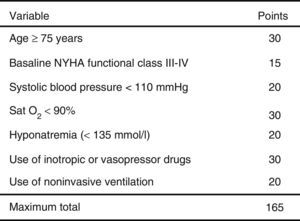
ResultsA total of 3640 patients were used in the derivation cohort and 102 (2.8%) died at 3 days. The final scale contained the following variables (maximum 165 points): age≥75 years (30 points), baseline NYHA III-IV (15 points), systolic blood pressure<110mmHg (20 points), room-air oxygen saturation<90% (30 points), hyponatremia (20 points), inotropic or vasopressor treatment (30 points) and need for noninvasive mechanical ventilation (20 points); with a ROC curve of 0.80 (95%CI 0.76–0.84; p<0.001). The validation cohort included 2957 patients (66 died at 3 days, 2.2%), and the scale obtained a ROC curve of 0.76 (95%CI 0.70–0.82; p<0.001). The risk groups consisted of very low risk (0–20 points), low risk (21–40 points), intermediate risk (41–60 points), high risk (61–80 points) and very high risk (>80 points), with a mortality (derivation/validation cohorts) of 0/0.5, 0.8/1.0, 2.9/2.8, 5.5/5.8 and 12.7/22.4%, respectively.
ConclusionsEAHFE-3D scale may help to predict the very short term prognosis of patients with acute heart failure in 5 risk groups.
Disponer de escalas pronósticas en la insuficiencia cardiaca crónica agudizada para detectar la mortalidad precoz es fundamental. El objetivo de este estudio es crear una escala pronóstica (escala EAHFE-3D) que estratifique el riesgo de muerte a muy corto plazo.
Pacientes y métodoSe utilizó el registro EAHFE, multipropósito y multicéntrico, con seguimiento prospectivo que incluye 6.597 pacientes con insuficiencia cardiaca crónica agudizada atendidos en 34 servicios de urgencias españoles entre 2007 y 2014. Se recogieron variables demográficas, antecedentes personales, datos del episodio agudo, destino final y mortalidad a los 3 días. La cohorte de derivación incluye pacientes seleccionados entre 2009 y 2011 en el registro EAHFE (n=3.640). La variable a estudio fue la mortalidad a los 3 días. Se creó una escala pronóstica (escala EAHFE-3D) con los resultados del estudio multivariante en función del peso de la OR. La escala fue validada utilizando una cohorte de pacientes incluidos en 2014 (n=2.957).
ResultadosSe analizaron 3.640 pacientes (102 muertos a los 3 días, 2,8%) en la cohorte de derivación. La escala final contiene las siguientes variables (máximo 165 puntos): edad≥75 años (30 puntos), NYHA basal iii-iv (15 puntos), presión arterial sistólica<110mmHg (20 puntos), saturación de O2<90% (30 puntos), hiponatremia (20 puntos), tratamiento inotropo o vasopresor (30 puntos) y necesidad de ventilación mecánica no invasiva (20 puntos), con un área bajo la curva ROC de 0,80 (IC 95% 0,76-0,84; p<0,001). La cohorte de validación incluye 2.957 pacientes (66 muertos a los 3 días, 2,2%) y la escala obtiene un área bajo la curva ROC de 0,76 (IC 95% 0,70-0,82; p<0,001). Los grupos fueron: muy bajo riesgo (0-20 puntos), bajo riesgo (21-40 puntos), riesgo intermedio (41-60 puntos), alto riesgo (61-80 puntos) y muy alto riesgo (>80 puntos), con una mortalidad (cohorte de derivación/validación) de 0/0,5, 0,8/1,0%, 2,9/2,8, 5,5/5,8 y 12,7/22,4%, respectivamente.
ConclusionesLa escala EAHFE-3D puede ser de ayuda para estratificar el pronóstico a muy corto plazo de los pacientes con insuficiencia cardiaca crónica agudizada en 5 grupos de riesgo.
Acute exacerbation of chronic heart failure (ECHF) is a frequent cause of consultation in hospital Emergency Care Departments (ECDs), and its prevalence increases with advancing age.1 The condition is associated to important mortality rates in hospital and after 30 days, reaching 5% and 10%, respectively. Repeated ECHF episodes are associated to progressive functional loss and increased mortality due to any cause.2
Acute exacerbation of chronic heart failure is a syndrome with a broad range of severity. On one hand we have patients that report to the ECD with low-risk ECHF and who might not need hospital admission. In fact, 24% of the patients attended due to an ECHF episode in Spanish ECDs are discharged directly from emergency care.3 A priority in the management of processes of this kind is adequate identification these patients, since failure to do so may result in an increase in inadequate admissions–with the consequent loss of efficiency in the use of the available resources.4–6 On the other hand, at the opposite extreme, we have very high-risk patients with important mortality over the very short term. If such individuals were adequately identified, they could benefit from more individualized medical care, and more adequate information could be offered to the relatives and/or caregivers. Furthermore, and always considering the patient basal situation and life expectancy, prompt identification could help us in the decision making process, with more aggressive management from the start, and better selection of monitoring beds or of admission to the Intensive Care Unit (ICU).7 In this scenario, failure to identify such patients can give rise to delays in immediate management and diagnostic procedures, a lack of continuous monitoring, inadequate discharge from emergency care or admission to inappropriate hospital wards–with an increased risk of adverse events.
To date, most studies that have developed risk stratification scales for patients seen due to ECHF have focused on in-hospital mortality, without specifying when death occurs, or on mortality over the middle (generally 30–60 days) or long term (up to 5 years).8–14 Furthermore, they generally only include patients admitted to hospital wards. To our knowledge, early mortality studies are very scarce,15 despite their importance in relation to decision making in emergency care, e.g., the intensity and timing of treatment, patient admission to the ICU, and the definition of a first prognostic impression with which to inform the patient and family.
Kawase et al. found elevated lactate concentrations (>3.2mmol/l) to be associated to increased early mortality, with longer ICU stays among those who survive.16 Lancellotti et al. in turn reported greater mortality among patients with ECHF and an elevated heart rate in the first 24–36h, and found that controlling the heart rate can improve the early mortality statistics.17 The European Society of Cardiology recommends and insists on the need for early and intensive patient management.18
Taking the above into account, a period of three days has been defined as the interval in which immediate measures adopted in the ICU can be most effective and have the greatest impact. However, there is no supporting scientific evidence referred to such a short period of time. The aim of the present study was to identify factors associated to mortality after three days and which can be quickly documented in the ECD, with the purpose of establishing a very early mortality risk stratification prognostic scale in patients attended due to an ECHF episode in the ECD.
Patients and methodsThe EAHFE-3D study is a secondary analysis of the Epidemiology of Acute Heart Failure in Emergency Departments (EAHFE) registry.3 It is a multipurpose cohort study of an analytical, non-interventional and multicenter nature with prospective follow-up that consecutively included all patients seen due to ECHF in 34 Spanish ECDs. The patient inclusion criteria were compliance with the diagnostic criteria for ECHF based on the presence of symptoms (dyspnea, orthopnea, paroxysmal nocturnal dyspnea) and signs (third sound, lung crepitants, jugular venous pressure>4cm, resting sinus node tachycardia, edemas, hepatomegalia, hepatojugular reflux), and radiological evidence of pulmonary congestion, requiring immediate treatment for stabilization. This study only excluded patients with ST-segment elevation acute coronary syndrome. The EAHFE study has had four patient enrollment phases. In order to generate the prognostic scale, use was made of the data collected in EAHFE-1 (from 15 April to 15 May 2007, with 1107 patients), EAHFE-2 (from 1 to 30 June 2009, with 1483 patients) and EAHFE-3 (from 7 November 2011 to 7 January 2012, with 3255 patients)–representing a total of 5845 patients enrolled in 29 ECDs. For the present post hoc study we included those patients with all the values needed to perform the intended analysis and corresponding to the follow-up period of three days. In order to validate the scale we used the data of the EAHFE-4 (from 1 January to 28 February 2014, with 2957 patients enrolled in 34 ECDs). The data collection methodology was the same in all four periods and in all centers, and has been published elsewhere.3,19 The study was approved by the Clinical Research Ethics Committees of the participating hospitals, and informed consent was obtained from the patients included in the study.
The patient baseline characteristics were recorded (age, gender, history of disease, previous treatment for heart failure, functional dependency as evaluated by the Barthel index, New York Heart Association [NYHA] functional class), along with information on the acute episode of ECHF–both clinical (dyspnea, orthopnea, paroxysmal nocturnal dyspnea, hepatomegalia, hepatojugular reflux, edemas, heart rate, systolic blood pressure [SBP]) and referred to complementary tests (sodium, creatinine, glomerular filtration rate calculated with the MDRD formula)20–and the treatment provided in emergency care. All the variables were dichotomized according to the cutoff point considered clinically most reasonable. The dependent variable of the study was mortality after three days. This information was obtained by telephone contact after discharge and through clinical follow-up of the hospitalized patients.
Qualitative variables were reported as absolute and relative frequencies, while quantitative variables were reported as the mean and standard deviation. Comparisons were made using the chi-squared test (or 2×2 tables with the Fisher exact test when the expected values were under 5) in the case of qualitative variables, and the Student t-test for independent measures in the case of quantitative variables. Those variables found to present significant differences in the univariate analysis, expressed as odds ratios (ORs), were entered in a logistic regression model. The variables that maintained statistical significance in the multivariate analysis were used to create a weighted scale according to their impact in the final model. The discrimination capacity of the model was assessed based on the area (AUC) under the receiver operating characteristic (ROC) curve. An analysis was subsequently made of the distribution curve of the EAHFE-3D scores according to mortality, with the arbitrary definition of 5 risk groups according to those cutoff points that made clinical sense. These cutoff points defined ascending risk categories (very low, low, intermediate, high and very high risk, respectively). Differences were considered statistically significant for p<0.05, or when the 95% confidence interval (CI) of the OR excluded the value 1, or when the AUC excluded the value 0.5. Validation was made with the EAHFE-4 cohort, and its AUC was calculated. Comparison of the AUC was carried out using the methodology of DeLong. The SPSS® version 19.0 statistical package was used, and the program STATA® 12.0 was employed for the comparison of AUC.
ResultsOf the 5845 episodes included in the EAHFE registry, a total of 3640 for which 100% of the information was available for the derivation cohort were analyzed. Of these patients, 101 (2.8%) died in the course of the three days after ECD consultation.
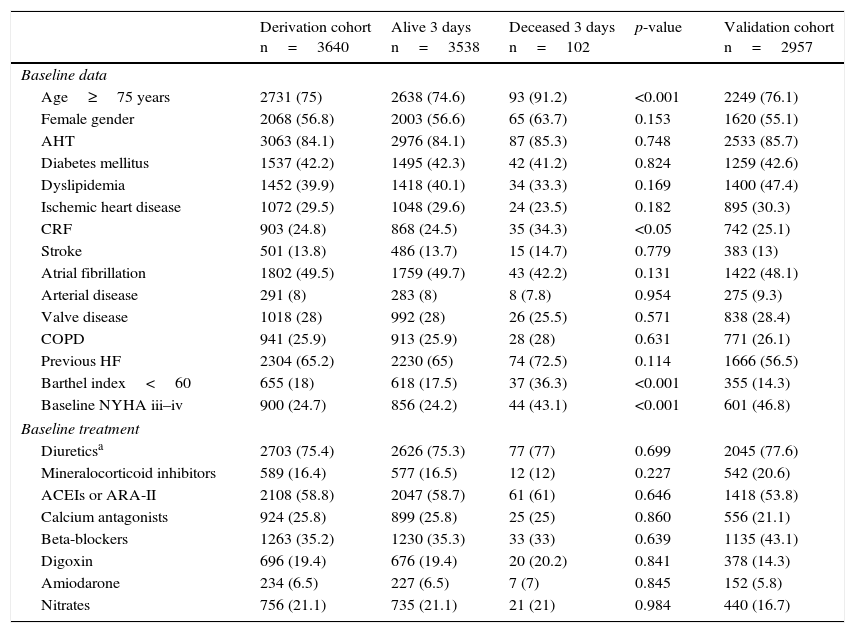
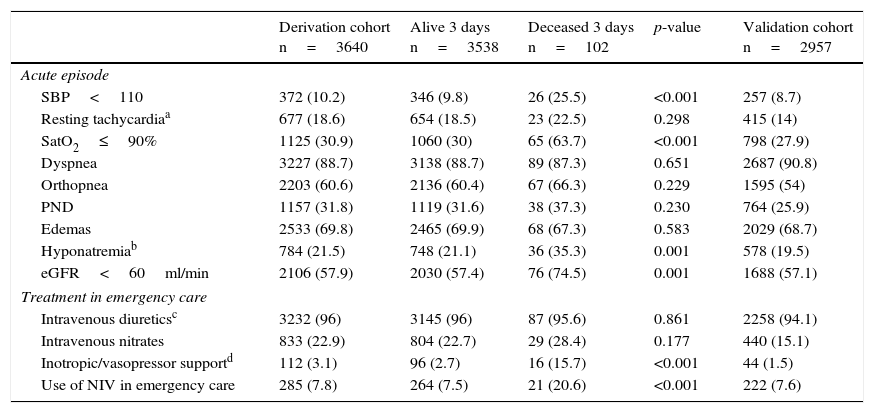
The characteristics of the derivation cohort of both groups and of the validation cohort are described in Tables 1 and 2. In the derivation cohort, of the 102 patients that had died after three days, 16.7% had been discharged home (n=17). Of these, 70.6% (n=12) again visited the ECD; as a result, 95.1% finally died in hospital (n=97). Regarding the destination of the patients that died, 8.9% (n=9) were considered amenable to intensive care and were admitted to the ICU. Of note in this regard is that up to 66% of the patients (n=57) were admitted to units dependent upon Internal Medicine. The early mortality predictors in the univariate analysis were: patient age≥75 years, the presence of chronic renal failure, functional dependency (Barthel index<60 points) and a baseline NYHA functional class of iii or iv. With regard to background treatment, no differences were observed between the two groups. In relation to clinical presentation, the identified early mortality predictors were: SBP<110mmHg, SatO2<90%, hyponatremia (sodium<135mmol/l), and an estimated glomerular filtration rate (eGFR)<60ml/min. Lastly, with regard to the treatment provided in emergency care, inotropic or vasopressor medication, and noninvasive ventilation (NIV), were correlated to increased mortality.
Univariate analysis of the baseline characteristics of the derivation cohort.
| Derivation cohort n=3640 | Alive 3 days n=3538 | Deceased 3 days n=102 | p-value | Validation cohort n=2957 | |
|---|---|---|---|---|---|
| Baseline data | |||||
| Age≥75 years | 2731 (75) | 2638 (74.6) | 93 (91.2) | <0.001 | 2249 (76.1) |
| Female gender | 2068 (56.8) | 2003 (56.6) | 65 (63.7) | 0.153 | 1620 (55.1) |
| AHT | 3063 (84.1) | 2976 (84.1) | 87 (85.3) | 0.748 | 2533 (85.7) |
| Diabetes mellitus | 1537 (42.2) | 1495 (42.3) | 42 (41.2) | 0.824 | 1259 (42.6) |
| Dyslipidemia | 1452 (39.9) | 1418 (40.1) | 34 (33.3) | 0.169 | 1400 (47.4) |
| Ischemic heart disease | 1072 (29.5) | 1048 (29.6) | 24 (23.5) | 0.182 | 895 (30.3) |
| CRF | 903 (24.8) | 868 (24.5) | 35 (34.3) | <0.05 | 742 (25.1) |
| Stroke | 501 (13.8) | 486 (13.7) | 15 (14.7) | 0.779 | 383 (13) |
| Atrial fibrillation | 1802 (49.5) | 1759 (49.7) | 43 (42.2) | 0.131 | 1422 (48.1) |
| Arterial disease | 291 (8) | 283 (8) | 8 (7.8) | 0.954 | 275 (9.3) |
| Valve disease | 1018 (28) | 992 (28) | 26 (25.5) | 0.571 | 838 (28.4) |
| COPD | 941 (25.9) | 913 (25.9) | 28 (28) | 0.631 | 771 (26.1) |
| Previous HF | 2304 (65.2) | 2230 (65) | 74 (72.5) | 0.114 | 1666 (56.5) |
| Barthel index<60 | 655 (18) | 618 (17.5) | 37 (36.3) | <0.001 | 355 (14.3) |
| Baseline NYHA iii–iv | 900 (24.7) | 856 (24.2) | 44 (43.1) | <0.001 | 601 (46.8) |
| Baseline treatment | |||||
| Diureticsa | 2703 (75.4) | 2626 (75.3) | 77 (77) | 0.699 | 2045 (77.6) |
| Mineralocorticoid inhibitors | 589 (16.4) | 577 (16.5) | 12 (12) | 0.227 | 542 (20.6) |
| ACEIs or ARA-II | 2108 (58.8) | 2047 (58.7) | 61 (61) | 0.646 | 1418 (53.8) |
| Calcium antagonists | 924 (25.8) | 899 (25.8) | 25 (25) | 0.860 | 556 (21.1) |
| Beta-blockers | 1263 (35.2) | 1230 (35.3) | 33 (33) | 0.639 | 1135 (43.1) |
| Digoxin | 696 (19.4) | 676 (19.4) | 20 (20.2) | 0.841 | 378 (14.3) |
| Amiodarone | 234 (6.5) | 227 (6.5) | 7 (7) | 0.845 | 152 (5.8) |
| Nitrates | 756 (21.1) | 735 (21.1) | 21 (21) | 0.984 | 440 (16.7) |
ARA-II: angiotensin II receptor antagonists; COPD: chronic obstructive pulmonary disease; AHT: arterial hypertension; HF: heart failure; ACEIs: angiotensin-converting enzyme inhibitors; CRF: chronic renal failure defined by creatinine>2mg/dl; NYHA: New York Heart Association.
Univariate analysis of the baseline characteristics of the derivation cohort.
| Derivation cohort n=3640 | Alive 3 days n=3538 | Deceased 3 days n=102 | p-value | Validation cohort n=2957 | |
|---|---|---|---|---|---|
| Acute episode | |||||
| SBP<110 | 372 (10.2) | 346 (9.8) | 26 (25.5) | <0.001 | 257 (8.7) |
| Resting tachycardiaa | 677 (18.6) | 654 (18.5) | 23 (22.5) | 0.298 | 415 (14) |
| SatO2≤90% | 1125 (30.9) | 1060 (30) | 65 (63.7) | <0.001 | 798 (27.9) |
| Dyspnea | 3227 (88.7) | 3138 (88.7) | 89 (87.3) | 0.651 | 2687 (90.8) |
| Orthopnea | 2203 (60.6) | 2136 (60.4) | 67 (66.3) | 0.229 | 1595 (54) |
| PND | 1157 (31.8) | 1119 (31.6) | 38 (37.3) | 0.230 | 764 (25.9) |
| Edemas | 2533 (69.8) | 2465 (69.9) | 68 (67.3) | 0.583 | 2029 (68.7) |
| Hyponatremiab | 784 (21.5) | 748 (21.1) | 36 (35.3) | 0.001 | 578 (19.5) |
| eGFR<60ml/min | 2106 (57.9) | 2030 (57.4) | 76 (74.5) | 0.001 | 1688 (57.1) |
| Treatment in emergency care | |||||
| Intravenous diureticsc | 3232 (96) | 3145 (96) | 87 (95.6) | 0.861 | 2258 (94.1) |
| Intravenous nitrates | 833 (22.9) | 804 (22.7) | 29 (28.4) | 0.177 | 440 (15.1) |
| Inotropic/vasopressor supportd | 112 (3.1) | 96 (2.7) | 16 (15.7) | <0.001 | 44 (1.5) |
| Use of NIV in emergency care | 285 (7.8) | 264 (7.5) | 21 (20.6) | <0.001 | 222 (7.6) |
PND: paroxysmal nocturnal dyspnea; eGFR: estimated glomerular filtration rate; SBP: systolic blood pressure; NIV: noninvasive ventilation.
Use of dobutamine, noradrenalin or dopamine at vasopressor doses (>5μg/kg/min). The definition of the variables can be consulted in the electronic supplementary material.
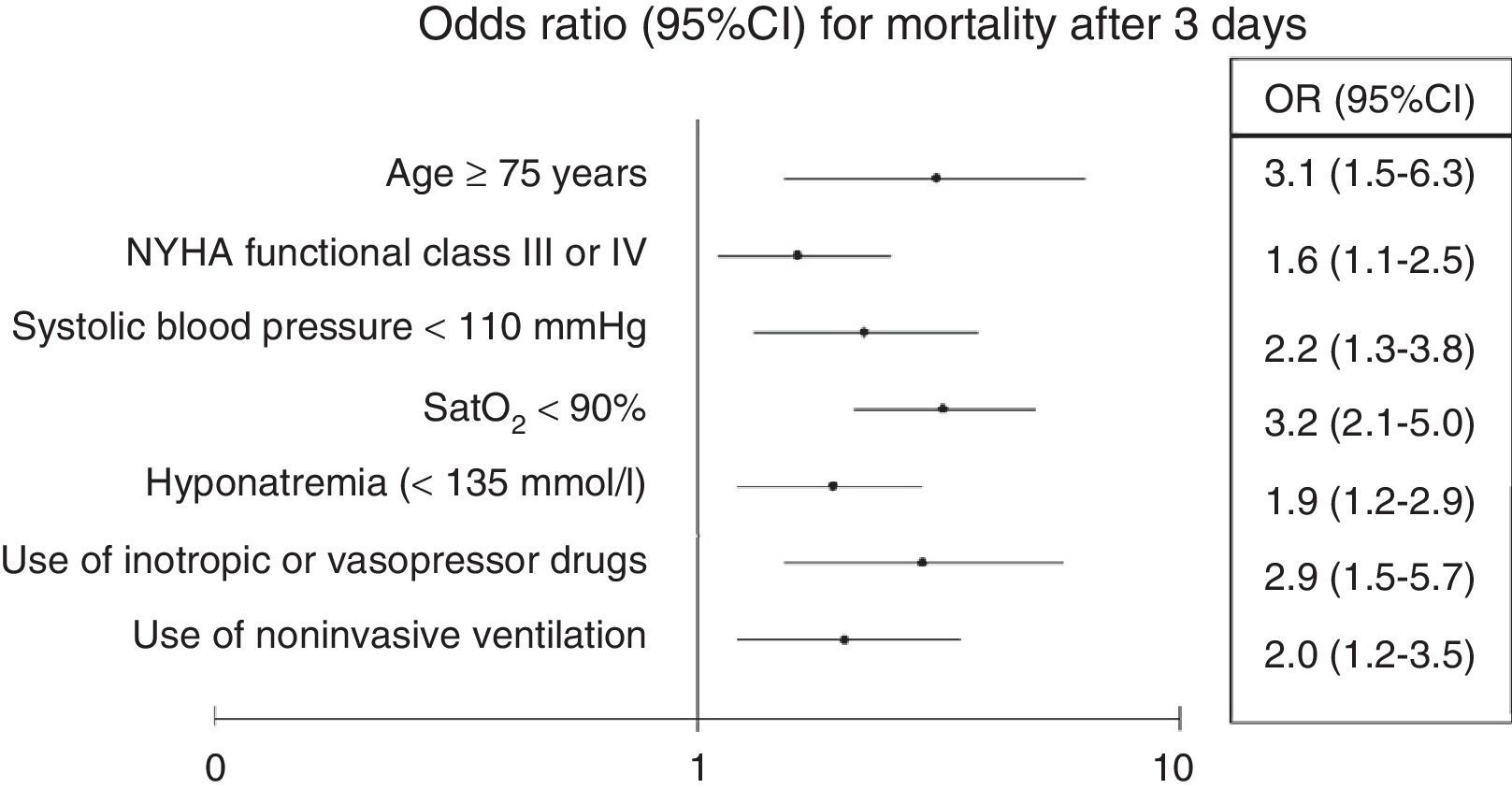
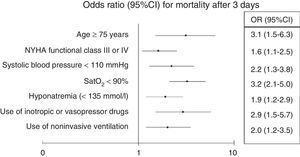
Adjustment of the multivariate model showed statistical significance to be retained for age≥75 years (OR 3.1; 95%CI 1.5–6.3; p<0.001), baseline NYHA functional class iii–iv (OR 1.6; 95%CI 1.1–2.5; p=0.03), SBP<110mmHg (OR 2.2; 95%CI 1.3–3.8; p=0.002), SatO2<90% (OR 3.2; 95%CI 2.1–5; p<0.001), hyponatremia (OR 1.9; 95%CI 1.2–2.9; p=0.005), inotropic or vasopressor treatment (OR 2.9; 95%CI 1.5–5.7; p=0.002), and the need for NIV (OR 2; 95%CI 1.2–3.5; p=0.01) (Fig. 1).
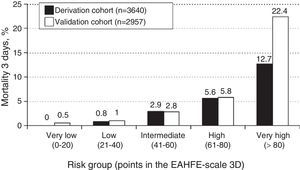
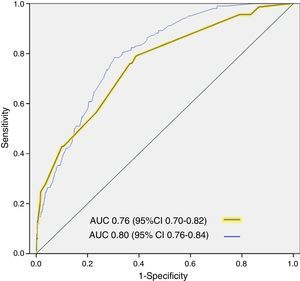
The cutoff points chosen for each of the 5 early mortality risk groups were: very low risk group 0–20 points; low risk group 21–40 points; intermediate risk group 41–60 points; high risk group 61–80 points; and very high risk group>80 points (Fig. 2). The number of patients included in each group was 432 (11.9%), 1329 (36.5%), 1080 (29.7%), 579 (15.9%) and 220 (6.0%), respectively, with a recorded mortality rate of 0, 0.8, 2.9, 5.5 and 12.7% (Fig. 3). The discriminatory capacity of the model was good, with an AUC of 0.80 (95%CI 0.76–0.84; p<0.001) (Fig. 4). The validation cohort in turn included 2957 patients, of which 66 died after three days (2.2%). The AUC of this model was 0.76 (95%CI 0.70–0.82; p<0.001) (Fig. 4) – the mortality rate by groups being 0.5, 1.0, 2.8, 5.8 and 22.4, respectively (Fig. 3). The difference between the AUC was 0.04. The DeLong nonparametric method showed no significant differences in the comparison of AUC (p=0.47).
DiscussionKnowledge of the short-term prognostic variables in ECHF is essential in the ECD in order to optimize management and monitoring of the patients, their destination, and the provision of information for both the patients and their families. A number of variables are related to increased mortality over the short (in-hospital mortality), middle (30 or 60 days) and long term (one year or more) in acute heart failure. Only the study of Lee et al. have assessed early mortality after 7 days, and the data of that study were used to create the EHMRG scale.15 The EAHFE-3D scale focused on identifying earlier mortality (after three days), independently of patient destination (discharge home or admission to hospital), since one of the aims was to detect imminent death risk with a view to assigning intensive treatments and resources to the appropriate patients, from the time of initial evaluation in emergency care. In this regard, a patient age of over 75 years, a baseline NYHA functional class of iii or iv, a systolic blood pressure of <110mmHg, a baseline SatO2 of <90%, hyponatremia and the use of inotropic or vasopressor drugs and NIV in emergency care were identified as independent variables associated to poorer outcome over the very short term.
The EAHFE-3D study intended to only use variables that are easy to obtain at the time of evaluating patients with ECHF in the ECD. Accordingly, on one hand natriuresis was discarded despite its important prognostic utility,21 because such monitoring is not available in all ECDs, and its usefulness is relative in patients with a reliable diagnosis of ECHF, since specificity is low. Accordingly, this scale could obviate biomarker costs.22,23
On the other hand, troponin monitoring was also discarded, for although it likewise has important prognostic utility, it is not requested on a routine basis, and the parameter is not determined in almost 50% of all cases of ECHF attended in emergency care.24 Undoubtedly, generalization in the ECD of the availability of natriuretic monitoring and of the request for troponin measurements could allow improvements in future versions of this model. Of note is the fact that only three of the 7 variables finally included in the EAHFE-3D scale coincide with three of the 10 variables included in the EHMRG scale15: age, systolic blood pressure and arterial SatO2. However, both scales obtain the same AUC (0.80), which confers good discriminatory capacity when applied in their respective settings. These AUC values may seem modest, though their statistical significance is clear, with p<0.001 in both cases.
Among the variables included in the EAHFE-3D scale, age is a universal indicator of poor prognosis in patients with acute heart failure, independently of the follow-up period considered.25–28 The increase in mortality among patients with ECHF associated to increasing age has been justified in terms of existing comorbidities, the frequency of factors associated to fragility, and even differences in immediate patient care conditioned by the presence of diseases, and the scarce published evidence on the treatments used.29 The rest of the variables have also been mentioned in previous studies. Hyponatremia has been related to patient prognosis in many studies, though in general the models involved refer to long-term mortality (between 1 and 5 years).30,31 One of the models that incorporate the variables of patients with ECHF at the time of admission to emergency care is the EFFECT scale,14 based on the analysis of 4031 patients attended in emergency care due to ECHF, and which assesses mortality after 30 days and one year. In this model, hyponatremia (sodium<136mmol/l) was correlated to mortality after 30 days (hazard ratio [HR] 1.53; 95%CI 1.14–2.05) and one year (hazard ratio 1.46; 95%CI 1.19–1.80). In the OPTIMIZE-HF registry,32 which included 48,162 patients with acute heart failure, 19.7% presented hyponatremia upon admission, and this was associated to a longer hospital stay and greater mortality (after 60 and 90 days) following discharge. Microvascular perfusion is seriously altered in patients with ECHF,33 and there are anomalies in microvascular oxygen utilization, compared with stable patients.34 Different studies have reported the usefulness of baseline SatO2 pulsioxymetric measurement as a complementary tool for establishing the severity of ECHF, since it evidences a close correlation between defects in gas exchange or pulmonary wedge pressure, and the severity of acute heart failure.35
Development of the EAHFE-3D scale allows the identification above 60 points of high and very high risk patients (with mortality rates after 3 days of 5.5% and the 12.7%, respectively), which are the individuals that should be attended in critical care, where in the event of shock or the need for vasoactive drugs, hemodynamic management with Doppler-echocardiography is recommended (investment in equipment and basic training) by the latest shock management guides.36–38 Low-mortality patients could benefit from early discharge home. Furthermore, the scale may be useful in individually deciding final patient destination in the most appropriate unit.39,40 In this regard, it is necessary to develop a local protocol with participation of the different specialties implicated in the process. Accordingly, these patients should be amenable to admission to units with strict clinical control, unless other elements–fundamentally limitation of therapeutic effort–dictate otherwise.41,42
The present study has certain limitations. On one hand, the study population does not include patients with ST-segment elevation acute coronary syndrome, which is correlated to increased mortality, though the proportion of this associated syndrome in the overall patients with ECHF admitted to emergency care is very small, and the management of such individuals corresponds to a different scenario. On the other hand, as has been commented above, the fact that biomarkers are not taken into consideration in the EAHFE-3D scale may have limited its performance, though this in turn has facilitated utilization of the scale in all ECDs, independently of their availability. Another aspect to be taken into account is that the application of NIV or the administration of vasopressor and inotropic drugs may be heterogeneous in the different ECDs, since the decision to use such agents is left to the criterion of the attending physician. Despite the mentioned limitations, we consider that the 3D-EAHFE scale easily identifies patients at high risk of very early mortality in the emergency care setting, and can contribute to improve care and define the prognosis in such cases.
Conflicts of interestThe ICA-SEMES research group has received support without restrictions and not directly related to this study from the companies Orion Pharma and Novartis.
This study has been possible in part thanks to aids from the Instituto de Salud Carlos III and FEDER (PI10/01918 and PI11/01021), and from the Generalitat de Catalunya (SGR 2009/1385).
Cristina Gil, Marta Fuentes (Hospital Universitario de Salamanca); Maria José Pérez-Durá, Eva Salvo, José Vallés (Hospital La Fe de Valencia); Rosa Escoda (Hospital Clínic de Barcelona); José Pavón, Ana Bella-Álvarez (Hospital Dr. Negrín de Las Palmas de Gran Canaria); Antonio Noval (Hospital Insular de Las Palmas de Gran Canaria); José M. Torres (Hospital Reina Sofía de Córdoba); Maria Luisa López-Grima, Amparo Valero (Hospital Dr. Peset de Valencia); Alfons Aguirre, Maria Àngels Pedragosa (Hospital del Mar de Barcelona); Maria Isabel Alonso, Helena Sancho, Paco Ruiz (Hospital de Valme de Sevilla); Antonio Giménez, José Miguel Franco (Hospital Miguel Servet de Zaragoza); Sergio Pardo (Hospital San Juan de Alicante); Ana Belen Mecina (Hospital de Alcorcón); Josep Tost (Consorci Sanitari de Terrassa); Jordi Fabregat (Hospital Mútua de Terrassa); Susana Sánchez (Hospital Río Ortega de Valladolid); Pascual Piñera (Hospital Reina Sofía de Murcia); Raquel Torres-Garate (Hospital Severo Ochoa de Madrid); Aitor Alquezar, Miguel Alberto Rizzi (Hospital Sant Pau de Barcelona); Fernando Richard (Hospital de Burgos); Javier Lucas (Hospital General de Albacete); Héctor Alonso (Hospital Marqués de Valdecilla de Santander); José Manuel Garrido (Hospital Virgen de la Macarena de Sevilla); Esther Rodríguez-Adrada (Hospital Clínico San Carlos de Madrid); Irene Cabello, Eva Lista, Ignasi Bardes (Hospital Universitari de Bellvitge de Barcelona).
Please cite this article as: Jacob J, Miró Ò, Herrero P, Martín-Sánchez FJ, Gil V, Tost J, et al. Predicción de la mortalidad a muy corto plazo de los pacientes con insuficiencia cardiaca crónica agudizada: escala EAHFE-3D. Med Intensiva. 2016;40:348–355.