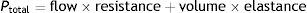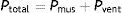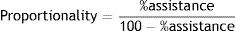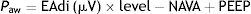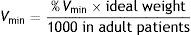Recent major advances in mechanical ventilation have resulted in new exciting modes of assisted ventilation. Compared to traditional ventilation modes such as assisted-controlled ventilation or pressure support ventilation, these new modes offer a number of physiological advantages derived from the improved patient control over the ventilator. By implementing advanced closed-loop control systems and using information on lung mechanics, respiratory muscle function and respiratory drive, these modes are specifically designed to improve patient–ventilator synchrony and reduce the work of breathing. Depending on their specific operational characteristics, these modes can assist spontaneous breathing efforts synchronically in time and magnitude, adapt to changing patient demands, implement automated weaning protocols, and introduce a more physiological variability in the breathing pattern. Clinicians have now the possibility to individualize and optimize ventilatory assistance during the complex transition from fully controlled to spontaneous assisted ventilation. The growing evidence of the physiological and clinical benefits of these new modes is favoring their progressive introduction into clinical practice. Future clinical trials should improve our understanding of these modes and help determine whether the claimed benefits result in better outcomes.
Los mayores avances en ventilación mecánica de los últimos años se han producido en el desarrollo de nuevos modos de ventilación asistida. En comparación con los modos tradicionales como la ventilación controlada-asistida o la presión de soporte, ofrecen una serie de ventajas fisiológicas así como un mayor control sobre el ventilador por parte del paciente. Basados en la utilización de algoritmos de control de asa cerrada que incorporan información de la mecánica, la actividad de la musculatura respiratoria y del estímulo respiratorio, estos modos están diseñados específicamente para mejorar la sincronía paciente-ventilador y reducir el trabajo respiratorio. Dependiendo de las características de funcionamiento específicas de cada modo, estos pueden ayudar en los esfuerzos respiratorios espontáneos del paciente de forma sincronizada en tiempo y magnitud, adaptarse a sus demandas, realizar protocolos automatizados de reducción del soporte y devolver al patrón respiratorio una variabilidad más fisiológica. El clínico tiene ahora a su disposición modos que permiten individualizar y optimizar la asistencia ventilatoria mecánica en la compleja transición de la ventilación controlada a la ventilación espontánea-asistida. La creciente evidencia de las ventajas fisiológicas y clínicas de estos nuevos modos así como las nuevas posibilidades de monitorización que ofrecen, están llevando a su paulatina introducción en la práctica diaria. Futuros estudios permitirán aumentar nuestro conocimiento acerca de estos modos y deberán determinar si sus beneficios se traducen en mejores resultados clínicos.
Mechanical ventilation (MV) is a life support measure that is used when the respiratory system of the patient is unable to meet the metabolic demands of the body. The indications of MV range from disease processes that affect gas exchange to simple “switching off” of the respiratory control system during anesthesia. Mechanical ventilation is usually started with a controlled ventilation phase during which the clinician takes full control of the ventilatory process, ensuring a minimum level of gas exchange and adequate muscle rest. Once the underlying disease condition has been corrected, a transition phase is started in which the patient gradually begins to participate in the ventilatory process. In this phase, which is referred to as assisted ventilation, the aim is to provide ventilatory support synchronized in time and magnitude with the inspiratory effort of the patient as the level of mechanical ventilation is gradually reduced.
The greatest advances in MV correspond to the development of new assisted ventilation modes. Impulsed by important technical innovations, these new modes offer theoretical advantages with respect to the traditional assisted ventilation modes such as assisted-controlled ventilation or pressure support ventilation. However, their slow introduction to clinical practice and the fact that their superiority in terms of clinical outcomes has not yet been firmly established have caused the traditional modes to remain the most widely used techniques.1
The present review describes new assisted ventilation modes that have been grouped as follows: (1) modes that adapt to the instantaneous inspiratory effort of the patient, such as proportional assist ventilation (PAV) and neurally adjusted ventilatory assist (NAVA); (2) automated modes that can be adapted to the patient demands, such as adaptive support ventilation (ASV) and the NeoGanesh system marketed as SmartCare™; and (3) modes that introduce biological variability in the ventilatory pattern, such as variable pressure support ventilation (V-PSV) or “noisy ventilation”.
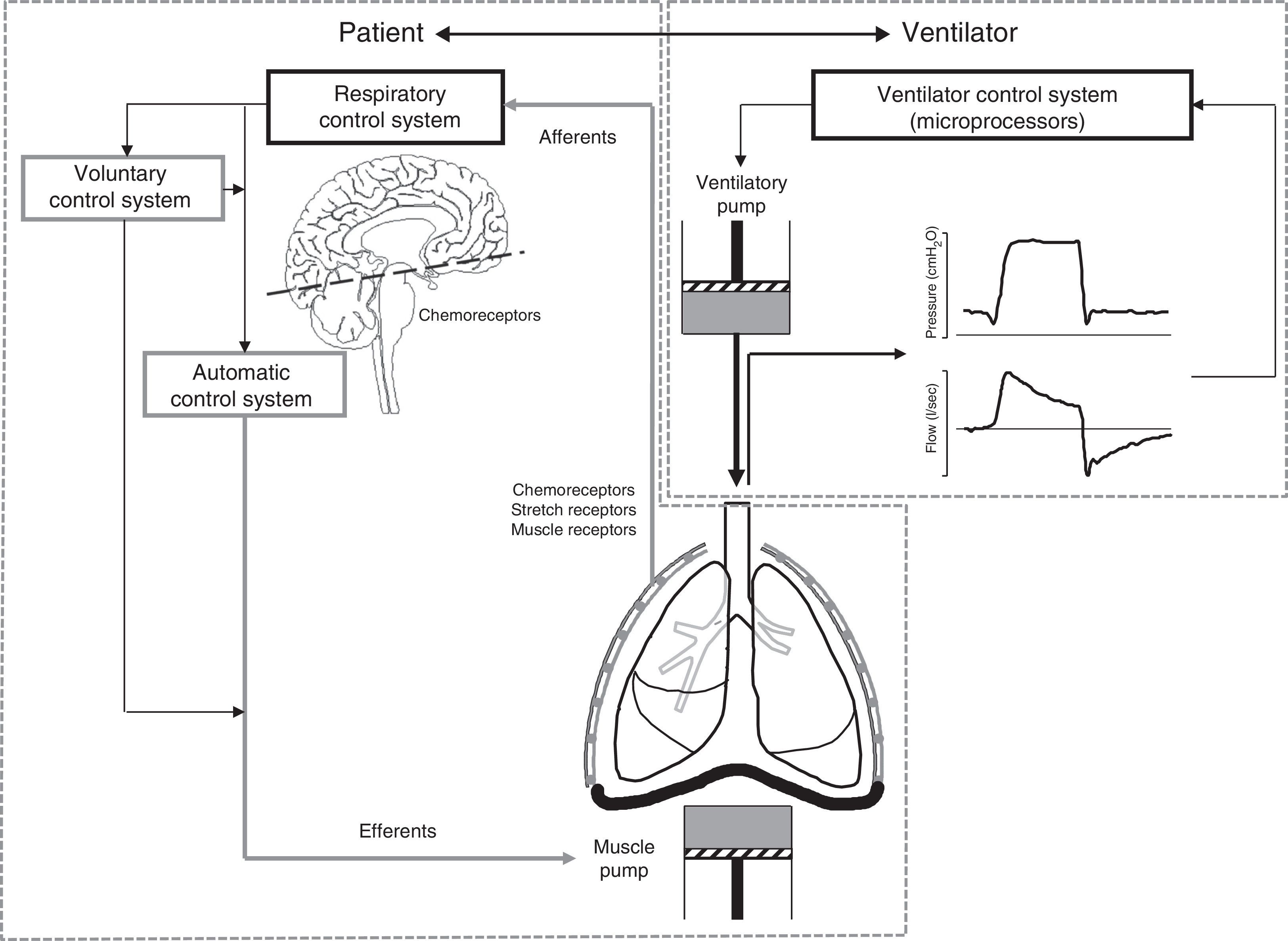
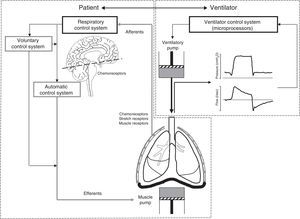
The challenges of assisted ventilationAssisted ventilation has the difficult task of harmonizing the operation of two complex systems, i.e., patient and ventilator–each with its own control center and ventilatory pump (Fig. 1). The respiratory control system (RCS) is composed of an automatic system and a voluntary system. The former integrates information from neurological and chemical peripheral afferents at brainstem level, while the voluntary or behavioral system in turn is located in supramedullary and cortical structures. In healthy individuals, the respiratory stimulus has three main origins: (1) chemical, mediated by changes in PaO2, PCO2 and pH; (2) metabolic, mediated by less well known mechanisms; and (3) a conscious origin that disappears during sleep phases.2 In effect, during sleep, the respiratory pattern is almost exclusively conditioned by chemical stimuli, which for example explains the apneas seen in response to minor changes in PCO2 in sedated patients.3 During the waking state, the voluntary control system is activated and influences the respiratory patterns in a variable and often unpredictable manner. As a result, patients subjected to assisted ventilation can develop complex respiratory patterns that affect interaction with the ventilator, thereby complicating mechanical assist.
Principles of patient–ventilator interaction. Assisted ventilation has the difficult task of harmonizing the operation of two complex systems, i.e., patient and ventilator–each with its own control center and ventilatory pump. The respiratory control system (RCS) is complex, and is composed of an automatic system and a voluntary system. The afferents transmit the stimuli from the sensors (central and peripheral chemoreceptors, stretch receptors and muscle receptors) to the control system, regulating the neural respiratory impulse. The automatic control system emits the efferents (motor neurons) that activate and regulate the muscle pump. The voluntary system in turn can modulate the activity of the automatic system or directly activate the muscle pump.
In order to activate the muscle pump, the automatic control system transmits the respiratory impulses along the efferents (motor neurons). The voluntary system not only interacts directly with the automatic system, but also has efferents that can directly activate the muscle pump without passing through the automatic control filter2 (Fig. 1). The difficulty of harmonizing the respiratory cycle generated by this complex RCS with the mechanical cycle of the ventilator is reflected by the fact that both are in manifest asynchrony in approximately 25% of all patients.4 An element that contributes to this situation is the fact that the traditional ventilation modes are rigid–delivering prefixed volumes or pressures without taking into account the frequent changes in patient demands or the changes between the sleeping and waking states. Moreover, in the case of assisted-controlled ventilation, the clinician assigns a fixed inspiratory time that rarely coincides with the physiologically variable time set by the respiratory control center (neural time).
Assist modes adapted to the instantaneous inspiratory effort of the patientThese modes are represented by PAV and NAVA, and have opened a new range of possibilities for assisted ventilation. Based on solid physiological principles, these techniques offer a series of theoretical advantages that make them particularly attractive for improving patient–ventilator synchrony. This is because in these modes the RCS of the patient takes control of the respirator and is free to determine its own respiratory pattern. Consequently, none of the entities such as volume, pressure and flow are pre-established; rather the ventilator simply assists the pattern chosen by the patient. In both of the mentioned modes the ventilator functions as an additional muscle, proportionally assisting the instantaneous efforts of the patient over the entire inspiratory phase. In addition, and in contrast to the other modes, ventilatory assist ceases at the same time as patient effort. This affords improved harmony between the mechanical and neural ventilatory times.
Upon taking control of the RCS, the ventilatory pattern recovers the characteristic variability of the natural respiratory pattern. Furthermore, under conditions in which the RCS is functionally intact, the afferents from the chemical and neural sensors modulate the intensity and characteristics of the respiratory impulse. This implies that both PAV and NAVA theoretically pose a lesser risk of under- or over-assistance, which often constitutes a cause of asynchrony with the traditional modes.5 Both assist modes require sufficient patient alertness and functional integrity of the RCS, which is affected by sedation.
Proportional assist ventilationProportional assist ventilation (PAV) was introduced in the early nineties,6 and represents a synchronized assist ventilation mode in which the ventilator provides pressure assistance proportional to the instantaneous effort of the patient.
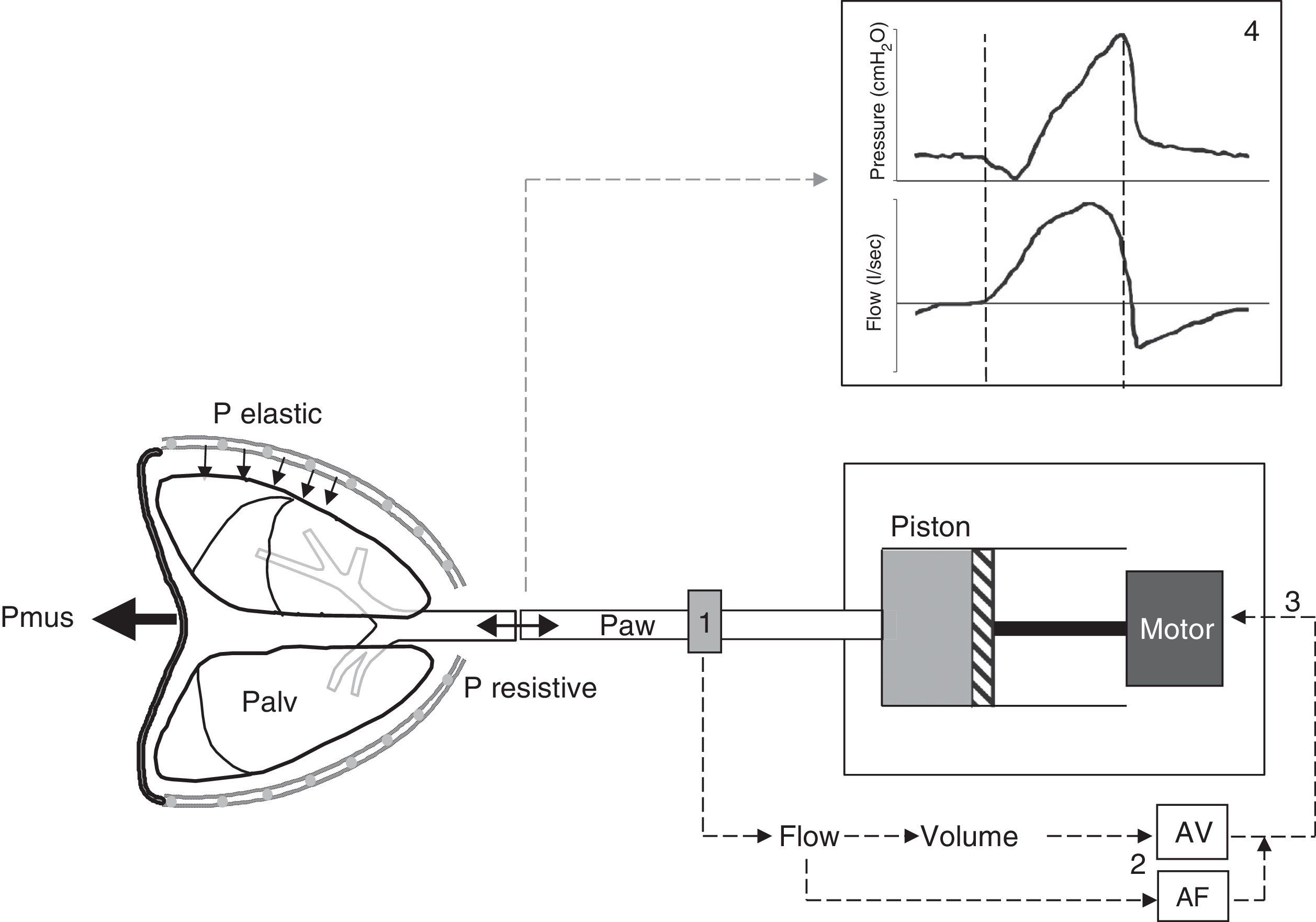
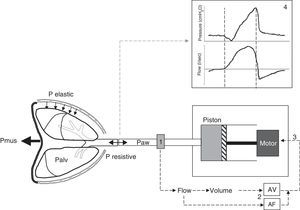
Principles of proportional assist ventilationIn the PAV system the ventilator detects the inspiratory effort of the patient by precisely measuring the flow and volume leaving the ventilator toward the patient. Both parameters are conditioned by the inspiratory decrease in alveolar pressure which the patient generates through muscle contraction. The flow and volume are amplified by respective adjustable gain controls, and the sum of both constitutes the control signal that generates the pressure response of the ventilator. The latter reacts with the rapid delivery of flow in response to this control signal (Fig. 2).
Schematic representation of the PAV system. The PAV mode affords assistance proportional to effort through the continuous measurement of the flow and volume (1) leaving the ventilator toward the patient, conditioned to the muscle pressure (Pmus) generated by the patient and which leads to a decrease in alveolar pressure (Palv). The flow and volume are amplified (AF and AV) by adjustable gain controls (2), and the sum of both signals conforms the input control signal (3) that generates the pressure response of the ventilator motor. The latter drives the piston, causing the ventilator to respond with rapid flow delivery to the patient in proportion to his or her Palv, overcoming the elastic and resistive pressure. The pressure-time and flow-time curves resulting from the mechanical cycle (4) show that the pressurization pattern is gradual, reaching the maximum value at the end of inspiration, and exhibiting proportionality at all times. Note that expiratory cycling coincides with the drop in inspiratory pressure, i.e., the cessation of inspiratory effort (second broken line), and the more physiological sinusoidal morphology of flow of the inspiratory phase.
The proportionality of the assistance is determined by the motion equation of the respiratory system. According to this equation, the total pressure that must be applied to insufflate the lung must overcome the resistive pressure (flow×resistance) and the elastic retraction pressure (volume×elastance) of the respiratory system:
During assisted ventilation, the total pressure is the sum of the pressure generated by muscle contraction of the patient (Pmus) and the pressure generated by the ventilator (Pvent).
The levels of flow and volume assistance are adjusted independently by the user. This requires an estimation of the passive mechanical characteristics, resistance and elastance, at the start of adjustment and on an intermittent basis. Once these are known, the pressure assist afforded by the ventilator is determined by the sum of the flow and volume assistance:
Because of the changing nature of respiratory mechanics, the system requires frequent measurement of elastance and resistance. There is consequently a risk of excessive or insufficient assistance in cases of estimation error or a lack of concordance between the estimated and the actual values. In the event of over-estimation, compensation is excessive, and the expiratory cycle may be delayed, prolonging assistance beyond the cessation of inspiratory effort on the part of the patient–this being known as the “run-away” phenomenon.7,8
A simplified and improved form has recently been introduced, called proportional assist ventilation with load-adjustable gain factors, or PAV+. This mode offers two essential improvements: (1) the noninvasive and semi-continuous measurement of respiratory mechanics, allowing automatic closed-loop adjustment of the assist level. This measurement is made by introducing brief pauses (300ms) at the end of inspiration every 8–15 respirations to estimate resistance9 and elastance10; and (2) the automatic adjustment of a single level of flow and volume assistance that becomes a constant fraction of the measured values of resistance and elastance.
Functioning of proportional assist ventilation with load-adjustable gain factors (PAV+)During ventilation in PAV+ mode, we simply need to adjust the percentage by which the ventilator must assist patient effort. Accordingly, an assist level of 70% means that the ventilator will contribute 70% to the total pressure reached, leaving the remaining 30% to the patient. The proportionality is simplified as follows:
For an assist level of 70%, the proportionality is 3; in other words, the system multiplies instantaneous pressure assistance by a factor of 3.
After activating the inspiratory trigger through pressure or flow, the inspiratory pressure progresses with the established proportionality, following a profile identical to that of Pmus. The result is gradual pressurization, reaching the maximum pressure only at the end of inspiration. In the moment in which the effort of the patient begins to decrease, the delivery of flow also decreases–expiratory cycling therefore generally coinciding with the cessation of patient effort.
PAV and PAV+: clinical characteristicsMany clinical studies have compared the physiological advantages of PAV versus conventional assist modes. Marantz et al.7 characterized the physiological response to PAV among patients dependent upon mechanical ventilation. They found that during PAV, in the absence of limitations imposed by respiratory mechanics, the RCS of the patient determines the tidal volume (Vt) and the frequency in response to variable assist levels. The patients tend to lower Vt and to increase the frequency in order to maintain the chosen minute volume. This results in a reduction of the inspiratory pressures.
With respect to pressure support ventilation (RSV), PAV has shown similar muscle discharge11–14 and better hypercapnia compensation.15 In response to an increase in elastic loading of 30%, Kondili et al.16 recorded greater efficiency in compensation (lesser increase of the work of breathing) with PAV+ than with PSV. Xirouchaki et al. compared the effectiveness of PSV versus PAV+ in maintaining critical patients dependent upon mechanical ventilation in assisted ventilation. They found PAV+ to significantly increase the probability of remaining with spontaneous ventilation, in addition to considerably reducing patient–ventilator asynchrony.17 Thanks precisely to a decrease in patient–ventilator asynchrony, Bosma et al. showed PAV to afford superior sleep quality, with fewer disruptions, in comparison with PSV.18
The PAV system depends on pneumatic triggering, and therefore has the same limitations for inspiratory cycling in patients with dynamic hyperinsufflation and intrinsic positive-end expiratory pressure (PEEP) as the traditional modes. Although expiratory cycling, based on flow, accompanies the cessation of inspiratory effort, expiratory asynchronies have been described particularly with high assist levels.19
The PAV mode can also be used in noninvasive ventilation (NIV). Compared with PSV, mainly in patients with chronic obstructive pulmonary disease (COPD), PAV usually affords higher levels of tolerance, a better physiological response, and fewer complications.20–22 However, PAV has not been associated with a decrease in the need for intubation in comparison with PSV. This could be related to the fact that leakage–the main cause of disadaptation and asynchrony during NIV23–equally affects triggering in PAV and in PSV.
Proportional assist ventilation and monitoringWith the PAV+ system we have semicontinuous monitoring of the elastance and resistance of the respiratory system. In addition to providing valuable evolutive information, it allows us to immediately assess the response to changes in the respiratory parameters or to quickly detect possible complications. The system is also able to estimate and monitor Pmus, which is the only unknown factor of the motion equation. Knowing Pmus, we in turn can calculate the work of breathing, helping to select an adequate assist level with a view to avoiding excessive muscle work or rest.24
Neurally adjusted ventilatory assistNeurally adjusted ventilatory assist (NAVA) is a new assisted ventilation mode synchronized and proportional to the effort of the patient that has become available only in the last few years.25 As control signal for both assist and for inspiratory and expiratory cycling of the ventilator, this mode uses the electrical activity of the diaphragm (EAdi). The latter is recorded via transesophageal electromyography using a modified nasogastric tube, also known as an EAdi catheter, which is similar in size and function to a conventional nasogastric tube but equipped with several microelectrodes at the distal tip for recording EAdi. Correct positioning of the catheter is carried out using the transesophageal electrocardiographic signal recorded through the same electrodes as a guide. The operator can check correct positioning (at the esophageal hiatus) on the ventilator screen, based on a simple algorithm.26
The electrical activity of the diaphragmThe utilization of EAdi for control of the ventilator has a series of theoretical advantages. In effect, EAdi is a signal that directly (i.e., without calculations or estimates) measures the efferents from the RCS, integrating the sum of time and space of the neural respiratory impulse that results in diaphragmatic activation.27 The amplitude of the signal depends on the degree of recruitment and on the intensity and frequency of triggering of the motor units, and consequently reflects the intensity with which the patient wishes to breathe.27,28 From its origin, the signal takes less than 20ms in triggering the mechanical response of the diaphragm29–this being about three to four times faster than the pneumatic trigger response time of modern ventilators. It is therefore the signal closest to the origin of the respiratory stimulus that current technology is able to offer.
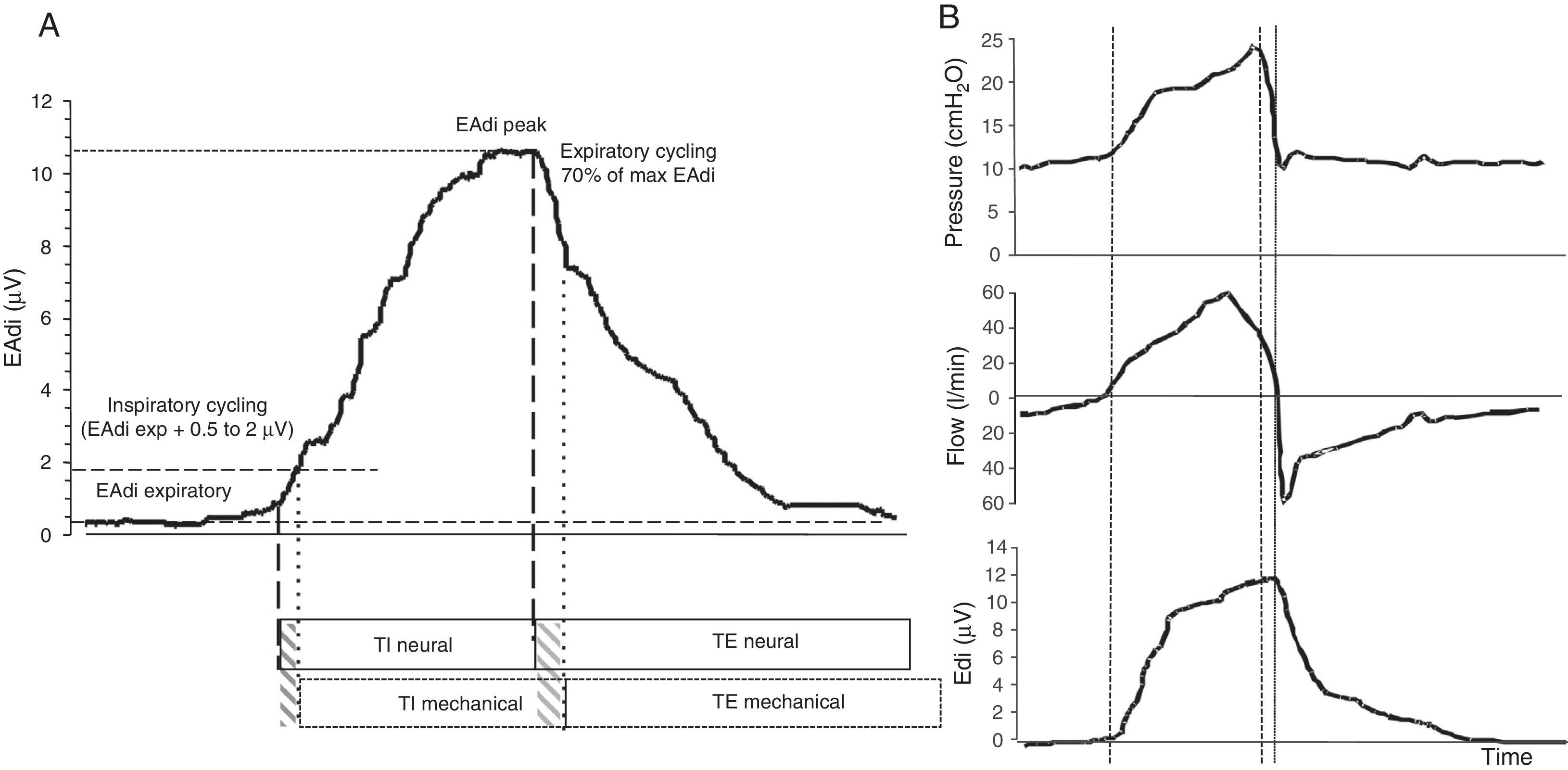
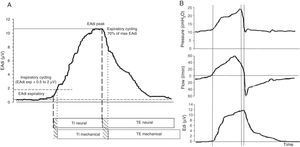
Functioning of the NAVA systemDuring NAVA, inspiratory cycling is determined by the detection of the elevation of EAdi over the expiratory level, with a sensitivity threshold determined by the operator. Expiratory cycling occurs when EAdi decreases to 70% of the maximum inspiratory value (Fig. 3). This allows adjustment of the duration of the mechanical inspiratory and expiratory times to the neural inspiratory and expiratory times of the patient determined by the RCS, in a way which no other ventilatory mode is able to do.30 In addition, the NAVA system eliminates the limitations of pneumatic triggering, since it is not affected by leakages or the presence of dynamic hyperinsufflation. This defines NAVA as the ventilatory mode which theoretically offers the greatest level of patient–ventilator synchrony.
EAdi signal and characteristic respiratory curves during ventilation in NAVA. (A) EAdi signal. The start of inspiration is given by the increase in EAdi activity (first broken line) from the expiratory activity, which under normal conditions is 0. At the point where EAdi reaches a threshold value (first dotted line), the ventilator starts assist until EAdi drops to 70% of the maximum value (second dotted line). The neural inspiratory time comprises the period between the two solid lines, and ends when EAdi reaches its maximum value. The mechanical ventilatory time comprises the period between the two broken lines (inspiratory and expiratory cycling). Note that although minimal, there is a phase lag in the time between the neural and mechanical times due to the imposed cycling criteria. (B) The curves corresponding to pressure, flow and EAdi of a cycle show the perfect inspiratory (first broken line) and expiratory cycling synchrony produced immediately after the start of the neural time of the patient, in relation to the cessation of inspiratory effort. In the same way as in PAV, pressurization is gradual, and in NAVA follows or parallels the morphology of the inspiratory phase of EAdi. The NAVA level is 1, and we can see that the end-inspiratory pressure reached is 22cmH2O, which corresponds to EAdi (=12)×NAVA level (=1)+PEEP level (=10).
In the same way as during PAV, the inspiratory assist is at all times proportional to the effort of the patient and is determined by a proportionality constant adjusted by the operator, called the NAVA level, which amplifies the instantaneous progression of EAdi during the inspiratory phase. The pressure in the airway (Paw) over the level of PEEP, in each moment during inspiration, is expressed as follows:
Different methods have been proposed for adjusting the NAVA level, which theoretically should be that affording an adequate level of muscle discharge. Brander et al. have described a method based on the response of Vt and Paw to ascending NAVA levels.31 Starting from low levels, the authors described a double response comprising a gradual increase to a certain NAVA level beyond which Vt and Paw reach a plateau. The optimum NAVA level would be that coinciding with transition from an ascending phase to the plateau phase of the Vt and Paw values. Roze et al. in turn have proposed adjustment to a NAVA level that reaches 60% of the maximum EAdi obtained after a standardized test with minimum assist (pressure support ventilation with 7cmH2O and PEEP 0) with a duration of 1h.32
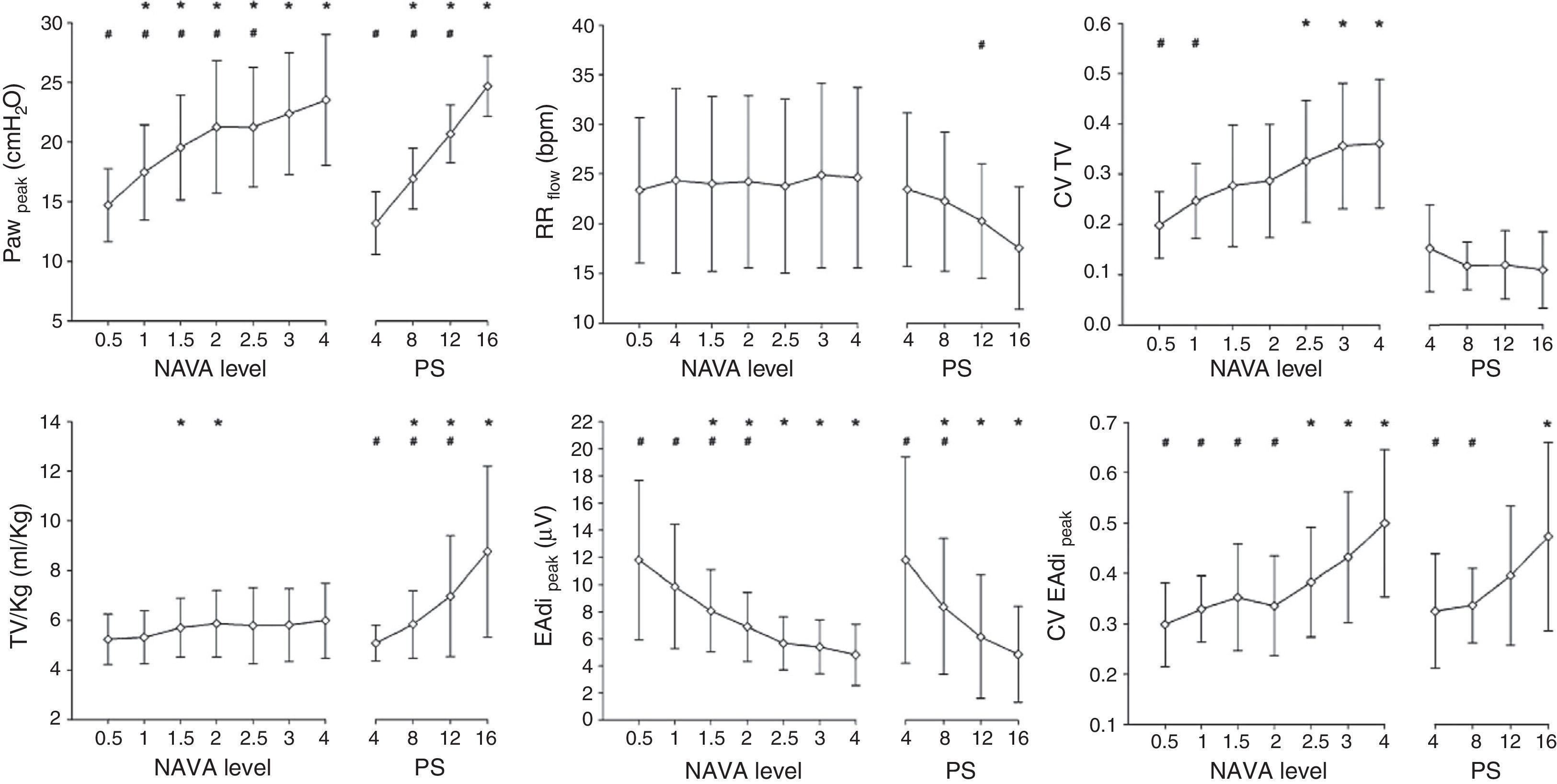
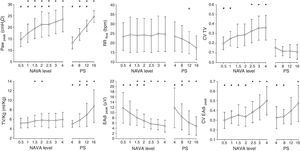
NAVA: clinical characteristicsSeveral clinical studies have evaluated and compared the physiological response to NAVA. These studies have consistently recorded significant improvement in patient–ventilator synchrony, a lesser over-assistance tendency, and greater variability of the respiratory pattern in comparison with PSV in different groups of patients.33–40 Ineffective effort, i.e., inspiratory effort of the patient that is not accompanied by mechanical assist, virtually disappears with NAVA.34 Likewise, in contrast to PSV, increments in assist level have been shown to exert less effect upon the inspiratory and expiratory cycling times,35 ensuring better synchrony over a broad assist range. Patroniti et al.38 have published a detailed description of the ventilatory pattern during NAVA. In patients with respiratory failure, the authors compared the response to increasing NAVA levels with increasing PSV levels (Fig. 4). With NAVA, the patients maintained similar Vt and respiratory frequency values, even with high assist levels, despite an increase in Paw, which corresponded to a decrease in EAdi. In contrast, during PSV, both Vt and pressure increased (up to >100% with the maximum level), while the frequency and EAdi decreased.
Effect of different NAVA levels and pressure support. Note that in NAVA, and in contrast to pressure support ventilation (PSV), greater assist levels do not increase the tidal volume or decrease the respiratory frequency, and the pressure in the airway reaches a plateau with higher assist levels–corresponding to a decrease in EAdi. The increase in assist is accompanied by increased variability in tidal volume in NAVA, while it decreases in PSV. PS: pressure support ventilation; EAdi: electrical activity of the diaphragm; CV EAdi peak: coefficient of variation of the electrical activity of the diaphragm; CV TV: coefficient of variation of the tidal volume; Paw: pressure in the airway; RR: respiratory frequency; TV/kg: tidal volume per kg ideal weight. *p<0.05 versus the lowest assist level for the same ventilatory mode. **p<0.05 versus the highest assist level for the same ventilatory mode.
In the same way as during PAV, studies with NAVA have shown that patients tend to select a protective tidal volume (6ml/kg) with moderate assist levels and a generally higher respiratory frequency.
The NAVA mode has been shown to facilitate assisted ventilation also in patients with seriously impaired respiratory function. In this respect, the NAVA mode reduced asynchrony in patients subjected to extracorporeal oxygenation support and with severely impaired lung distensibility37 versus PSV, and achieved better auto-regulation of PCO2 during weaning from extracorporeal oxygenation41–in both cases maintaining protective ventilatory parameters with low Vt values.
Because of its operating characteristics, NAVA may be particularly interesting in the context of NIV, since it is not affected by leakages. In this regard, Piquilloud et al.42 and Bertrand et al.43 reported a significant reduction of asynchronies with NAVA versus PSV during NIV both in patients with exacerbated COPD and in hypoxemic patients.
NAVA and monitoringThe EAdi signal offers new and interesting possibilities in respiratory monitoring. By affording a direct and continuous measure of the central respiratory stimulus of the patient, the signal allows us to evaluate the response to changes in assist level, detect apneas, evaluate sedation effects, and also assess the neural respiratory stimulus. EAdi is the best tool available for monitoring patient–ventilator synchrony, since it offers direct information on the neural inspiratory and expiratory times and their relation to the mechanical times. It allows us to determine the neural frequency (the real frequency of the patient), thereby enhancing the value of this variable in determining the degree of patient stress or wellbeing. A number of indices derived from the EAdi signal have recently been described. Neuroventilatory efficiency, measured as Vt/EAdi, indicates the capacity of the diaphragm to generate volume, standardized with respect to the neuronal stimulus. In one same parameter it integrates information on the respiratory stimulus, diaphragmatic function, and respiratory loading, and has been shown to be a good predictor of weaning.44,45 Neuromechanical efficiency, measured as Paw/EAdi during an occlusion in which the patient inhales against a closed valve, provides an estimate of the capacity of the diaphragm to generate force in relation to the neural inspiratory effort. Based on neuromechanical efficiency, Bellani et al. have derived a method to estimate Pmus from EAdi, thereby producing more objective information for determining the best NAVA level.46
Automated modes adaptable to patient demandsThese modes encompass closed-loop control modes that incorporate algorithms and control rules which transfer physiological and clinical reasoning principles to automated assist protocols. According to different physiological and clinical objectives, these modes automatically adjust the pressure or minute-volume levels administered to the patient, adapting to the needs of the latter over time. Adaptive support ventilation (ASV) performs cycle-to-cycle adjustments of tidal volume (through changes in pressure) and respiratory frequency, adapting them to changes in respiratory mechanics. NeoGanesh or SmartCare™ in turn performs adjustments, in cycles of several minutes, in delivered pressure support ventilation, adapting the levels to the changing conditions of the patient. The aim is to simulate clinical reasoning in order to avoid under- or over-assistance and to achieve a decrease of the automated support.
Adaptive support ventilationAdaptive support ventilation (ASV), described in the early nineties, is based on the physiological principle described by Otis and Mead47,48 which establishes that for a given level of alveolar ventilation there is an optimum respiratory frequency that results in less work of breathing–a kind of “law of minimum effort”. According to this principle, in order to reach one same alveolar ventilation level at very low frequencies, we require a greater Vt, increasing the work to overcome the elastic load of the respiratory system. In contrast, at high frequencies, the work of breathing must increase to overcome the resistive load, with a pattern characterized by rapid shallow breathing. Between these two extremes lies the optimum combination of frequency and volume for achieving the desired alveolar ventilation.
Functioning of ASVUnlike the other examined modes, ASV in fact is a mixed mode that can function as a controlled or assisted mode according to the contribution of the patient.
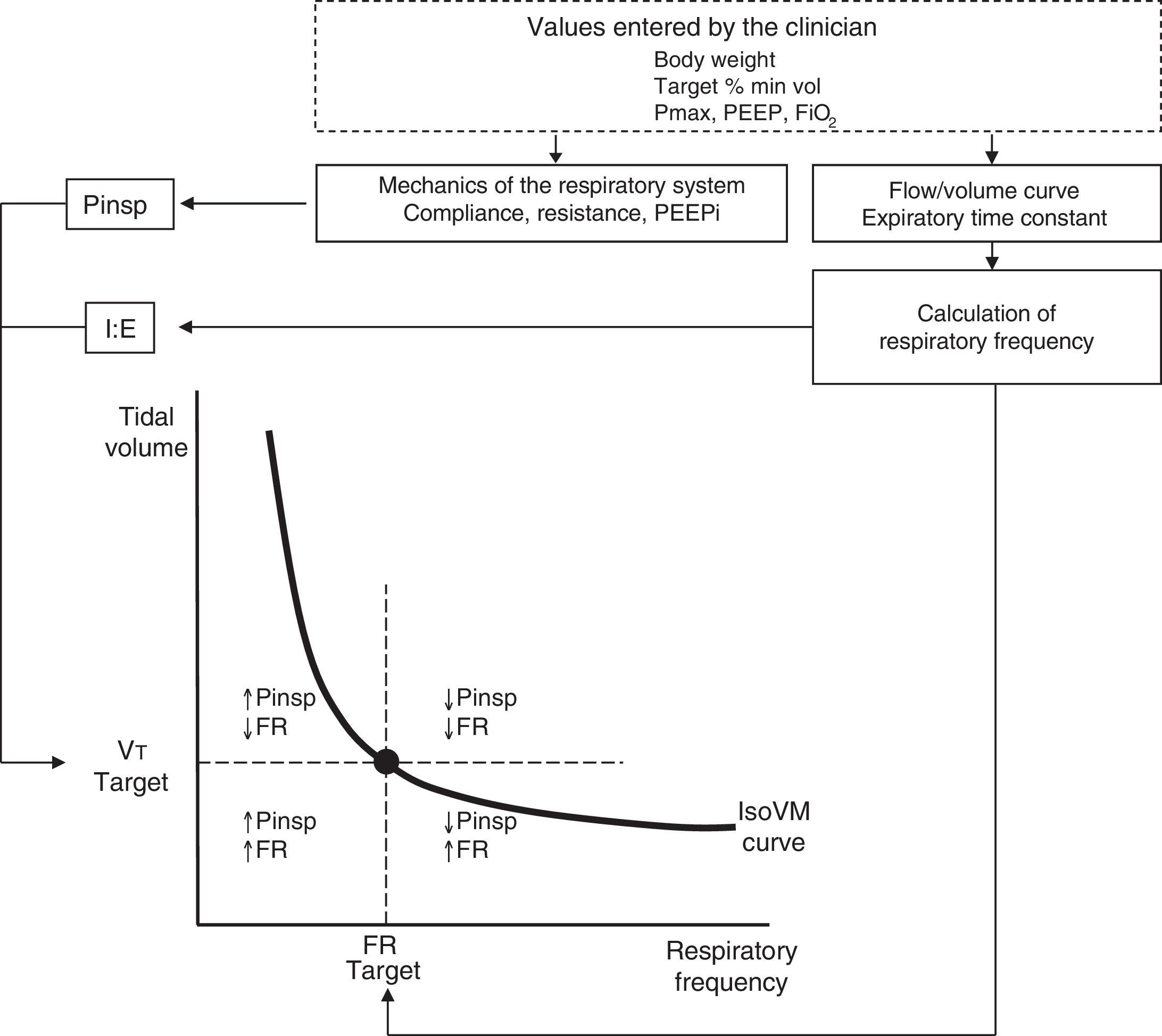
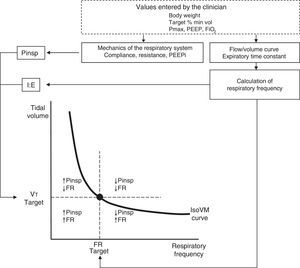
Figure 5 schematically represents the principles of the functioning and control system of ASV. The operator establishes a target percentage minute-volume based on the body weight of the patient.
Functioning of ASV. Before starting, the clinician enters the data referred to patient weight, percentage minute-volume (estimated a priori according to the patient and disease condition), FiO2, PEEP and the maximum inspiratory pressure limit (Pmax). Analysis of the flow-volume curve determines the expiratory time constant, and minimum squares fitting is used to calculate the respiratory mechanics and the presence of intrinsic PEEP. The closed-loop control algorithm of the ASV system adjusts the inspiratory pressure according to the iterative equation derived from Otis and Mead. The combinations of target minute-volume and frequency are continuously adjusted to reach and keep the patient on the minute-isovolumetric curve (IsoVM).
Under normal conditions, % Vmin is 100%, with the possibility of choosing between 25 and 300%, depending on the conditions of the patient.
It should be remembered here that minute-volume is the sum of the alveolar ventilation volume (the “effective” volume) and the dead space volume. Accordingly, ASV incorporates the estimation of dead space in its algorithm, and which the system assumes to be 2.2ml/kg. The ASV system then adjusts the level of pressure and respiratory frequency cycle-to-cycle, following its algorithm to maintain the ventilatory pattern according to the established target minute-volume, in consistency with the mechanical characteristics of the respiratory system and the spontaneous respiratory frequency of the patient. Inspiratory cycling uses the conventional pneumatic trigger by pressure or flow, while expiratory cycling is by flow as in the case of PSV.
ASV: clinical characteristicsDue to its “mixed” nature, ASV has been studied both as controlled mode and as assist mode. Most clinical studies have focused on examining ASV under passive ventilation (controlled) conditions, comparing it with other modes, and specifically evaluating whether ASV yields protective parameters (low Vt and Paw) in an automated and efficient way.
As assist mode (which is what interests us in this review), ASV has been studied mainly as a mode designed to facilitate weaning. It has been shown to be a safe and effective technique that simplifies the weaning process in the postoperative period of heart surgery49–51 and in patients with COPD,52 and is moreover associated with a lesser consumption of resources. In comparative studies, ASV has not been found to shorten the mechanical ventilation times in heart surgery,50,51 though shortened times have been recorded in COPD patients, where Kirakli et al. observed a shortening of the weaning time of over 24h compared with PSV.53
The best comparative clinical study to date on the effect of ASV upon patient–ventilator synchrony was published by Tassaux et al. In comparison with synchronized intermittent ventilation (SIMV-PSV), these authors found ASV to improve synchrony, reducing the muscle load for a similar delivered minute-volume.54
The ASV mode has recently received improvements, with addition to the algorithm of closed-loop control for end-expiratory CO2 (etCO2)55 and oxygen saturation. The result is an evolved ASV system called IntelliVent™, which allows us to implement a protective ventilatory strategy in both the control phase and in assistance to weaning.56
Automated adjustment of pressure support: NeoGanesh-SmartCare™NeoGanesh and its commercial version SmartCare™ constitute an automated, knowledge-based weaning technique. The control algorithm incorporates rules for action based on clinical reasoning, in an attempt to reproduce the PSV adjustments which the clinician would decide in the same context.
Functioning of SmartCare™The control algorithm of the system uses the values of Vt, respiratory frequency and etCO2. These values are averaged every two minutes (every 5min in the case of changes in pressure level) and provide the algorithm with a “ventilatory diagnosis”. The system responds as follows: (1) it reduces the level of PSV in the case of diagnosed over-assist (e.g., the combination of high Vt with low frequency and etCO2); (2) it increases assist in the event of insufficient assistance (increasing frequency together with other additional criteria); and (3) it introduces no changes in the case of normal ventilation. The aim is to move the patient toward a zone of respiratory wellbeing in order to start the weaning process. This zone of wellbeing is derived from the patient characteristics (body weight, type of illness, size of the endotracheal tube, type of humidifier). The values are entered by the clinician in the ventilator, and determine the limits of Vt, frequency and etCO2, and the PSV adjustments required. The automated weaning protocol involves automated adaptation of the PSV level followed by an automated PSV reduction phase, and finally an automated spontaneous breathing test.
SmartCare™: clinical characteristicsSmartCare™ is able to facilitate the weaning process, reducing resource consumption and shortening the time on mechanical ventilation. Clinical studies have reported somewhat discordant findings in relation to such benefits, depending on whether the control group included57 or did not include58 weaning protocols and sufficient resources (patient/nurse ratio).59 In the most recent multicenter study involving 92 patients with over 24h of mechanical ventilation, automated weaning shorted the duration of mechanical ventilation by one day, and also lessened the need for tracheostomy compared with a protocolized conventional weaning group.60
Variable pressure support ventilation (noisy ventilation)Variability is an intrinsic characteristic not only of the respiratory system but also of any complex biological system, and the loss of such variability is generally associated with functional impairment.61 There is a growing evidence of the beneficial effect of variability, understood as cycle-to-cycle changes in Paw and Vt and/or respiratory frequency, upon the respiratory system.62 All the new assist modes described thus far introduce respiratory variability, the latter being determined to one degree or other by the patient. Variable pressure support ventilation (V-PSV) introduces random variability in the levels of pressure support ventilation, resulting in a ventilatory pattern that is variable but independent of the demands of the patient and his or her inspiratory effort.
Functioning of variable ventilationV-PSV (noisy ventilation) is based on the recurrent application of a set of 600 pressure values generated on a random basis. These values follow a normal distribution, with a mean and standard deviation adjusted to achieve the desired level of variability (measured by the coefficient of variation; in general 1±0.3, to afford a variability of 30%).63 The mean pressure value is adjusted to obtain a Vt of 6ml/kg, and the pressure limits are determined by the adjusted upper pressure limit and the expiratory pressure level (PEEP or CPAP). The clinician can adjust the level of variability between 0 and 100%, and the system maintains a stable mean pressure.
Experimental studies have consistently shown beneficial physiological effects, such as improved gas exchange and respiratory mechanics. A relevant aspect is the possible benefits in terms of lung protection.64 The mechanisms underlying the improvement in respiratory mechanics are not fully clear, but an alveolar recruitment effect has been postulated, together with possible stimulation of the production and release of surfactant.65
Although an attractive mode, the lack of clinical data means that many questions still need to be answered before the true clinical usefulness of the technique can be established. As an example, what pattern or level of variability would have been most appropriate for a given situation? In this respect, Spieth et al.63 used an experimental surfactant depletion model to show that the best choice for improving respiratory mechanics and gas exchange corresponds to a coefficient of variation of 30%, which interestingly coincides with the normal respiratory variability values during spontaneous ventilation.63 In patients we will have to determine whether this level of variability is also optimum, and whether extrinsic variability offers advantages with respect to the intrinsic variability of the patient (such as that introduced in PAV or NAVA), as well as explore the effects upon patient–ventilator synchrony.
ConclusionsThese are very interesting times for mechanical ventilation. The constant technological advances have allowed the development of new assisted ventilation modes with the capacity to adapt to the changing patient needs. The new modes allow the patient a total control of the ventilatory process, causing the ventilator to act as an accessory muscle in synchrony with patient inspiratory effort. New modes that incorporate increasingly complex closed-loop or knowledge-based control systems are paving the way toward gradual automatization of the mechanical ventilation process. It can be expected that such modes and automatization will gradually find their way into routine clinical practice. The results of future studies will help us to better define their advantages, indications and benefits in assisting patients subjected to mechanical ventilation.
Conflicts of interestThe author serves as a consultant to Maquet Critical Care.
Please cite this article as: Suarez-Sipmann F, por el Grupo de Trabajo de Insuficiencia Respiratoria Aguda de la SEMICYUC. Nuevos modos de ventilación asistida. Med Intensiva. 2014;38:249–260.