Edited by: Juan Antonio Llompart-Pou - Specialist in Intensive Care Medicine, Doctorate in Health Sciences, Universitat Illes Balears. Department of Intensive Care Medicine, Trauma and Neurocritical ICU, Hospital Universitari Son Espases. Palma, Spain
Last update: November 2025
More info"The superficial changes The deep also changes The way of thinking changes Everything in this world changes"
Oxygen (O2) is essential for cell survival, hence, its levels and delivery to tissues are of critical importance.1 Oxygen is transported in the bloodstream driven by cardiac output in two ways: a) dissolved (5%) and b) bound to its carrier protein (95%), hemoglobin (Hb). On the other hand, O2 transfer to the tissues depends on saturation and the O2/Hb dissociation curve.1
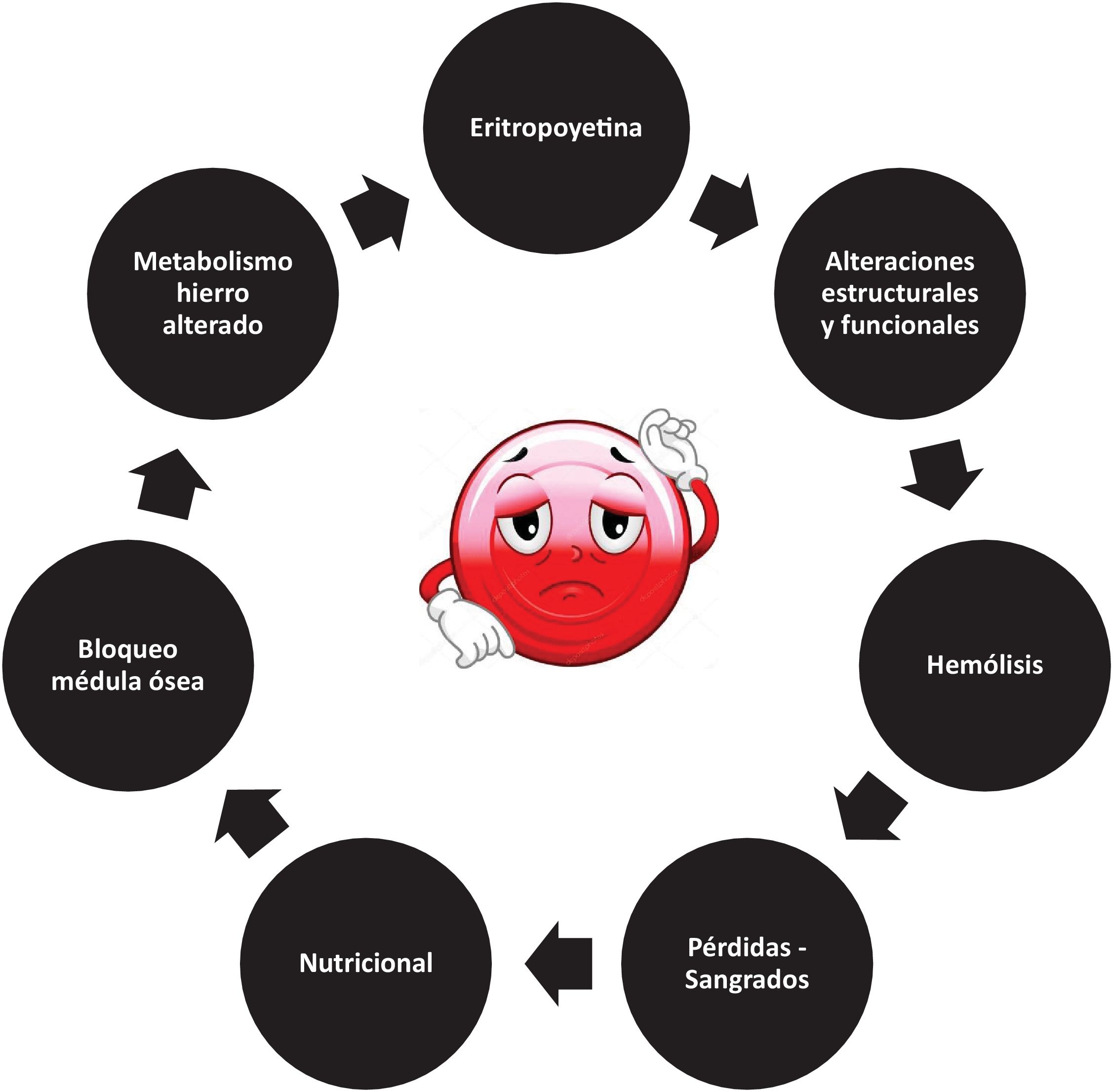
Anemia, as defined by the World Health Organization (WHO), is highly prevalent in critically ill patients, with an estimated 60% presenting with Hb levels < 12 g/dl at admission.2 The etiology of anemia is multifactorial, ranging from objectifiable blood losses to the inherent physiology of the erythrocyte, which undergoes a series of changes in its structure, metabolism and production that predispose to the development of anemia3 (Fig. 1).
Transfusion of whole blood or packed red blood cells is one of the most classic tools when seeking to optimize O2 transport (TO2). However, there is still disagreement about when, how much and what the goals of transfusion should be, especially when considering the risks and benefits of this practice.4,5
Hemoglobin and transfusion in the critically ill patientReviewing and knowing the past allows us to better understand the present and build the future. In this context, Shoemaker marked the starting point by noting that mortality in high-risk postoperative patients increased when the hematocrit levels were below 32%, especially when the cardiovascular reserves were compromised.6 In these individuals, erythrocyte transfusion was significantly associated with improved O2 transport and consumption (VO2).6 Subsequently, the concepts of tissue hypoxia and oxygen debt resurfaced as a consequence of the analysis of hemodynamic variables in these patients.7 As a result, various resuscitation strategies based on optimizing TO2 and VO2 were developed, including the transfusion of packed red blood cells with Hb levels > 10 g/dl.8 This strategy was abandoned after later studies showed more risks than benefits.9
In 1998, the results of the TRICC, a Canadian multicenter, prospective and controlled trial, were published. This study included critically ill patients with Hb levels <9 g/dl in the first 72 h of admission, with the transfusion of packed red blood cells when Hb < 7 g/dl (restrictive group) versus another group (liberal group) that was transfused when Hb < 10 g/dl.10 Lower mortality was observed in the restrictive group in individuals <55 years of age (p = 0.02) and when the APACHE II score was <20 (p = 0.03), with the exception of those subjects with impaired cardiac function (p = 0.69)10 — with similar data being reproduced in the TRISS study.11
One of the fundamental aspects to be highlighted in both of the above studies is that patients with acute brain injury were not represented, which is why this population subgroup is excluded from the restrictive transfusion strategy in the recommendations of the European Society of Intensive Care Medicine.12
Hemoglobin and transfusion in acute brain injuryPhysiological rationale for maintaining adequate hemoglobin levelsUnder physiological conditions, the brain consumes 20–25% of the body's total O2 and glucose, due to its incessant activity. In the context of injury, the brain, lacking reserves of energy substrates, becomes vulnerable to energy deficits and increases its demand.13 In this sense, any alteration in the O2 supply chain (including Hb) could cause cerebral tissue hypoxia - one of the detrimental mechanisms of secondary brain damage.14
Regarding the pathophysiology of Hb-related tissue hypoxia, the following causes stand out: (a) anemic: this occurs when the Hb levels are insufficient to transport O2 consistent with the cellular demands (each gram of Hb transports 1.34 ml of O2); (b) high affinity: triggered by physiological imbalances that shift the O2 dissociation curve to the left, making cellular delivery difficult (hypothermia, hypocapnia, metabolic alkalosis, 2,3-diphosphoglycerate deficiency in transfusions of blood that has been stored for long periods); and (c) poor quality Hb, characterized by an inability to transport O2 (carboxyhemoglobin, methemoglobin).14,15
Preliminary clinical evidence: when uncertainty is the only certaintyThe optimal Hb level in acute brain injury is not clear. Both the presence of anemia and the transfusion procedure have been associated with increased complications, mortality and poorer functional outcomes.16–20
The most robust clinical evidence highlights the limitations and methodological problems of the studies analyzed and concludes that the available information is uncertain, confusing and inconclusive regarding the effect of the liberal versus the restrictive transfusion strategy on mortality and the functional outcomes.21–26
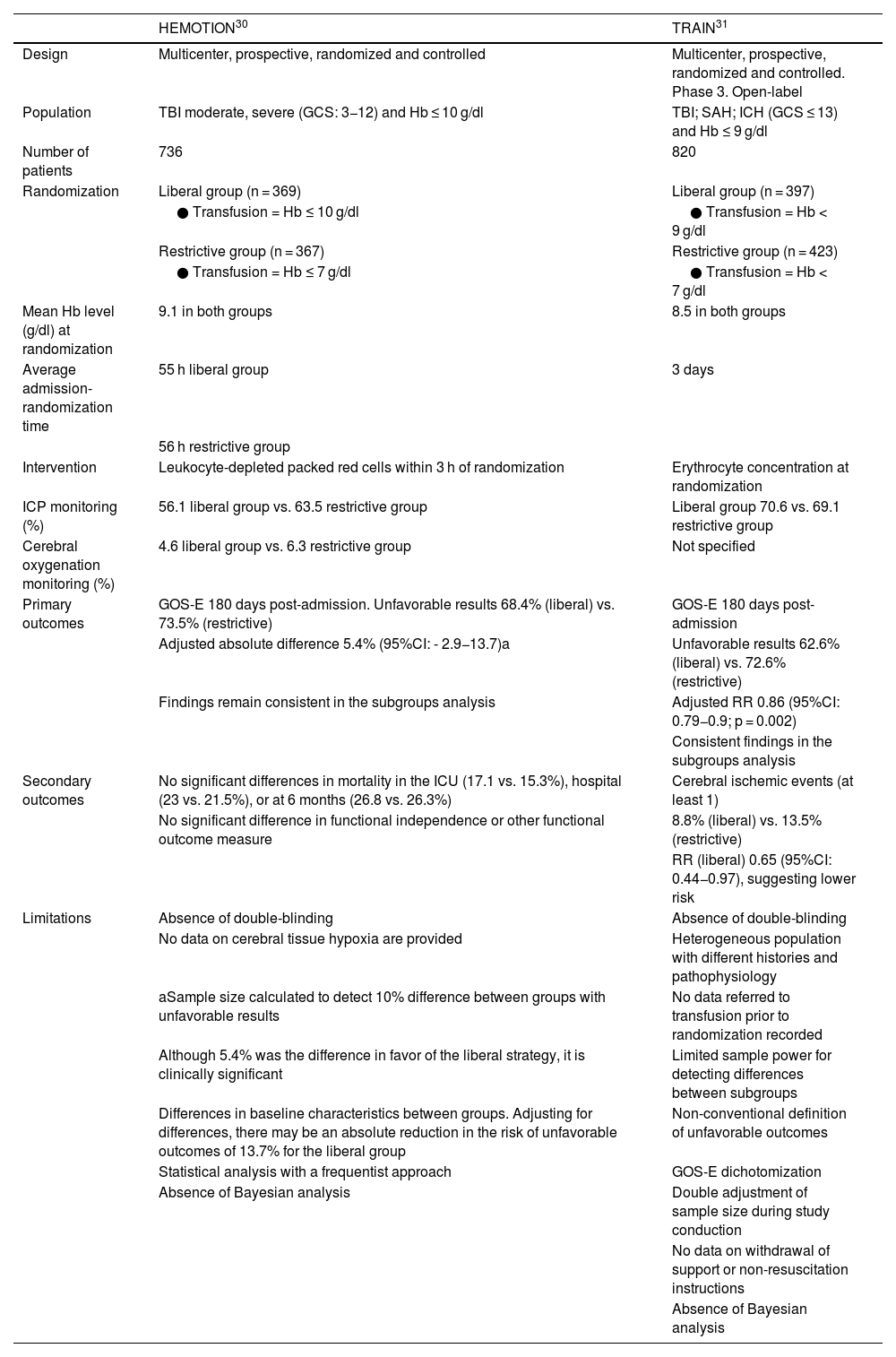
Recent trials: Is there light at the end of the tunnel?The answer is "train in motion", an allegorical phrase referring to TRAIN and HEMOTION, two recent studies that have attempted to clarify some of the existing doubts.27,28 The main characteristics, results, strengths and weaknesses of these two studies are summarized in Table 1. The conclusions are similar and point to a "paradigm shift", establishing that the liberal strategy aimed at maintaining Hb > 9 g/dl is associated with better functional outcomes.27,28
Characteristics of the HEMOTION and TRAIN studies.
| HEMOTION30 | TRAIN31 | |
|---|---|---|
| Design | Multicenter, prospective, randomized and controlled | Multicenter, prospective, randomized and controlled. Phase 3. Open-label |
| Population | TBI moderate, severe (GCS: 3−12) and Hb ≤ 10 g/dl | TBI; SAH; ICH (GCS ≤ 13) and Hb ≤ 9 g/dl |
| Number of patients | 736 | 820 |
| Randomization | Liberal group (n = 369) | Liberal group (n = 397) |
| ● Transfusion = Hb ≤ 10 g/dl | ● Transfusion = Hb < 9 g/dl | |
| Restrictive group (n = 367) | Restrictive group (n = 423) | |
| ● Transfusion = Hb ≤ 7 g/dl | ● Transfusion = Hb < 7 g/dl | |
| Mean Hb level (g/dl) at randomization | 9.1 in both groups | 8.5 in both groups |
| Average admission-randomization time | 55 h liberal group | 3 days |
| 56 h restrictive group | ||
| Intervention | Leukocyte-depleted packed red cells within 3 h of randomization | Erythrocyte concentration at randomization |
| ICP monitoring (%) | 56.1 liberal group vs. 63.5 restrictive group | Liberal group 70.6 vs. 69.1 restrictive group |
| Cerebral oxygenation monitoring (%) | 4.6 liberal group vs. 6.3 restrictive group | Not specified |
| Primary outcomes | GOS-E 180 days post-admission. Unfavorable results 68.4% (liberal) vs. 73.5% (restrictive) | GOS-E 180 days post-admission |
| Adjusted absolute difference 5.4% (95%CI: - 2.9−13.7)a | Unfavorable results 62.6% (liberal) vs. 72.6% (restrictive) | |
| Findings remain consistent in the subgroups analysis | Adjusted RR 0.86 (95%CI: 0.79−0.9; p = 0.002) | |
| Consistent findings in the subgroups analysis | ||
| Secondary outcomes | No significant differences in mortality in the ICU (17.1 vs. 15.3%), hospital (23 vs. 21.5%), or at 6 months (26.8 vs. 26.3%) | Cerebral ischemic events (at least 1) |
| No significant difference in functional independence or other functional outcome measure | 8.8% (liberal) vs. 13.5% (restrictive) | |
| RR (liberal) 0.65 (95%CI: 0.44−0.97), suggesting lower risk | ||
| Limitations | Absence of double-blinding | Absence of double-blinding |
| No data on cerebral tissue hypoxia are provided | Heterogeneous population with different histories and pathophysiology | |
| aSample size calculated to detect 10% difference between groups with unfavorable results | No data referred to transfusion prior to randomization recorded | |
| Although 5.4% was the difference in favor of the liberal strategy, it is clinically significant | Limited sample power for detecting differences between subgroups | |
| Differences in baseline characteristics between groups. Adjusting for differences, there may be an absolute reduction in the risk of unfavorable outcomes of 13.7% for the liberal group | Non-conventional definition of unfavorable outcomes | |
| Statistical analysis with a frequentist approach | GOS-E dichotomization | |
| Absence of Bayesian analysis | Double adjustment of sample size during study conduction | |
| No data on withdrawal of support or non-resuscitation instructions | ||
| Absence of Bayesian analysis |
GCS: Glasgow Coma Scale; GOS-E: Glasgow Outcome Scale-Extended; Hb: hemoglobin; ICH: intracerebral hemorrhage; SAH: subarachnoid hemorrhage; CI: confidence interval; ICP: intracranial pressure; RR: relative risk; TBI: traumatic brain injury; ICU: intensive care unit.
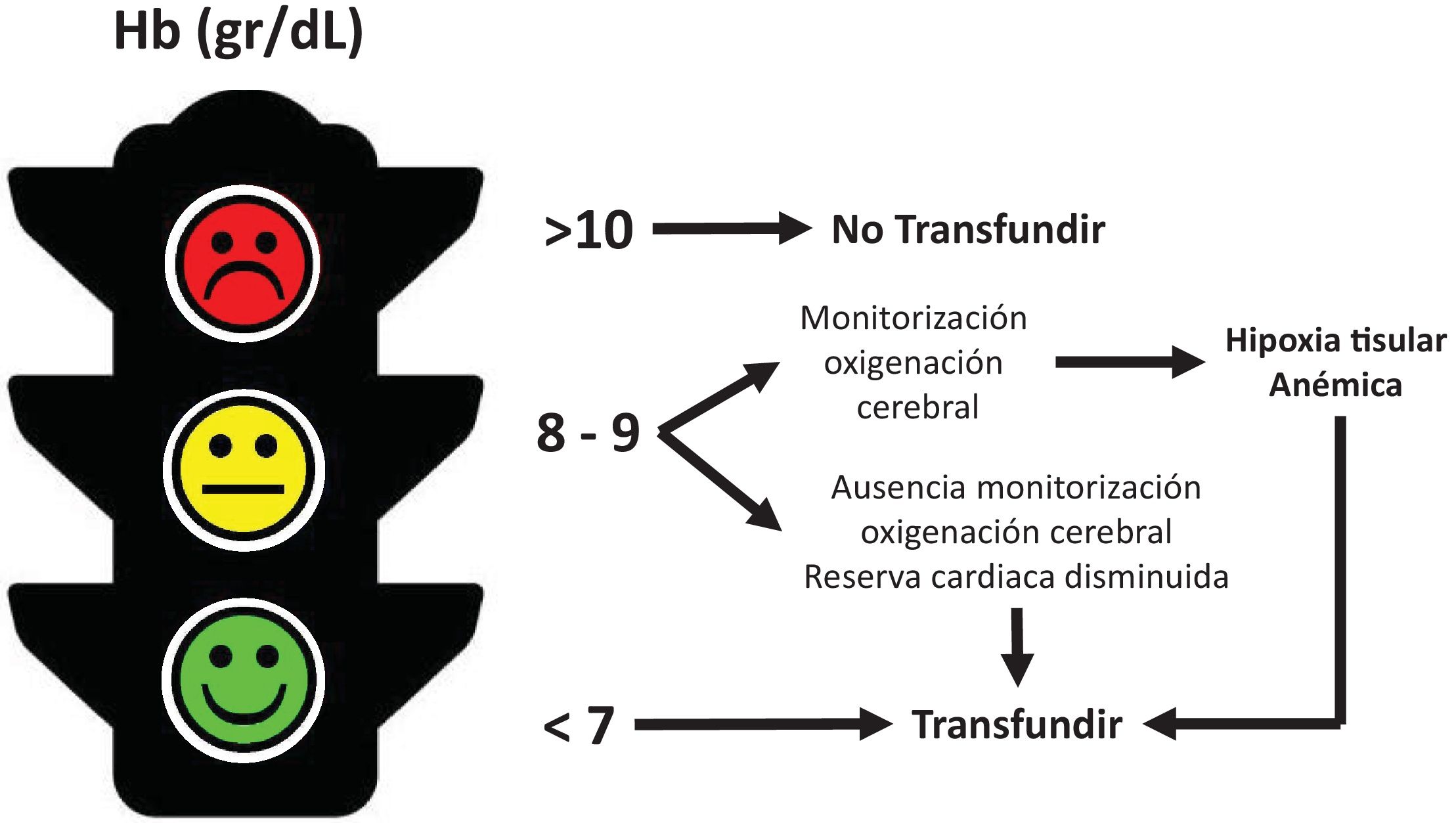
Transfusion carries benefits and risks that should be carefully weighed. Transfusion should be a cost-effective and well-considered intervention; however, the use of Hb concentration alone as a guide to indicate transfusion is inadequate and even dangerous, as it may limit or lead to unnecessary transfusions. When to transfuse remains an empirical and arbitrary decision. How to monitor transfusion effectiveness is a topic that is rarely discussed (Fig. 2).
Physiological studies using positron emission tomography (PET) have shown that transfusion with Hb levels < 10 g/dl increases arterial O2 content and decreases cellular O2 extraction, while cerebral blood flow and metabolism remain unchanged.29,30
Transfusion of packed red blood cells (not stored for prolonged periods) improves invasive cerebral oxygenation (PtiO2) only in the presence of tissue hypoxia31,32; however, its effects on cerebral metabolism are variable or null.33–35
Non-invasive monitoring of cerebral oxygenation with near-infrared spectroscopy (NIRS) has not yet been able to detect an effect of red blood cell transfusion.36,37
Finally, how do we monitor the quality of the Hb delivered? Undoubtedly, it is not just "a number to be achieved", so we should rethink this aspect and systematically include the assessment of basic physiological variables that allow us to determine whether or not the patient's Hb and the Hb delivered are suitable for delivering oxygen to the tissues.
Conclusion and authors' opinionAnemia is a prevalent secondary adverse factor associated with acute brain injury. Its deleterious role lies in the fact that it compromises cerebral TO2, which may induce tissue hypoxia. The transfusion procedure (with leukocyte-depleted fresh red blood cells) requires a prior risk-benefit assessment. Based on recent evidence, it seems prudent to maintain the Hb levels at around 10 g/dl. However, when we transfuse, we must demonstrate its efficacy, and this is not done simply by analyzing "a number".
We believe it essential to maintain basic physiologic variables that ensure adequate oxygen transport and delivery to tissues, especially in settings where advanced neuromonitoring resources are lacking or limited. When both invasive and non-invasive techniques are available to assess cerebral oxygenation, we believe it is necessary to use them as an additional, but not the only, element in determining the transfusion strategy.