Surgery represents one of the main therapeutic references in the world, affording greater survival and life expectancy for many patients. In general, the estimated postoperative mortality is low (around 1–4%). Thirteen percent of the surgical procedures have a high risk of complications, accounting for 80% of all postoperative deaths. Recently, there have been significant advances related to organizational aspects, new anesthetic and surgical techniques, prognostic scales, perioperative management and greater participation and involvement of the patient. This new series of Medicina Intensiva will address fundamental aspects of how Departments of Intensive Care Medicine can add value to the surgical process, in a coordinated manner with other services. Institutional policies are required to ensure the detection of patients at risk in hospitalization wards, with early admission to the ICU of those patients in whom admission is indicated, adapting the treatment in the ICU and optimizing the criteria for discharge. The detection and prevention of post-ICU syndrome in patients and relatives, and the follow-up of ICU discharge and hospitalization in a multidisciplinary manner can reduce the sequelae among critical surgical patients, improving the outcomes and quality of life, and restoring the patient to society. In future publications of this series directed to the surgical patient, updates will be provided on the perioperative management of some of the most complex surgeries.
La cirugía representa uno de los principales pilares terapéuticos en todo el mundo, ofreciendo una mayor supervivencia y esperanza de vida para muchos pacientes. En general, se estima una mortalidad postoperatoria baja, alrededor del 1-4%. Un 13% de los procedimientos quirúrgicos tienen un riesgo alto de complicaciones, representando un 80% de las muertes postoperatorias. Recientemente, se han producido avances significativos relacionados con aspectos organizativos, nuevas técnicas anestésicas y quirúrgicas, escalas pronósticas, manejo perioperatorio y una mayor participación e implicación del paciente. En esta nueva serie de MedicinaIntensiva se abordarán aspectos fundamentales de cómo los servicios de medicina intensiva pueden aportar valor al proceso quirúrgico, de forma coordinada con otros servicios. Se requieren políticas institucionales que aseguren la detección de pacientes en riesgo en plantas de hospitalización, el ingreso precoz en UCI de aquellos pacientes en los que está indicado, adecuando el tratamiento en la UCI y optimizando los criterios al alta. La detección y prevención del síndrome post-UCI en pacientes y familiares, y el seguimiento al alta de UCI y de hospitalización de forma multidisciplinar, pueden reducir las secuelas del enfermo crítico quirúrgico, mejorando los resultados y la calidad de vida y restituyendo al paciente a la sociedad. En futuras publicaciones de esta serie dirigida al paciente quirúrgico se presentarán actualizaciones del manejo perioperatorio de algunas de las cirugías de mayor complejidad.
Surgery is one of the cornerstones of treatment throughout the world, offering increased survival and life expectancy for many patients. A total of 3595 million surgeries were performed in 2012, with an increase of over 38% versus 2004 in all countries – independently of the economical setting. Increased life expectancy has been correlated to surgery above 1533 operations per 100,000 inhabitants.1
Demographic changes such as aging of the population have a significant negative impact upon surgical outcomes. It has been estimated that the elderly population requires surgery with a four-fold greater frequency than the rest of the population. The number of patients undergoing surgery is expected to increase 25% by the year 2020. During this same period, the population of elderly people will increase 50%. Thus, the demographic data of surgical patients reflects a tendency toward more elderly patients and a greater presence of comorbidities.2
In general, postoperative mortality is estimated to be low (about 1–4%).3 Thirteen percent of all operations involve a high risk of complications, accounting for 80% of all postoperative deaths and representing over three million deaths a year.4 The International Surgical Outcomes Study5 has reported an adverse events rate of 16.8% among surgical patients. Furthermore, many patients who survive discharge from hospital, after suffering adverse events, develop functional sequelae and poorer long-term survival – with a two-fold increase in mortality risk at 5 years.6
The risk of perioperative adverse events depends on the patient disease condition before surgery, the prevalence of comorbidities and the urgency of the operation, as well as on the magnitude, type and duration of the surgical procedure.
Recently there have been significant advances in the global surgical process, related to organizational aspects, new anesthetic and surgical techniques, prognostic scales, perioperative management and increased patient participation and implication.7
Perioperative medicine is defined as a “medical care system with a multidisciplinary, integral and patient-centered approach that seeks to provide the best care possible for surgical patients from the moment of indication of surgery until full recovery, with the explicit purpose of improving the outcomes and reducing the complications”. It includes collaborative planning of the operation with the patient, preoperative evaluation, the optimization of comorbidities, the standardization of care, individualization and planning at discharge. All this requires the implicated professionals to work as a team, with effective communication and shared responsibilities.8 This new domain in medicine in turn demands specific competences that must be acquired by the professionals in order to guarantee the best outcomes in complex patients undergoing different types of surgery.9
In the last decades there have been two clearly significant advances that affect the surgical outcomes: minimally invasive surgery and multimodal rehabilitation programs – also known as fast track or enhanced recovery after surgery (ERAS) protocols. Both of these advances seek to reduce surgical aggression and facilitate postoperative recovery.10
Multimodal rehabilitation in surgery comprises a combination of perioperative strategies supported by evidence-based medicine and aimed at improving recovery after surgery. The benefits of these protocols impact upon the patient (lesser morbidity, improved quality of life, better patient experience) and on the healthcare system (shortening of hospital stay).11 These protocols establish recommendations related among other aspects to normal core body temperature, fluid management, hemodynamic monitoring, nutrition and early mobilization, minimization of nausea and vomiting, optimization of hydration, the provision of adequate analgesia, and the reduction of delirium particularly in elderly patients. However, it is the systematic and joint application of all these interventions that finally achieves the positive outcomes. The application of these protocols has been shown to offer benefit mainly in patients subjected to major colon surgery,12 and although at present there are few ERAS protocols available for other types of surgery, they are increasingly adopted in many countries, especially in the industrialized world.
Intensive care unit admission criteriaThe assessment of perioperative risk in surgical patients is crucial for the making of decisions related to the procedure, preoperative optimization, and the intra- and postoperative management of these patients, and this in turn will have an impact upon the final outcomes. Risk stratification is complex and depends on multidimensional factors of a surgical and anesthetic nature, and referred to the individual patient. This in turn demands the intervention of different specialists. A number of tools are currently available to help predict risk. The classical preoperative scales have been questioned, and others have been introduced that encompass both the intraoperative and the postoperative period, in addition to contemplating functional condition, the concept of frailty and the use of biomarkers. In the near future, intelligent perioperative data systems might allow the use of real time and personalized dynamic risk algorithms.13
Postoperative admission to the intensive care unit (ICU) has been regarded as a standard of care in certain types of high risk surgery, and in some countries such as the United Kingdom, these cases account for 40% of all admissions to such units.14 In Spain, surgical patients also represent an important percentage of all admissions to the ICU. In the ENVIN survey, 32.8% of the 138,999 patients admitted to intensive care were surgical patients, and of these 61.4% had undergone elective surgery.15
It is necessary to plan and organize postoperative care taking into account the limited resources, with the purpose of securing improved clinical benefit, safety and efficiency, and implicating the patient in the decision making process.
Different international guides have established recommendations for the postoperative admission of high risk patients to the ICU.16,17 These guides advise local policies to establish the admission criteria, adapted to the organizational characteristics and resources available in each institution.
Non-randomized studies have shown that admission to the ICU can improve the prognosis of surgical patients and even lower the costs by reducing the postoperative complications.18,19 Patients admitted in the immediate postoperative period have a better prognosis than patients admitted late and on an emergency basis after the operation.20,21 Recently, some studies have questioned the elective admission of high risk surgical patients to the ICU – this probably being related to the existence of different organizational models (open and closed ICU) and to the availability of resources in intensive care and hospitalization in different countries.22,23 Other studies have not found the routine postoperative admission to the ICU of elective surgery patients with severe comorbidities to have an impact upon the final outcomes.24
The European Surgical Outcomes Study (EuSOS), an international trial conducted in 28 European countries involving 498 hospitals and 46,539 patients, has evaluated the non-cardiac surgery outcomes in Europe, with the identification of a greater than expected mortality rate (4%).3 In this study, only 5% of the patients were admitted to the ICU on an elective basis. Emergency admission to these Units was associated to greater mortality than in the case of elective admissions. Most of the deceased patients (73%) were not admitted to the ICU after surgery, and of those who were admitted, 43% died after being discharged to the hospital ward.
These findings suggest deficiencies in the allocation of critical care resources, as well as failure to rescue surgical patients that experience worsening in the ward. Some authors have considered “rescue failure” to be a postsurgery patient quality indicator in hospital wards, and suggest that very few patients should die after elective surgery without having considered their admission to the ICU.4 In Spain, where over 70% of all critical patient beds are assigned to Departments of Intensive Care Medicine,25 high risk surgical patients are admitted to the ICU more often than in other countries (12.5% versus 8%).
Despite the difficulty of conducting randomized clinical trials to demonstrate the impact of postoperative surgical patient admission to the ICU, we need more evidence in order to establish criteria defining which surgical patient subgroups truly stand to obtain benefit from such admission. This in turn must be combined with organizational policies to ensure adequate patient flow at hospital level, optimizing the available resources through adequate management of discharges to allow the assigning of surgical patients to the best place possible, and avoiding the cancelation of elective surgeries due to a lack of beds in the ICU.
Management of the surgical patient in the intensive care unitIn recent years there have been significant advances in our knowledge of the perioperative care of surgical patients, with the identification of relevant areas that constitute a priority for future research and application to clinical practice.26 In some instances, knowledge gained in the intensive care setting has made it possible to explore practices that have been shown to be effective in intraoperative patient management. Admission of the surgical patient to the ICU must be adapted to the scientific evidence that has emerged in recent years, with due adherence to the ERAS protocols, affording continuity to the overall surgical process.
Some studies have suggested that the routine procedures carried out in the ICU, with emphasis on the use of invasive devices and diagnostic and therapeutic interventions, can have an adverse impact upon the prognosis of patients admitted to these Units.27 In the same way as in other areas of medicine, intensive care medicine has adopted more restrictive policies in recent years regarding needless interventions that may place patient safety at risk.28 On the other hand, recommendations based on scientific evidence have been made seeking – among other aspects – to reduce the days on mechanical ventilation or prevent delirium.29 Lastly, the Zero projects endorsed by the Spanish Society of Intensive and Critical Care Medicine and Coronary Units (Sociedad Española de Medicina Intensiva, Crítica y Unidades Coronarias [SEMICYUC]) have been shown to significantly reduce the incidence of infections related to the use of devices in patients admitted to the ICU.30,31
Humanization in the ICU has promoted initiatives that favor patient-centered medicine, considering integral management of the patients and their families, covering not only their physical needs but also their psychological and emotional requirements. Surgical patients may benefit from these policies that favor aspects such as the flexibilization of visiting hours, the presence and participation of the family, effective communication, integral patient wellbeing, the prevention and management of post-ICU syndrome, and end of life care.32 Although most patients are discharged to the hospital ward, a proportion die in the ICU. Palliative care therefore must be viewed as an integral part of perioperative intensive care medicine33 – all without neglecting the professionals, who should be offered strategies to prevent and lessen occupational weariness or burnout.
Table 1 summarizes the main considerations referred to the management of surgical patients in the ICU. Some of them are pending the results of ongoing research studies that may offer more scientific evidence.
Recommendations for postoperative patient management in the intensive care unit (ICU).
| Recommendations | |
|---|---|
| Adequate fluid management | Avoid hypovolemia, excessive hydration, and assess volemia. Adequacy of noninvasive monitoring |
| Hemodynamic management | Adjusted to objectives. Consider advanced monitoring in specific cases |
| Delirium | Detection, prevention and management of delirium |
| Pain control | Monitoring of pain. Avoid excessive use of opioids, analgesia regional |
| Sedation | Monitor sedation and dynamic protocols. Daily suspension of sedation according to protocol |
| Renal damage | Avoid hypotension, hypovolemia, excessive hydration and nephrotoxic agents |
| Respiratory complications | Consider regional analgesia, incentivizing of respiratory function, avoid hyperoxia |
| Blood and coagulation | Restrictive transfusion policy (except patients at risk). Transfusion algorithms and management of coagulation according to thromboelastogram and thromboelastometry |
| Mechanical ventilation | Adjust positive end expiratory pressure (PEEP), tidal volume and driving pressure. Daily weaning tests |
| Antibiotic use | Postsurgery prophylaxis protocol. Early treatment according to protocol in sepsis/septic shock |
| Infection associated to medical care | Zero protocols (bacteremia, pneumonia, urethral catheterization, multiresistance) |
| Mobilization | Early mobilization and rehabilitation |
| Nutrition | Consider early nutrition |
| Thromboembolic prophylaxis | Adjusted to protocol |
| Teamwork | Transfer of information, multidisciplinary rounds, daily objectives, checklists, real-time random analyses, etc. |
| Humanization | Flexibilization of visiting hours. Presence and participation of the family |
| Adequacy of end of life care | Limitation of life support |
| Incorporation of palliative care medicine | |
| Living wills | |
| Organ and tissue donation | |
| Management | Evaluation and auditing of outcomes |
In recent years it has become manifest that we need tools to allow the early detection of patients at risk of worsening in the conventional hospital ward. This has led to the introduction of rapid response teams (of variable composition and involving different activation systems) and models of ICUs without walls (characterized by multidisciplinary teamwork and the automated detection of patient severity by integrating clinical and laboratory test parameters).34
In surgical patients, the risk of death in the first 30 days after the operation is 1000 times greater than during surgery itself.35 It therefore would be very useful to be able to establish early identification of those individuals at risk in this particular patient population.
Although different studies have demonstrated the impact of these systems in preventing cardiac arrest, non-elective admission and mortality,36,37 other studies have reported negative or inconclusive findings.38–40 Their ultimate impact upon the outcomes is therefore not clear.
A number of multiparametric scales have been described and validated in surgical patients. Most include variables such as heart rate, blood pressure, respiratory frequency, temperature and oxygen saturation, and others incorporate parameters such as diuresis, altered mental state or concern of the professional about the condition of the patient. A recent study has compared the validity of three scales in 32,527 surgical patients: the Modified Early Warning Score (MEWS), the National Early Warning Score (NEWS) and the Electronic Cardiac Arrest Risk Triage (eCART). This study has observed a greater adverse event predicting capacity with the latter scale, which in addition to clinical parameters also includes laboratory test values.41
There are some limitations to these detection systems. In general, discriminating power is measured based on the area under the receiver operating characteristic curve (AUROC), and although the latter affords global information on the studied population, it is not valid for individual decision making in clinical practice. The sensitivity of the tool measures the probability that an adverse event will occur, but its specificity is given by the incidence of adverse events in the patient sample. In surgical patients, the incidence of adverse events is lower than in other patient subgroups. As a result, in order to assess the usefulness of these scales, some authors propose the use of concepts such as the “missed events rate” (events that occur and are not detected by the tool) and the “non-events rate” (the number of activations that are not followed by an adverse event), and suggest that both should be close to 0%.42 This has been evidenced in the study published by Kellet and Kim,43 where the AUROC for mortality at 48h was 0.86, but the mortality rate was only 0.04%. A score of 7 on the scale was related to a missed events rate of 69% and a non-events rate of 99%. Thus, despite a good AUROC, almost none of the alarms were followed by adverse events in the first 48h, while most of the real events were not detected. Other studies have shown that although these systems increase the number of activations, they do not condition lessened mortality. In this regard, Hillman et al.38 found only 9% of the calls to be related to adverse events. Furthermore, it has been suggested that these systems can distract the attention of professionals from other patients at risk.44 The use of Wi-Fi monitoring systems may prove useful, but they still generate an important number of false alarms. In a recent study, such systems generated 3.3 alarms/hour – most of which corresponded to technical or measurement errors.45 Lastly, other studies have found that the implementation of such systems is not always adequate. Specifically, in a study analyzing compliance with the protocol, only 27% of the patients with adverse events were adequately monitored in the previous 24h, and only 29–58% of the calls were followed by an adequate clinical response.46
In future other tools such as big data and machine learning may prove useful, with greater discriminating capacity referred to patients at risk of suffering adverse events, though they are not yet available for clinical practice.47
In conclusion, despite the need for detection and response systems applicable to surgical patients at risk of worsening in the conventional hospital ward, it is necessary to continue assessing the validity and usefulness of such systems in clinical practice. Lastly, in order to obtain the best results, we not only need tools but also the multidisciplinary effort, awareness and training of the professionals involved.
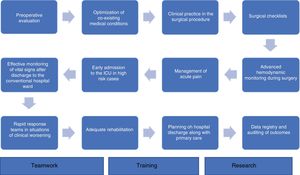
Perioperative medicine as a process. Final considerationsQuality perioperative care, viewed as a global process, is essential in order to improve the outcomes of surgical patients. It includes adequate preoperative evaluation, the optimization of co-existing medical conditions, good clinical practice in the surgical procedure, the use of surgical checklists, advanced hemodynamic monitoring during surgery, the management of acute pain, early admission to the ICU in high risk cases, effective monitoring of vital signs after discharge to the conventional hospital ward, the intervention of rapid response teams in situations of clinical worsening of the patient, and the planning of hospital discharge along with primary care. In this scenario, data registry and the auditing of outcomes constitute a key element for quality improvement48 (Fig. 1).
From the Department of Intensive Care Medicine it is possible to contribute value to the surgical process in a coordinated manner with other Departments.49 Institutional policies are needed to ensure the detection of high risk patients in the hospital ward, with early admission to the ICU of those patients in which admission is indicated, and the optimization of management in the ICU and the criteria at discharge. The detection and prevention of post-ICU syndrome in patients and families, and follow-up at discharge from the ICU and hospitalization from a multidisciplinary perspective can reduce the sequelae of the critical surgical patient – improving outcomes and quality of life with a view to reincorporating the patient to society.50
Future publications of this series addressing the surgical patient will present updates on the perioperative management of some of the more complex surgeries.
The Department of Intensive Care Medicine and its professionals constitute a crucial link in perioperative medicine, and can add value to the different stages of the surgical process (Table 2).
Participation of the Department of Intensive Care Medicine in perioperative medical practice.
| 1. Risk stratification. Preoperative assessment of high risk patients in collaboration with other specialties |
| 2. Offer of ICU beds for high risk patients, avoiding the cancelation of surgeries |
| 3. Participation and continuity of the ERAS protocols during admission to the ICU |
| 4. Reduction of risk associated to healthcare |
| 5. Patient-centered medicine. Humanization of care |
| 6. Post-ICU prevention and support |
| 7. Equipment and systems for the detection of high risk patients in conventional hospital wards |
| 8. Follow-up at hospital discharge. Post-ICU consultations |
| 9. Research: contributing scientific evidence to the process |
| 10. Registry and auditing of outcomes |
ERAS: enhanced recovery after surgery; ICU: intensive care unit.
In its “Strategic plan 2018–2022”, the SEMICYUC advocates quality of the surgical process, establishing specific actions aimed at offering the best care for surgical patients and their families, collaborating with other specialties and disciplines to guarantee a process that is effective, safe, accessible, efficient and respectful of the patient values.
It is necessary to support research and training in this emerging area, which in recent years has been implicated in very important physiopathological and technological advances, modifying the classical concept of the surgical patient. The scientific evidence will have to answer many of the questions which these changes have raised, considering that the concept “prevention is better than healing” is applicable to perioperative medicine, seeking to optimize the outcomes and avoiding the damage associated to healthcare.51 Having said all this, it cannot be forgotten that in patient-centered medicine, technology can never replace well trained professionals that think about and care for their patients.52
Financial supportNone.
Conflicts of interestNone.
Please cite this article as: Martín Delgado MC, Gordo Vidal F. Medicina intensiva perioperatoria. Med Intensiva. 2019;43:427–434.