Acute respiratory distress syndrome (ARDS), first described in 1967, is characterized by acute respiratory failure causing profound hypoxemia, decreased pulmonary compliance, and bilateral CXR infiltrates. After several descriptions, the Berlin definition was adopted in 2012, which established three categories of severity according to hypoxemia (mild, moderate and severe), specified temporal aspects for diagnosis, and incorporated the use of non-invasive ventilation. The COVID-19 pandemic led to changes in ARDS management, focusing on continuous monitoring of oxygenation and on utilization of high-flow oxygen therapy and lung ultrasound. In 2021, a New Global Definition based on the Berlin definition of ARDS was proposed, which included a category for non-intubated patients, considered the use of SpO2, and established no particular requirement for oxygenation support in regions with limited resources. Although debates persist, the continuous evolution seeks to adapt to clinical and epidemiological needs, and to the search of personalized treatments.
El síndrome de dificultad respiratoria aguda (SDRA), inicialmente descrito en 1967, se caracteriza por insuficiencia respiratoria aguda con hipoxemia profunda, disminución de la distensibilidad pulmonar e infiltrados bilaterales en la Rx de tórax. En 2012 la definición de Berlín, estableció tres categorías en base a la hipoxemia (SDRA leve, moderado y grave), precisando aspectos temporales y permitiendo el diagnóstico con ventilación no invasiva. La pandemia de COVID-19 llevó a reconsiderar la definición, enfocándose en el monitoreo continuo de la oxigenación y la oxigenoterapia de alto flujo. En 2021, se propuso una Nueva Definición Global de SDRA, basada en la definición de Berlín pero incluyendo una categoría para pacientes no intubados, permitiendo el uso de SpO2/FiO2 y la ecografía pulmonar para el diagnóstico, y sin ningún requerimiento de soporte especial de la oxigenación en regiones con recursos limitados. Aunque persisten debates, la evolución continua busca adaptarse a las necesidades clínicas y epidemiológicas, y personalizar tratamientos.
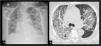
Conceptually, ARDS consists in an acute respiratory failure caused by inflammatory pulmonary edema, characterized by increased vascular permeability with extravasation of fluids into the interstitial space with consequent flooding of the alveolar spaces.1 The loss of aerated lung tissue, due to atelectasis in gravity-dependent areas produced by the increased weight of the overlying lung tissue, leads to profound oxygenation impairment secondary to increased intrapulmonary shunt and alveolar dead space, along with a marked decrease in respiratory system compliance. ARDS is also characterized by the presence of lung infiltrates on CXR (CXR) and computed tomography (CT) scans (Fig. 1A and B). Anatomically and pathologically, the main feature of ARDS is an histological pattern known as diffuse alveolar damage (DAD), which includes the presence of hyaline membranes, edema, type I and II alveolar cell necrosis, and hemorrhage.2,3 This description constitutes the "conceptual model of ARDS" and reflects how clinicians "perceive" the syndrome.4
ARDS can develop secondary to a multitude of risk factors of pulmonary (direct) and extrapulmonary (indirect) cause. This etiological heterogeneity likely reflects the activation of different mechanisms of injury (Table 1).5
Risk factors associated with ARDS.
| Pulmonary |
|
| Extra-pulmonary |
|
Another crucial aspect is the lack of gold standard for defining ARDS. Even DAD is not pathognomonic since it might not be identified in all clinically diagnosed ARDS cases.6 Given the pathophysiological complexity of ARDS and the diverse causes that generate it -although all ultimately converge in the activation of proinflammatory mechanisms- a definition to standardize diagnosis, clinical management, and use of various therapeutic approaches was required. Additionally, the definition of a disease promotes the development of related scientific research.
The definitions of ARDS over timeThe original definition of ARDS dates to 1967 and was issued by Petty and Ashbaugh, who described a group of 12 patients with acute respiratory failure, profound hypoxemia secondary mainly to intrapulmonary shunt, bilateral infiltrates on CXR and decreased thoraco-pulmonary compliance, with no history of chronic respiratory failure, and in absence of left ventricular failure.6 This condition occurred after exposure to what they called a "catastrophic event" (which are now the risk factors for ARDS), of pulmonary or extrapulmonary origin (Table 2). Because of its clinical and radiological similarity to respiratory distress syndrome secondary to surfactant deficiency in newborns, the authors named this entity "Adult Respiratory Distress Syndrome". Petty and Ashbaugh's definition identified what is currently known as severe ARDS, since from the point of view of oxygenation impairment it referred to patients with PaO2 < 50 mmHg with an inspired oxygen fraction (FiO2) > 0.6; that is, with deep hypoxemia, evidenced by a PaO2/FiO2 ratio of approximately 80 mmHg.7
Adult respiratory distress syndrome definition, according to Ashbaugh et al.7
| Pneumonia (bacterial, viral) |
|
|
| Excluding |
|
|
| Respiratory distress clinically evidenced through |
|
|
| Evidence of chest radiography with chest radiographs with evidence of |
|
| Presence of impaired gas exchange |
|
|
| Impaired distensibility of the respiratory system present |
|
Since this initial approach, several modifications of the definition have been made. In 1988, Murray et al. proposed the Lung Injury Score (LIS) with the intention of quantifying the severity of the syndrome. The LIS was the average value of 4 variables, expressed as a score from 0 to 4 reflecting increasing severity: hypoxemia (defined as the PaO2/FiO2 ratio); extent of pulmonary infiltrates on chest radiograph (in quadrants), thoraco-pulmonary compliance (ml/cmH2O) and use of positive end-expiratory pressure (PEEP, cmH2O). ARDS was defined by an LIS > 2.5.8 The LIS was adopted for use, but the definition remained a construction of few experts.
In 1994, the American-European Consensus Conference (AECC) definition was published, in which the syndrome known until then as Adult Respiratory Distress Syndrome was considered as compounded by two conditions of progressive severity: acute lung injury (ALI) and ARDS (Acute Respiratory Distress Syndrome), defined by the compromise of oxygenation: PaO2/FiO2 ≤ 300 and ≤200, respectively (Table 3).9 The acute characteristics of the syndrome and the exclusion of cardiovascular causes for pulmonary edema development were maintained.
ARDS different definitions.
| Variable | AECC (1994)9 | Berlín (2012)4 | Kigali (2016)19 |
|---|---|---|---|
| Timing | Acute onset | Within 1 week of a known clinical insult or new or worsening respiratory symptoms | Within 1 week of a known clinical insult or new or worsening respiratory symptoms |
| Chest image | Bilateral infiltrates on frontal chest radiograph | Bilateral opacities not fully explained by effusions, lobar/pulmonary collapse or nodules on chest radiograph or CT scan | Bilateral opacities not fully explained by effusions, lobar/pulmonary collapse or nodules on radiography, computed tomography or lung ultrasonography |
| Edema cause | Cardiogenic edema should be ruled out. Wedge pressure < 18 mmHg if measurable, or absence of clinical or ultrasound signs of left atrial hypertension. | Respiratory failure not fully explained by heart failure or fluid overload. Need objective evaluation (e.g., echocardiography) to exclude hydrostatic edema if no risk factors are present. | Respiratory failure not fully explained by heart failure or fluid overload. Need objective evaluation (e.g., echocardiography) to exclude hydrostatic edema if no risk factors are present. |
| Oxygenation | 2 categories: a) ALI: PaO2/FiO2 ≤ 300b) ARDS: PaO2/FiO2 ≤ 200 | ARDS Mild: 200 < PaO2/FiO2 ≤ 300 ARDS Moderate: 100 < PaO2/FiO2 ≤ 200 ARDS Severe: PaO2/FiO2 ≤ 100 | ARDS: SpO2/FiO2 ≤ 315 |
| PEEP | Independent of PEEP level | At least 5 cmH2O through invasive mechanical ventilation (or noninvasive in mild ARDS). | No PEEP required |
The AECC definition, like the LIS score, suffered from several criticisms, centered mainly on two aspects. First, experts highlighted the diagnostic difficulties for the evaluation of CXR infiltrates on CXR, due to the great intra- and interobserver variation. In addition, the decision to exclude any standardized level of PEEP for oxygenation assessment produced further variability, secondary to PEEP great impact on the definition of ARDS. Patients could quickly move from one category of hypoxemia to another, without implying a real change in the underlying disease and in its severity.10–12
The publication of the Berlin definition published in 2012 produced other significant changes (Table 3).4 The timing of onset of the acute respiratory failure was incorporated to the preexistent AECC definition: ARDS had to appear within 1 week of exposure to a risk factor. In addition, clarification was added for the origin of edema and of lung images, allowing definition by CT. Three mutually exclusive categories of ARDS severity were established based on the PaO2/FiO2 ratio, evaluated with a minimum PEEP level of 5 cmH2O. In cases of mild ARDS the possibility of considering ARDS in patients who met the diagnostic criteria and were receiving noninvasive ventilation (NIV) was acknowledged. Up till then, ARDS could only be diagnosed in patients undergoing invasive mechanical ventilation.
The Berlin definition was a great advance. One of its strengths was its empirical validation in 3670 patients, unlike previous consensus definitions which only involved agreement between experts. An attempt was also been made to make the new definition compatible with previous ones, especially with the AECC. Another positive feature is Berlin definition’s predictive validity for mortality. Thus, as severity of ARDS increases, an increase in mortality and comorbidities was observed. The experts sought to ensure that the variables defining ARDS were easily measurable, that is to say, that the application of the definition was feasible. For example, the increase in extravascular lung water was considered the variable that best reflected ARDS, but its incorporation into the definition was discarded due to the technical difficulties and expensive technology involved. In addition, the experts established a "conceptual model" of ARDS, which was mentioned at the beginning of this review.
Berlin's definition, however, had limitations. The first is that it requires the use of a minimum level of PEEP for diagnosis, either with invasive or noninvasive ventilation―in the latter case in mild ARDS only. The assessment of bilateral infiltrates on CXR continues to lack intra- and interobserver reproducibility (reliability), and the consideration of a 7-day interval within which the syndrome should develop after exposure to a risk factor is completely arbitrary.13
Another historical debate, which is reinforced after the presentation of each new definition, is whether the syndrome called ARDS really exists or is simply a compilation of multiple, very heterogeneous diseases causing acute hypoxemic respiratory failure. Thus, all definitions of ARDS would necessarily be "unsatisfactory and superficial".13–16
Despite these criticisms, the utilization of AECC definition and the homogenization it implied helped to establish crucial therapeutic achievements which decreased mortality, such as protective ventilation. In addition, the use of high PEEP, compared to the conventional approach of intermediate PEEP, was shown to have no benefit.17,18 The Berlin definition was also widely adopted.
In 2016, in a study conducted in Kigali, Rwanda, the researchers noted that with the previous definitions no patient with acute hypoxemic respiratory failure could be diagnosed as having ARDS, because blood gas measurements were unavailable; it was therefore impossible to know the PaO2/FiO2.19 Only a single daily measurement of peripheral oxygen saturation measured with pulse oximetry (SpO2) was available. The typical images of ARDS could also not be considered for diagnosis since less than 50% of patients with hypoxemia had access to CXR. However, lung ultrasound was available. Furthermore, less than 30% of the hypoxemic patients could receive mechanical ventilation, due to the lack of ventilators; therefore, the PEEP ≥ 5 cmH2O criteria could not be applied either. Finally, due to lack of beds, only 30% of patients with hypoxemic acute respiratory failure could be admitted ICU; thus, patients with less severe ARDS were possibly underdiagnosed. These profound deficiencies in critical care provision are also present in other low-resource areas.
The researchers proposed the Kigali definition of ARDS as a modification of the Berlin definition (Table 3):
- •
The PaO2/FiO2 ≤ 300 required for the diagnosis of ARDS was replaced by SpO2/FiO2 ≤ 315, based on the acceptable linear correlation between PaO2 and SpO2, provided that SpO2 is ≤ 97%, there are no hemoglobin abnormalities, and that peripheral perfusion is adequate. The Rice equation reflects this relationship:
SpO2 /FiO2 = 64 + 0–84 (PaO2/FiO2)20
However, above the 97% threshold, changes in PaO2 generate minimal impact on SpO2, due to the shape of the oxyhemoglobin dissociation curve; therefore, in the flat part of the curve the correlation between both methods of oxygenation assessment is lost.
- •
Lung ultrasound was added as diagnostic method for diagnosis of the syndrome, in the absence of access to CXR or lung CT. Even though these variables were not incorporated at that time into the Berlin definition as it would have been appropriate, what occurred shows the importance of constituting panels of experts with members from all regions of the world. The Berlin definition of ARDS, like the previous AECC definition, had been designed by experts from high-income countries (mostly North America and Europe) without the involvement of researchers from middle- and low-income countries.21
The Kigali definition also reflected the recent expansion of ultrasound as a diagnostic method in the ICU, which might be more reliable than CXR for evaluation of lung infiltrates when trained operators are involved.22,23
The SARS-CoV-2 pandemic that devastated the world and generated maximum stress on health care systems, particularly on the ICUs and emergency departments, prompted a reevaluation of the definition of ARDS. Thousands of patients with acute hypoxemic respiratory failure secondary to COVID-19 were simultaneously admitted to hospitals worldwide; about 15–20 % presented severe disease and 5% required admission to the ICU.24 This trigged the need of continuous monitoring of oxygenation in patients who could rapidly worsen and require some type of respiratory support, from oxygen therapy to invasive mechanical ventilation. Thus, the usual PaO2/FiO2 monitoring with arterial blood gases, which is intermittent, laborious, invasive and resource-intensive, was replaced by the continuous, noninvasive and less costly SpO2 measurement, capable of detecting changes rapidly.
Additionally, during the pandemic, the use of high-flow nasal cannula (HFNC), which was increasingly used in acute hypoxemic respiratory failure,25 became widespread.
As a result of these changes, in 2021, 54 years after Petty and Ashbaugh's publication, an update of the definition of ARDS that could also be universally applicable was proposed. An evolution from the "expert consensus" to "a scientific system of categorization" using approaches adopted in other fields of knowledge to build definitions of syndromes (also called "constructs") for which there are no gold standards, was deemed crucial.26 Lack of a gold standard for definition not only occurs in ARDS but also in other well-known syndromes such as fragility, heart failure, and irritable bowel syndrome, among others.27
For these reasons, the construction of the New Global definition followed a rigorous methodology: description of the clinical phenomenon (or syndrome, or "construct") to be "captured"; justification of the changes proposed for the new definition; explicit criteria for the selection of experts for the panel, which should reflect not only expertise but gender, cultural, geographic, socioeconomic, and ethnic diversity; and specification of the method by which agreements would be reached. Usually, an agreement implies a majority of at least 70% when voting to accept or discard a variable or a statement.
Another objective was to reevaluate the conceptual model of ARDS recommended by the Berlin definition and to develop a New Global Definition of ARDS (Table 4).28 The feasibility of the New Global Definition was also evaluated, i.e., whether its components are easily measurable, both in clinical situations and in research, and can be measured worldwide. The reliability (or reproducibility), namely, the ability of the definition to diagnose the same patient equally in different scenarios and when applied by different professionals, was also analyzed. Finally, the validity of the new definition was explored, that is to say, its ability to reflect what clinicians really want to identify. These concepts include surface validity (the ability to identify the characteristics that are obviously part of ARDS and together distinguish patients with the condition from those without it); and predictive validity (whether the definition predicts outcomes, such as mortality, which should be more frequent in patients with the syndrome compared to those without it).29
Diagnostic criteria for the New Global Definition of ARDS.
| Conceptual model | ARDS is an inflammatory, diffuse, acute, lung injury precipitated by a predisposing risk factor, such as pneumonia, nonpulmonary infection, trauma, transfusion, burns, aspiration, or shock. The resulting injury leads to increased pulmonary vascular and epithelial permeability, pulmonary edema, and severity-dependent atelectasis, all of which contribute to loss of aerated lung tissue. The clinical features are arterial hypoxemia and diffuse radiological opacities associated with increased shunt, increased alveolar dead space and decreased pulmonary compliance. The clinical presentation is influenced by medical treatment (position, sedation, paralysis, PEEP and fluid balance). Histological findings vary and may include intra-alveolar edema, inflammation, hyaline membrane formation and alveolar hemorrhage. |
| Criteria that apply to all ARDS categories | |
| Risk factors and edema cause | Precipitated by an acute predisposing risk factor, such as pneumonia, nonpulmonary infection, trauma, transfusion, aspiration, or shock. Pulmonary edema is not attributable exclusively or primarily to cardiogenic pulmonary edema/fluid overload, and hypoxemia or gas exchange abnormalities are not primarily attributable to atelectasis. However, ARDS can be diagnosed in the presence of these conditions if a predisposing risk factor for ARDS is also present. |
| Timing | Acute onset or worsening of hypoxemic respiratory failure within one week of the estimated onset of the predisposing risk factor or new or worsening respiratory symptoms. |
| Chest image | Bilateral opacities on chest radiograph and computed tomography or bilateral B-lines and/or consolidations on ultrasound that are not fully explained by effusions, atelectasis or nodules/masses. |
| Criteria applied to specific ARDS categories | |||
|---|---|---|---|
| Non-intubated ARDS | Intubated ARDS | Definition modified for countries with limited resources | |
| Oxygenation | PaO2/FiO2 ≤ 300 or SpO2/FiO2 ≤ 315 (if SpO2 < 97 %) in HFNC with flow > 30 L/min. oNIV/CPAP ≥ 5 cmH2O of PEEP. | Mild:200 < PaO2/FiO2 ≤ 300 or235 < SpO2/FiO2 ≤ 315 (si SpO2 < 97%).Moderate:100 < PaO2/FiO2 ≤ 200 or148 < SpO2/FiO2 ≤ 235 (si SpO2 < 97%).Severe:PaO2/FiO2 ≤ 100 orSpO2/FiO2 ≤ 148 (si SpO2 < 97%) | SpO2/FiO2 ≤ 315 (if SpO2 < 97 %)No PEEP or minimum oxygen flow is required for diagnosis in resource-limited settings. |
In addition, it was intended that the New Global Definition of ARDS should be supported by different critical care societies worldwide. After a meticulous review process, the following updates were incorporated into the Berlin consensus definition:
New Global Definition of ARDS28Conceptual modelThe conceptual model presented in the Berlin definition1,2,4 was preserved, and it was added that clinical presentation might be greatly influenced by the medical treatments administered: change in position, sedation, paralysis, PEEP, and fluid balance.
Timing of diagnosis and consideration of extrapulmonary risk factorsRegarding the time of disease onset and exposure to the risk factor, the 7 days established by the Berlin criteria were retained. ARDS is an inflammatory edema due to increased permeability, excluding cardiogenic factors or hydric overload. However, ARDS can be diagnosed in the presence of these conditions if a predisposing risk factor for ARDS exists and if hydrostatic factors are not considered to be the main cause of hypoxemia.
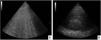
Chest imagesImaging criteria should include bilateral infiltrates on CXR or CT. Additionally, lung ultrasound (evidence of B-lines or consolidation, Fig. 2A and B) is incorporated in this update. Whichever modality is used, it should suggest loss of aeration not fully explained by lobar collapse, pulmonary nodules or pleural effusion.
CXR is the most widely used modality in critically ill patients. However, one of its limitations is the existence of high interobserver variability for identifying bilateral opacities. In fact, this was demonstrated when a CXR was evaluated by the same experts in mechanical ventilation and ARDS.30 A recent study showed an improvement in the interpretation of CXR using the RALE (Radiographic Assessment of Lung Edema) score. It quantifies the number of affected quadrants in each hemithorax in 0–4 points, together with the radiographic density of each quadrant, assigning 1–3 points to it.31 The RALE score showed high interobserver agreement (r = 0.83 [0.8−0.85], p < 0.0001 for 488 studies), and correlated with biomarker concentrations and with progression to prolonged mechanical ventilation.31
Therefore, the New Global Definition of ARDS integrates lung ultrasound to detect the loss of aeration, especially when CXR or CT is not available. This technique is especially useful when the operator is trained to detect bilateral consolidations and noncardiogenic pulmonary edema. In the modified definition for resource-limited countries, the lack of operator expertise could lead to overdiagnosis of ARDS, since PEEP is eliminated as a diagnostic criterion.
Although the use of lung ultrasound for this purpose could be questioned, there is evidence that supports it as an appropriate complement for imaging diagnosis of ARDS.32 On the other hand, a very recent multicenter study evaluated pulmonary edema with the LUS (Lung Ultrasound Score) score for diagnosis of ARDS. It was demonstrated, through a model then applied successfully to a validation cohort, that LUS has a very good diagnostic performance and could detect ARDS correctly, comparable to that of expert evaluators. these conclusions, however, require validation in larger numbers of patients.23
OxygenationThe creation of three new categories of ARDS with the aim of broadening the definition in line with the knowledge gathered during the COVID-19 pandemic was one of the major innovations. Thus, three groups were established:
- •
ARDS in non-intubated patients.
- •
ARDS in intubated patients.
- •
Modified ARDS category for resource-limited settings.
The non-intubated category includes patients with NIV (already considered in the Berlin definition) and also with HFNC. In addition, to ensure that the definition is applicable in regions with scarce resources, a third category was created. For this purpose, the Kigali modification was taken as a reference for settings where advanced ventilatory support devices are not available.
This flexibility of the criteria has benefits as well as possible disadvantages. For example, it might be possible to make the diagnosis of ARDS earlier, from the moment the patient is receiving HFNC support. This allows the early implementation of different treatments, which might even prevent progression to invasive mechanical ventilation.33 Some special considerations are needed to keep in mind when utilizing HFNC. With respect to the FiO2 delivered, there is a relationship between the flow programmed in the device and the patient's inspiratory tidal flow, which might impact on the FiO2 delivered and cause errors in the calculation of PaO2/FiO2.34 Moreover, the inclusion of HFNC in the diagnosis of ARDS might be questioned due to its capacity to generate pressure. In the new definition of ARDS the authors assume that 30 L/m of flow achieves 5 cmH2O of PEEP; however, the airway pressure generated by HFNC will be determined by the programmed flow and patient factors, such as compliance of the respiratory system and whether the mouth is open or closed.33 However, it is noteworthy that patients with severe hypoxemia who receive HFNC might continue to meet ARDS criteria after being intubated, although they can be considered as a less severe form of ARDS.34,35
The gold standard for severity assessment is the use of arterial blood gases to determine PaO2. However, as previously mentioned, the wide use of SpO2 and the SpO2/FiO2 index has proven a valid alternative for diagnosis, given that patients diagnosed by either SpO2/FiO2 or PaO2/FiO2 have similar clinical characteristics and outcomes.36 Although the absolute value of the SpO2 may differ from that of the arterial oxygen saturation measured invasively, the good correlation between both methods and with PaO2 has led to the universal adoption of continuous SpO2 measurement, and its subsequent incorporation to the New Global Definition of ARDS as a standard of care. Likewise, SpO2/FiO2 cut-off points have been included for mild, moderate and severe ARDS.20,37,38
Based on these previous points, the committee agreed to allow the use of SpO2/FiO2 as an alternative to PaO2/FiO2 for the diagnosis of ARDS. Sensitivity and specificity of SpO2/FiO2 for the diagnosis of ARDS according to the AECC definition are good (around 85% for both) but the specificity drops sharply (56%) for PaO2/FiO2 values between 300 and 200.20 In addition, the devices used for SpO2 measurement (digital pulse oximeters) have considerable margins of error in the recordings, which exposes patients to potential misdiagnosis.38,39
When balancing the benefits and limitations listed, the committee felt that the widespread availability of pulse oximetry in all healthcare settings outweighed the disadvantage of overlooking or miscategorizing hypoxemia in some patients. And, most importantly, the overall effect of the New Global Definition will be to increase health equity in settings where ARDS is currently underdiagnosed.
The New Global Definition of ARDS will have a significant impact on epidemiological studies and on interventional clinical trials, since by reflecting a global panorama, additional data on risk factors, disease course and outcomes of different treatments in different populations will be available. However, it might increase diagnostic sensitivity, as patients with atelectasis, for example, could be diagnosed as ARDS in low-resource regions.29 The incorporation of non-intubated patients also increases the heterogeneity of the population diagnosed as ARDS, but might allow, as already mentioned, earlier diagnosis and therefore rapid initiation of treatment. In the future, the identification of distinct ARDS subphenotypes (subgroups of patients that can be reliably discriminated from other subgroups based on a pattern of measurable properties) might reduce the impact of heterogeneity on clinical, physiological, or biological effects of any treatment.40
Issues not included in the New Global DefinitionThere are several topics that might have been considered in this new global definition. For example, the discussion about prognosis enrichment, which might help to optimize validity and applicability of the results obtained in clinical trials on ARDS. As such, the consideration of stricter inclusion criteria, such as limiting participation to intubated patients with moderate to severe ARDS, or to those meeting the definition of ARDS with a programmed PEEP value of 10 cmH2O, could identify more homogeneous subgroups of patients. In turn, this would improve the understanding of clinical variability and facilitate translation to clinical practice of the findings.
Another aspect that was not addressed is how to assess unilateral infiltrates on CXR. In clinical practice, they are considered an exclusion criterion for ARDS, which may preclude the application of protective ventilation strategies.14 However, it is known that CXR is susceptible to biases and limitations, as bilateral infiltrates may go unrecognized especially when taken with the equipment commonly used in ICUs, and in patients with pre-existing lung disease.41 Another common misconception is that patients with unilateral infiltrates on mechanical ventilation have better oxygenation and lower mortality, compared to those with bilateral infiltrates and ARDS.42 Yet data from the LUNG SAFE study reveal that while patients with unilateral infiltrates usually have lower initial severity, mortality does not differ significantly from that of patients with ARDS. Furthermore, in patients with unilateral infiltrates, ventilatory monitoring variables such as driving pressure are important risk factors for developing ARDS, highlighting the importance of implementing protective ventilatory strategies also in this population.43
On the other hand, there is still debate over some issues. Researchers could choose to focus on subjects with ARDS who continue to meet the definition beyond 24 h if they wish to exclude those who improve rapidly or introduce a stabilization period as proposed by Guerin in the PROSEVA study, therefore achieving more homogeneous subgroups to evaluate a response to a therapeutic maneuver.44–46 Other subcategories of ARDS (subgroups and subphenotypes) that were not included in the current definition have been proposed to assess possible different responses to treatment. These subcategories might be defined by biomarker levels, types of images (focal or diffuse lung infiltrates), recruitment potential, or physiological variables such as inspiratory pressure, elasticity behavior, or the ventilatory ratio.47–53
Final considerationsThe New Global Definition of ARDS is an extension of the Berlin definition in matters of diagnosis and monitoring of the disease. The category of intubated patients preserves the previously described PaO2/FiO2 severity cut-off points but allows the use of equivalent SpO2/FiO2 cutoff values in situations where arterial blood gases are not available. The two new categories added: ARDS in non-intubated patients, and ARDS in patients assisted in regions with low health resources, constitute a relevant innovation, but it is especially highlighted that the diagnostic modifications proposed for these regions should be considered only in them. It is recommended that researchers continue to report their data based on the Berlin definition when possible. The epidemiological impact of the modified definition for low-resource areas is still uncertain. Looking ahead, there is great hope that the identification of ARDS subphenotypes may facilitate the assessment of response to different treatments and the development of personalized treatments.
Conflict of interestGAP has received funding for teaching programs from Medtronic LATAM and Vapotherm Inc. USA.
To Emiliano Descotte of the Hospital Británico of Buenos Aires for his disinterested contribution with the ultrasound images.