Cardiogenic shock (CS) is a heterogeneous syndrome with high mortality and a growing incidence. It is characterized by an imbalance between the tissue oxygen demands and the capacity of the cardiovascular system to meet these demands, due to acute cardiac dysfunction. Historically, acute coronary syndromes have been the primary cause of CS. However, non-ischemic cases have seen a rise in incidence. The pathophysiology involves ischemic damage of the myocardium and a sympathetic, renin-angiotensin-aldosterone system and inflammatory response, perpetuating the situation of tissue hypoperfusion and ultimately leading to multiorgan dysfunction. The characterization of CS patients through a triaxial assessment and the widespread use of the Society for Cardiovascular Angiography and Interventions (SCAI) scale has allowed standardization of the severity stratification of CS; this, coupled with early detection and the “hub and spoke” approach, could contribute to improving the prognosis of these patients.
El shock cardiogénico (SC) es un síndrome heterogéneo con elevada mortalidad y creciente incidencia. Se trata de una situación en la que existe un desequilibrio entre las necesidades tisulares de oxígeno y la capacidad del sistema cardiovascular para satisfacerlas debido a una disfunción cardiaca aguda. Históricamente, los síndromes coronarios agudos han sido la causa principal de SC; sin embargo, los casos no isquémicos han aumentado en incidencia. Su fisiopatología implica el daño isquémico del miocardio, una respuesta simpática, del sistema renina-angiotensina-aldosterona e inflamatoria, que perpetúan la situación de hipoperfusión tisular conduciendo finalmente a la disfunción multiorgánica. La caracterización de los pacientes con SC mediante una valoración triaxial y la universalización de la escala SCAI ha permitido una estandarización de la estratificación de la gravedad del SC que, sumada a la detección precoz y el enfoque “Hub and Spoke”, podrían contribuir a mejorar el pronóstico de los pacientes en SC.
Cardiogenic shock (CS) is a potentially fatal syndrome characterized by cardiac dysfunction and systemic hypoperfusion. The incidence of the syndrome is growing, and the mortality rate remains high; CS therefore poses a significant challenge for intensivists, due to its complexity as a heterogeneous clinical syndrome in which individualized and early patient management is crucial.
In this context, this first article of the series “Update in cardiogenic shock” explores the epidemiological and pathophysiological aspects, risk stratification, and early detection strategies referred to CS.
EpidemiologyThe prevalence of CS varies depending on the definition used, the clinical setting and the data compilation period involved. Based on a recent national registry,1 CS accounts for 6% of all admissions to Spanish Intensive Care Units (ICUs), with an in-ICU mortality rate of 32%. Both the prevalence and mortality data at the Spanish national level are consistent with the information provided by different European registries,2–5 with mortality rates of between 30%–60%.
Historically, acute coronary syndrome (ACS) has been the most common cause of CS (CS-ACS). The estimated incidence of CS-ACS among patients who have suffered an ischemic event is 4%–12%.6 A French registry found the incidence of CS-ACS to decrease in the period 2005–2015 from 5.9% to 2.8%, with a mortality rate that did not experience significant changes (40%–50%).7 In contrast, in Spain during a similar period (2003–2015), the incidence of CS-ACS remained constant between 5.7%–6.4%, though mortality decreased significantly from 82% to 67.1%.8
Overall, the contribution of CS-ACS has decreased in the last two decades in parallel to an increase in CS of other etiologies. The main causes of non-ischemic CS (CS-NoACS) include non-ischemic cardiomyopathy, arrhythmias and severe valve disease.9 Patients with CS-NoACS have a greater burden of pre-existing heart failure, as well as atrial and ventricular arrhythmias, pulmonary hypertension and chronic kidney disease.9 However, the comparative data on mortality between CS-ACS and CS-NoACS present some discrepancies. In the CardShock registry,10 patients with CS-ACS did not show significant differences in mortality concerning patients with CS-NoACS (40% versus 24%, respectively; P = .06), though ACS as a cause of CS was found to be an independent predictor of mortality (odds ratio [OR] 7.4; 95% confidence interval [95%CI] 1.9%–29.8%). On the other hand, a more recent German study recorded a significantly higher 30-day mortality rate in patients with CS-NoACS, with a hazard ratio (HR) of 1.14, (95%CI 1.04–1.24).11
DefinitionsCardiogenic shock traditionally has been defined as a situation characterized by low cardiac output (cardiac index [CI] < 2.2 l/min/m2), with sustained arterial hypotension (systolic blood pressure [SBP] ≤ 90 mmHg or mean blood pressure [MBP] ≤ 60 mmHg) under conditions of euvolemia, hypoperfusion and pulmonary congestion (pulmonary artery occlusion pressure [PAOP] ≥15 mmHg).12,13 However, agreement on the diagnostic criteria or definition is lacking, and CS may be observed without arterial hypotension. On the other hand, in some cases, the diagnosis is established only through the identification of clinical parameters, since there are no advanced hemodynamic monitoring systems that can obtain the described parameters.13
Thus, the most recent guides and publications describe CS as a clinical syndrome resulting from an imbalance between the tissue oxygen demands and the capacity of the cardiovascular system to satisfy such demands, due to acute cardiac dysfunction.12–16
Cardiogenic shock should be understood as a continuum extending from a pre-shock or compensated shock scenario to refractory CS. Pre-shock has been described as a clinical situation characterized by hypotension without evidence of hypoperfusion, and which will evolve towards CS if adequate treatment is not provided.13,17 On the other hand, refractory CS is the most serious manifestation of CS. It is characterized by persistent hypoperfusion and multiorgan dysfunction despite the application of etiological treatment and adequate pharmacological and/or mechanical supportive measures.12,14,17
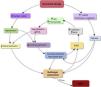
PathophysiologyThe pathophysiology of CS (Fig. 1) is originated by an index event that results in cardiac dysfunction due to decreased contractility, mechanical complications or altered homeostasis in a patient with chronic heart disease and ventricular dysfunction.
The leading cause of CS is ischemic heart disease. Accordingly, the most frequent sequence is pump failure (reversible or otherwise) secondary to acute thrombosis of a coronary artery that perfuses an extensive myocardial territory, without collateral circulation. It has been estimated that for CS-ACS to develop, a loss of myocardial mass of about 40% is required, with the anterior descending artery being the most frequently affected vessel – though multivessel disease may be present in 60% of the cases.18 The magnitude of the infarcted or recoverable myocardium, as well as the neurohormonal and metabolic compensatory mechanisms, influence the speed with which CS develops and progresses.19
In CS-NoACS, the index event is not so clearly identifiable. This is the case for example in cardiomyopathy with ventricular dysfunction, where a supraventricular arrhythmia, a subtle increase in congestion, or anemization can cause compensated chronic heart failure (HF) to evolve towards CS.
In both cases (CS-ACS and CS-NoACS), the decrease in myocardial contraction capacity to below the threshold required to sustain cardiac output in accordance with the physiological demands results in changes at cardiac and systemic level that seek to maintain cardiac output. If these adjustments prove ineffective, a vicious circle is generated, leading to multiorgan failure and the death of the patient.
Sympathetic and renin-angiotensin-aldosterone system responseWhen cardiac output decreases sufficiently, both systemic and coronary perfusion are lowered. Coronary perfusion moreover drops through a double mechanism, since there is also an increase in intraventricular diastolic pressure. The decrease in myocardial perfusion in turn induces or worsens ischemia, generating a vicious circle of increased cardiac dysfunction and lowered cardiac output.
At the systemic level, the baroreceptors activate a sympathetic response with the release of catecholamines that elevate the heart rate, venous tone and arterial vasoconstriction, to increase peripheral and coronary perfusion. However, this in turn causes an increase in afterload, with the consequent rise in filling pressures and overload of the already damaged myocardium.16,19 In addition, due to the increase in arteriolar and venous vasoconstriction, a gradient is produced that results in fluid displacement from the non-stressed to the stressed compartment, generating increased congestion.
Renal hypoperfusion activates the renin-angiotensin-aldosterone axis.15 Angiotensin produces peripheral vasoconstriction, contributing to the increase in cardiac afterload. In turn, aldosterone causes water and sodium retention, seeking to increase the circulating volume – an effect that adds to the secretion of antidiuretic hormone, thereby worsening congestion.
Anaerobic metabolism and systemic inflammatory responseWhen these mechanisms are exceeded and cardiac output is unable to meet the metabolic and oxygen demands of the tissues, the latter enter anaerobic metabolism, generating lactic acidosis and depletion of the adenosine triphosphate (ATP) reserves. This in turn leads to worsening of cardiac output, which moreover can be adversely affected by arrhythmias or mechanical complications.
Ischemia sets in and a proinflammatory state is generated in the form of a systemic inflammatory response syndrome. This state is characterized by the release of cytokines, tumor necrosis factor-alfa (TNF-α), free radicals and nitric oxide synthase (NOS), producing vasodilatation through microvascular resistance to catecholamines20 and increased depression of myocardial function secondary to a decrease in beta receptor expression. Another substance released in the context of hypoperfusion is interleukin-6 (IL-6), which is independently associated with early mortality in patients with acute myocardial infarction.21
MicrocirculationThe pro-inflammatory state produces endothelial dysfunction, resulting in capillary leakage that contributes to multiorgan dysfunction.22 Under normal conditions, the glycocalyx covers the luminal surface of the endothelium, preserving its integrity and avoiding platelet aggregation and coagulation. When the glycocalyx loses its integrity, endothelial activation occurs, with platelet and leukocyte adhesion and activation of the coagulation cascade, producing thrombosis and microvascular occlusion.23
These microcirculatory disorders may appear from the early stages of CS, when no macrocirculatory alterations have yet developed, and are associated with a poorer prognosis.24
The activation of leukocytes and monocytes results in the secretion of large amounts of myeloperoxidase, which in turn generates reactive oxygen species (ROS), damaging the myocardial cells and altering the vascular tone.20 On the other hand, the mitochondrial DNA of the damaged cells that is released and circulates in blood induces a potent inflammatory response mediated by Toll-like receptor 9 (TLR-9), which identifies it as a foreign material in a way similar to the response that occurs in septic shock when bacterial material is detected.25
Multiorgan dysfunctionAs hypoperfusion becomes generalized and anaerobic metabolism sets in, several initially reversible organ dysfunctions develop. Hypoxia and metabolic acidosis of lactic acid origin produce a decrease in vascular resistance at the arteriolar level, worsening vasoplegia and amplifying the inflammatory response.12
Liver and renal dysfunction secondary to hypoperfusion and congestion is associated with metabolic, ionic and water alterations and coagulopathy. The neurological exploration may reveal drowsiness and coma or, contrarily, psychomotor agitation. At the pulmonary level, congestion favors the appearance of edema and therefore of hypoxia, which will affect both the already ischemic myocardium and the rest of the organs. Gastrointestinal hypoperfusion in turn facilitates bacterial translocation, favoring the appearance of sepsis, and bleeding.
The initial cardiac dysfunction, tissue hypoperfusion and multiorgan dysfunction generate increasingly greater hypotension that worsens the ischemia, perpetuating a vicious circle that becomes irreversible.16,26 Once this point of no return has been reached, and even if tissue perfusion is restored by the measures adopted to revert the condition, it is unlikely that the death of the patient can be avoided.
Classification systems and prognostic stratificationThe classification of CS may improve the outcomes by guiding the intensity and timing of patient management.27 Traditionally, CS has been classified based on clinical and hemodynamic criteria (Fig. 2). However, this classification is not suited to the current profile of patients with CS, and has scant therapeutic implications. On the other hand, the INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support), initially designed for the stratification of patients with advanced heart failure, classifies patients into 7 levels according to their hemodynamic profile and degree of target organ damage. In this classification, patients with CS can only be classified into stages 1 and 2; it is therefore not able to adequately discriminate severity in patients with CS.28
Several mortality risk factors in CS have been identified, with the severity of shock being one of the most potent predictors and the factor most widely used in the existing classifications.27,29 There is great heterogeneity in the definition of severity, however, and the lack of standardization constitutes a limitation both for communication between care teams and for the comparison of results in the research setting.
Of the individual severity parameters associated with the prognosis of CS, mention should be made of the following:
- 1
Grade of hypoperfusion. Lactate concentrations > 4–5 mmol/l upon admission imply a poorer prognosis, particularly when the levels exceed 10 mmol/l. A lack of lactate clearance and more acid pH levels have also been associated with a poor prognosis.10,30–33
- 2
Hemodynamic condition of the patient. Arterial hypotension, tachycardia, low cardiac output, loss of contractility (low stroke volume, cardiac work index), right ventricle dysfunction and systemic congestion.10,28,29,33,34
- 3
Intensity of the circulatory support measures. A poorer prognosis is associated with a need for more vasoactive drugs, in terms of both the number of vasopressors and inotropic agents, and their dosage.35 Indices such as the Vasoactive Inotropic Score or the Norepinephrine Equivalent Dose have been associated with mortality, integrating the arterial pressure reached and the need for mechanical circulatory support (MCS) as a modifier of amine dosage.36–38
- 4
Cardiac arrest (CA) recovery. Independently of its duration and the care level at which it occurs, and particularly when associated with anoxic brain damage, the prognosis is poor even when shock improves with treatment.13,39,40
- 5
Secondary inflammatory response grade.41
To determine the prognosis of CS we also must take into account several non-modifiable factors inherent to the patient. In this regard, age is the factor with the strongest impact on the prognosis. Other characteristics, such as frailty, comorbidities or gender and race are less consistent between different studies in terms of impact.42–44
The SCAI scaleAt present, the most popular classification for CS in clinical practice and in research is that of the Society for Cardiovascular Angiography and Interventions (SCAI).13 This scale stratifies the patients into 5 levels or stages of severity (A, B, C, D, E) using parameters referred to the clinical, hemodynamic and biochemical situation (perfusion and organ function), with cardiac arrest recovery acting as an unfavorable prognostic modifier represented under subtype A (Table 1).
Descriptors of the different grades of the Society for Cardiovascular Angiography and Interventions (SCAI) classification.13
| Shock grade | Physical examination | Laboratory findings | Hemodynamic parameters |
|---|---|---|---|
| A “At risk” | • No signs of lung edema | • Lactate in normal range | • Normotension |
| • No signs of hypoperfusion | • Normal renal function | • CI ≥ 2.5 | |
| • SvO2 ≥ 65% | |||
| • No vasoactive drugs | |||
| B “Beginning” | • Jugular ingurgitation | • Lactate in normal range | • Hypotension (SBP < 90 or MBP < 65 mmHg) |
| • Isolated crepitants | • Minimally altered renal function | • Tachycardia (HR > 100/min) | |
| • No signs of hypoperfusion | • Elevated BNP | • CI ≥ 2.2 | |
| • SvO2 ≥ 65% | |||
| • No vasoactive drugs | |||
| C“Classic” | • Jugular ingurgitation | • Lactate ≥ 2 mmol/l | • Hypotension with need for vasoactive drugs |
| • Crepitants | • Increase × 2 in basal creatinine | • CI < 2.2 | |
| • Signs of hypoperfusion | • Elevated BNP | • PCP > 15 mmHg | |
| • Need for NIV or CPAP | • Altered liver profile | • PAPI < 1.85 | |
| • CPO ≤ 0.6 | |||
| D “Deteriorating” | • Same as in grade C | • Same as in grade C | • Same as in grade C with the need for multiple vasopressors or MCS |
| E“Extremis” | • Invasive mechanical ventilation | • Multiorgan failure | • Hypotension despite grade C and D support |
| • Near loss of pulse | • pH ≤ 7.2 | • Refractory VF/VT | |
| • Need for MV | • Lactate ≥ 5 mmol/l | • Electrical activity without pulse | |
| • Need for defibrillation | |||
| • Cardiovascular collapse |
In the case of cardiac arrest, in any of the grades, we add subtype “A”. CI: Cardiac index; SvO2: Mixed venous oxygen saturation; BNP: Brain natriuretic peptide; SBP: Systolic blood pressure; MBP: Mean blood pressure; HR: heart rate; NIV: Noninvasive ventilation; CPAP: Continuous positive airway pressure; PAPI: Pulmonary artery pulsatility index, defined as the ratio between the difference in systolic and diastolic pressure of the pulmonary artery and the right atrial pressure; CPO: Cardiac power output (defined by the formula [MBP x cardiac output]/451); MCS: Mechanical circulatory support; VF: Ventricular fibrillation; VT: Ventricular tachycardia.
Several studies have validated the scale in different centers and countries, applied to both CS-ACS and CS-NoACS. These publications show SCAI to be correlated to the patient prognosis, with greater short and long-term mortality corresponding to higher stages, even on stratifying the population according to the diagnosis, non-modifiable risk factors, intensity of treatment, shock phenotype and comorbidity. This capacity has been demonstrated when the scale is used upon admission, with the poorest values in the first 24 h, or even with the poorest values during patient stay. The short-term mortality rate is 3% in stage A and increases to 7% in stage B, with a four-fold increase in stage C (10%–35%), and reaches 25%–68% in stage D and 45%–85% in stage E.
The SCAI could have a favorable impact when used as a triage tool, offering a window of opportunity for early detection, treatment adjustment or even patient transfer to a reference center in order to avoid CS progression and improve the prognosis.39
Nevertheless, the SCAI still presents some points of controversy that need to be resolved, such as the definition of normotensive CS in stage B, the cut-off points of the hemodynamic and laboratory test variables used, the degree of neurological involvement for introducing subtype A in the case of cardiac arrest, or the use of the scale in other types of shock.45
Triaxial assessment of patients with CSA review of the SCAI shows that even within each stage there are different levels of severity with different prognoses, conditioned to certain characteristics of the patient and the clinical situation. These factors act as risk modifiers; consequently, the use of a triaxial assessment model is proposed, taking into account patient severity according to the SCAI stage, the shock phenotype and the risk modifiers39,45 (Fig. 3).
Triaxial assessment of patients with cardiogenic shock (CS).
*Echocardiographic alterations: Left ventricular ejection fraction (LVEF) < 40% and early mitral valve insufficiency.
**Hemodynamic alterations: Cardiac index (CI) < 1.8 ml/kg/m2, Systolic volume index (SVI) < 35 ml/m2, Cardiac power output (CPO) < 0.6 W.
LV: Left ventricle; RV: Right ventricle.
The shock phenotype refers to the concrete hemodynamic profile of the patient. It is defined using clinical, biochemical, echocardiographic and invasive monitoring criteria, with the distinction of three differential prognostic profiles44,46:
- 1
Non-congestive, characterized by isolated left side dysfunction without congestion and with little organ damage.
- 2
Cardiorenal, characterized by significant left-side dysfunction, renal damage and pulmonary congestion.
- 3
Cardiometabolic or hemometabolic, characterized by right ventricle dysfunction, systemic congestion, lactic acidosis and multiorgan dysfunction.
Using the phenotype and SCAI, the mortality rate progresses from 6% in non-congestive stage C to 29% in cardiometabolic stage C – exceeding mortality in non-congestive stage D (17%). A greater inflammatory component with the corresponding loss of peripheral vascular tone is likewise related to a poorer prognosis. Use has been made of the neutrophil/lymphocyte ratio (NLR) to estimate inflammatory activity, and in this regard, considering a value of <3.5 in the controls, the ratio is associated with a 1.05-fold increase in mortality for every 3.5 points of increase in NLR. Stage B with inflammation presents greater mortality than stage C without inflammation.46
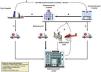
Detection of cardiogenic shockAs has been commented, CS is the most severe form of acute heart failure and is characterized by rapid worsening that leads to multiorgan failure secondary to tissue hypoperfusion. This process may prove reversible provided it is detected early and the required measures are adopted.14,47 However, the detection of CS (Fig. 4) may be complex, since it involves different phases (identification, hemodynamic evaluation and stratification) and requires the intervention of different professionals and/or care levels which in some cases may need resources not available in all centers that attend critically ill patients.48 As occurs in other time-dependent disorders (acute myocardial infarction, stroke, trauma, sepsis), correct management requires the definition of early detection criteria and intra- and inter-hospital coordination based on a code system. In this regard, a network care model for CS has been proposed, known as “Hub and Spoke”, where once the condition has been detected, rapid adjustment of the measures to the needs of the patient can be made (Fig. 5). This requires fluid communication between centers, consensus-based protocols, and the presence of a transportation team qualified for the care and transfer of patients with CS.49–51 With regard to the reference center (Hub), improved outcomes are achieved when detection and evaluation are made by a multidisciplinary team (Shock Team), with a clear definition of the role of each of its members (Table 2), and led by a specialist in cardiological critical care (Shock-Doc) who coordinates the therapeutic decisions and intervenes in the entire process.52–54
Patient flow of the Cardiogenic Shock Care Network.
Adapted from M. Martínez-Sellés et al.50 A: In order to secure early stabilization of a patient with CS not associated with acute myocardial infarction (AMI) diagnosed out of hospital, the patient can be transferred to the closest available level 3 hospital if transfer to a level 1 or 2 center takes over 30 min in comparison with transfer to the level 3 center. B: A patient diagnosed with CS outside the hospital setting or who is admitted to a level 3 hospital should be transferred to a level 1 or 2 center, depending on the transfer times, particularly in the context of acute coronary syndrome. C: A patient diagnosed with CS outside the hospital setting or who is admitted to a level 3 hospital may be transferred to a level 1 center if the need for high-complexity care is anticipated. D: Activation of the ECMO team. A mobile unit may be activated from the level 1 center towards the different available reference centers (levels 1 and 2) if a high-complexity mechanical circulatory assist device needs to be implanted to ensure a safe transfer. CS, cardiogenic shock; ECMO, extracorporeal membrane oxygenation; SBP, systolic blood pressure.
Members and functions of the “Shock team”.
| Member of the Shock team | Functions |
|---|---|
| Out of hospital/Emergencies/Hospitalization (physician and nurses in charge) | • First contact with the patient |
| • Diagnostic suspicion and first care | |
| • Activation of CS code | |
| • Transfers to level 2 and 3 centers | |
| Cardiological ICU: | • Coordination of the process (Shock-Doc) |
| Intensivist | • Identification, stratification and diagnosis of CS |
| Critical care nurses | • Medical treatment |
| • Monitoring, planning and early decision of MCS/VAD | |
| • Patients with MCS/VAD transport team (mobile ECMO) | |
| • Short duration percutaneous MCS/VAD implantation | |
| • Invasive hemodynamic monitoring and organ support (MV, CRRT) | |
| • Postinterventional and postoperative control | |
| • Neurological evaluation | |
| • Evaluation of long-duration VAD/heart transplantation | |
| • Adjustment of measures/LSTA/End of life care/Organ donation | |
| Cardiology: | • Identification, stratification and diagnosis of CS |
| Clinical cardiologist | • Medical treatment in Cardiology |
| Heart failure cardiologist | • Monitoring, planning and early decision of MCS/VAD |
| • Diagnostic support (TTE/TEE) at MCS/VAD implantation | |
| • Postinterventional and postoperative control | |
| • Evaluation of long-duration VAD/heart transplantation | |
| Hemodynamics: | • Coronary or structural interventionism |
| Hemodynamist | • Decision of early MCS/VAD implantation |
| Interventional nurses | • Short duration percutaneous VAD implantation |
| • Postinterventional monitoring and withdrawal of percutaneous MCS/VAD | |
| Cardiovascular surgeon: | • Decision of early MCS/VAD implantation |
| Surgery nurses | • Short and medium-duration percutaneous or surgical MCS/VAD implantation |
| Anesthesiology | • Evaluation of long-duration VAD/heart transplantation |
| • Postoperative monitoring and correction of surgical complications | |
| • Medical treatment, hemodynamic evaluation and support during surgical VAD implantation and heart transplantation | |
| • Evaluation of long-duration VAD/heart transplantation | |
| Perfusion team: | • Assembly, priming and connection of short and medium-duration MCS/VAD circuit |
| Perfusion or critical care nurses | • Partial or total change of short and medium duration MCS/VAD |
| • Intra- and inter-hospital transfer (mobile ECMO) | |
| • Postinterventional monitoring |
CS: Cardiogenic shock; MCS: Mechanical circulatory support; VAD: Ventricular assist device; ECMO: Extracorporeal membrane oxygenation; MV: Mechanical ventilation; CRRT: Continuous renal replacement therapy; LSTA: Life support treatment adjustment; TTE: Transthoracic echocardiography; TEE: Transesophageal echocardiography.
Several experiences have shown that establishing care protocols based on the organization of a multidisciplinary team with the early start of mechanical circulatory support (MCS), based on uniform and early diagnostic criteria, reduces mortality related to CS.50,55,56
Cardiogenic shock patient identification phaseThe detection of CS should begin at first medical contact with the patient (including out-of-hospital contact), and should be based on the evaluation of clinical signs (SBP < 90 mmHg or MBP < 60 mmHg, lethargy, coldness, diuresis < 30 ml/h) and laboratory test data (serum lactate > 2 mmol/l, BNP or NT-proBNP > 100 or 300 pg/ml, elevated urea and/or creatinine) indicative of hypoperfusion.47
It must be taken into account that the clinical signs are not specific to CS. In this regard, patients with advanced heart failure may present clinical and hemodynamic profiles that emulate CS without the latter actually being present. Likewise, hypertensive patients or individuals in the early stages of CS when pressure compensation of hypoperfusion is still active might not show hypotension, and in high-risk pre-shock patients the imminent development of shock might go unnoticed.
With regard to the laboratory test data, it should be noted that while lactate is a good indicator of the degree of hypoperfusion, has a high prognostic value, and is correlated to the degree of organ recovery and survival, it also may be elevated in other situations (diabetic ketoacidosis, trauma, liver failure, use of adrenaline, linezolid, propofol) that must be considered in the differential diagnosis.57 In the case of BNP, we also must take into account that elevations can be observed in a broad range of cardiac and non-cardiac conditions; the determination of BNP therefore should be made in cases where no firm diagnosis is available.47 On the other hand, troponin can be useful for the diagnosis of ACS. Table 3 describes the different diagnostic tests to be performed on patients with CS.
Complementary tests in patients with cardiogenic shock.
| Test | Timing | Findings | Diagnostic value |
|---|---|---|---|
| Lactate | I/D | • ≥2.0 mmol/l | • Grade of hypoperfusion/CS |
| • ≥5.0 mmol/l | |||
| ECG | I/D*/A | • Ischemia/myocardial lesion | • ACS |
| • Conduction disorders | • Cardiomyopathy | ||
| • Arrhythmias | • Arrhythmias | ||
| Echocardiogram | I/D*/A | • Ventricular function | • Cardiomyopathy |
| • Segmental alterations | • ACS | ||
| • High pressures LV | • Stress myocardiopathy (Takotsubo) | ||
| • Pulmonary hypertension | • Pulmonary congestion (ALE) | ||
| • Pericardial effusion | • PTE/congestion | ||
| • Valve disorders | • Tamponade | ||
| • Mechanical disorders | • Valve diseases/endocarditis | ||
| • IVC/cardiac rupture/mitral valve rupture | |||
| Chest X-ray Pulmonary ultrasound | I/D | • B lines | • Pulmonary congestion (ALE) |
| • Alveolar edema | • Infection/pneumonia | ||
| • Alveolar infiltrate | • Heart failure | ||
| • Pleural effusion | • Pneumothorax | ||
| • Pneumothorax | |||
| Natriuretic peptides (BNP, NT-proBNP) | I/A | • BNP > 100 pg/ml | • Negative predictive value |
| • NT-proBNP> 300 pg/ml | |||
| Troponin I | I/D* | • >0.4 ng/ml | • Myocardial damage (ACS) |
| Serum creatinine | I/D*/A | • >1.2 mg/dl | • Hypoperfusion/CS |
| Ions (Sodium, Potassium, Chloride) | I/D*/A | • Hyponatremia | • Signs of chronic HF |
| • Hyperkalemia | |||
| Procalcitonin | I | • >0.5 ng/ml | • High bacterial infection predictive value |
| TSH | I | • Hypo-hyperthyroidism | |
| Blood count | I/D*/A | • Anemia | • Signs of chronic HF |
| Coagulation study D-dimer | I | • Elevation of times or DD | • Sign of hypoperfusion |
| • DD negative predictive value PTE | |||
| Arterial blood gases (pH, pO2, pCO2, SatO2) | I/D* | • pH < 7.35 | • Hypoperfusion |
| • Respiratory failure (SatO2 < 90%, pO2 < 100 mmHg and/or pCO2 > 45 mmHg) | • Pulmonary congestion | ||
| • Respiratory failure |
According to clinical course. I: Admission; D: During admission; A: Discharge; ECG: Electrocardiogram; CS: Cardiogenic shock; ACS: Acute coronary syndrome; LV: Left ventricle; ALE: Acute lung edema; PTE: Pulmonary thromboembolism; IVC: Interventricular communication; BNP: Brain natriuretic peptide; NT-ProBNP: N-terminal prohormone of BNP; HF: Heart failure; TSH: Thyroid stimulating hormone; DD: D-dimer; pO2: partial pressure of O2; pCO2: partial pressure of CO2; SatO2: Arterial O2 saturation.
During the entire initial CS evaluation phase, it is essential to start an etiological study that subsequently will be completed in the next phase, seeking the presence of a potentially reversible triggering or coexisting cause that should be treated immediately (acute myocardial infarction code),43,55 or which implies the activation of other time-dependent disease conditions (sepsis code).
Hemodynamic evaluation phaseOnce the signs of hypoperfusion have been established, careful patient evaluation is necessary, using continuous monitoring of cardiac rhythm, respiratory rate, oxygen saturation and blood pressure, and the etiological study should be completed.14,58,59 In this regard, a 12-lead electrocardiogram should be obtained to evidence myocardial ischemia or alterations in cardiac rhythm capable of accounting for the patient's condition, and an echocardiographic study should be made to identify possible underlying causes and explain the associated pathophysiological process.43,47 These tests are essential and are systematically recommended in the evaluation of shock patients, particularly when considering that if the underlying cause is not identified and treated, the outcome usually proves fatal despite adequate hemodynamic management of CS.60,61
Initial echocardiographic evaluation, which may be performed on a point-of-care basis,62 is required to clarify the type of shock, with a due evaluation of the function of both ventricles, determination of the presence of diseases conditioning shock (Table 3), and conduction of a first hemodynamic evaluation to assess the presence of elevated ventricular filling pressures.14,61 Then, and especially in cases of progressive worsening or high SCAI levels, an echocardiographic evaluation should be made (transthoracic or transesophageal) by an expert with advanced knowledge of ultrasound to confirm the etiology, assess the hemodynamic situation, guide volume response based on static and dynamic parameters,61,63,64 and orientate pharmacological treatment and the use of MCS devices (ECMO) and/or ventricular assist measures (counterpulsation balloon, Impella®).65,66 In addition to echocardiographic assessment, pulmonary ultrasound67 and chest X-rays are very useful, since they allow us to evaluate the degree of pulmonary congestion, discard other disease conditions that could explain the clinical condition, and confirm the positioning of catheters and cardiac devices.14
In cases of hemodynamic instability and/or progression of the disorder, it is advisable to use invasive arterial monitoring and place a central venous catheter for continuous measurements of pressures and biochemical parameters, and guidance at the start of initial treatment.14,43,68
The use of a pulmonary artery catheter for initial shock evaluation is controversial and is probably not advisable on a systematic basis – reserving it for selected patients that fail to respond to initial therapy, or situations in which greater precision is needed in evaluating the shock phenotype in order to guide patient management.12,43
Stratification phaseAs we have seen, the stratification of patients with CS based on the SCAI scale and identification of the different phenotypes allows rapid risk assessment, unifies communication between care levels, and thus improves the patient prognosis. Since progression over the continuum of SCAI levels is a dynamic process that incorporates new information as it becomes available, this phase should be started from first contact with the patient, overlapping in time with the hemodynamic evaluation and identification phases (Fig. 4).
ConclusionsPatients with CS present a high mortality risk despite the therapeutic advances made. The early reversion of hypoperfusion is the cornerstone of the treatment of a heterogeneous syndrome in which non-ischemic causes have become increasingly important in recent years. Early detection and prognostic stratification, based on the SCAI scale and the recognition of specific phenotypes, are crucial for the management of CS. This approach, along with the code model based on the “Hub and Spoke” formula, allows patient care through multidisciplinary teams, with coordinated protocols focused on individualized decision-making, improving intra- and inter-hospital coordination, and reducing patient mortality.
Ethical approval and informed consentNot applicable.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Financial supportNone.