The impact of postoperative intensive care upon patient outcomes was evaluated by retrospectively investigating the rate of poor outcomes among miscellaneous elective surgical patients with severe comorbidities.
DesignA retrospective cohort study was carried out.
SettingUniversity hospital.
PatientsSurgical patients with severe comorbidities.
InterventionThe outcomes of 1218 surgical patients treated in intensive care units (ICUs) and postsurgical wards (ICU group vs. non-ICU group) were reviewed for poor outcomes (i.e., no discharge or death). A propensity score analysis was used to generate 248 matched pairs of ICU-admitted patients and controls.
Variables of interestPoor outcome rates on postoperative day 90 and mortality on postoperative days 30 and 90.
ResultsNo significant between-group differences were observed in terms of poor outcomes on postoperative day 90 [ICU vs. non-ICU: 33/248 (13%) vs. 28/248 (11%), respectively; ICU odds ratio (OR): 1.19, 95% confidence interval (CI), 0.71–2.01, p=0.596] or in between-group differences in terms of mortality on postoperative days 30 and 90 [ICU vs. non-ICU: 4/248 (1.6%) vs. 2/248 (0.8%) on postoperative day 30 and 5/248 (2.0%) vs. 3/248 (1.2%) on day 90, respectively; ICU OR (95% CI), 2.00 (0.37–10.9) and 1.67 (0.40–6.97) for postoperative 30- and 90-day mortality, respectively (p=0.683 and 0.724)]. Low preoperative body weight was negatively correlated to patient outcomes [OR (95% CI): 0.82/10kg (0.70–0.97), p=0.019], whereas regional analgesia combined with general anesthesia was positively correlated to patient outcomes [OR (95% CI): 0.39 (0.69–0.96), p=0.006]. Extra ICU admission was correlated to poor patient outcomes [OR (95% CI): 4.18 (2.23-7.81), p < 0.0001].
ConclusionsPostoperative ICU admission failed to demonstrate any meaningful benefits in patients with severe comorbidities undergoing miscellaneous elective surgeries.
Se evaluó el impacto de los cuidados intensivos postoperatorios sobre los desenlaces de los pacientes investigando de forma retrospectiva la tasa de desenlaces desfavorables en un grupo variado de pacientes con comorbilidades graves que se sometieron a cirugías programadas.
DiseñoEstudio retrospectivo de cohortes.
ÁmbitoHospital universitario.
PacientesPacientes quirúrgicos con comorbilidades graves.
IntervencionesSe revisaron los desenlaces de 1.218 pacientes quirúrgicos tratados en unidades de cuidados intensivos (UCI) y plantas posquirúrgicas (grupo UCI frente a grupo no UCI) en busca de desenlaces desfavorables (esto es, ausencia de alta o muerte). Se llevó a cabo un análisis de puntuación de la propensión para generar 248 parejas de pacientes ingresados en la UCI y sus respectivos pacientes de control.
Variables de interésTasas de desenlaces desfavorables al día 90 tras la intervención y mortalidad a los 30 y 90 días de la intervención.
ResultadosNo se observaron diferencias significativas entre los grupos en cuanto a desenlaces desfavorables el día 90 tras la intervención (UCI frente a no UCI: 33/248 [13%] frente a 28/248 [11%], respectivamente; oportunidad relativa [OR]: 1,19; intervalo de confianza [IC] del 95%: 0,71-2,01; p=0,596) ni diferencias entre los grupos en términos de mortalidad al cabo de 30 y 90 días tras la intervención (UCI frente a no UCI: 4/248 [1,6%] frente a 2/248 [0,8%] el día 30 tras la intervención y 5/248 [2,0%] frente a 3/248 [1,2%] el día 90, respectivamente; OR UCI [IC del 95%]: 2,00 [0,37-10,9] y 1,67 [0,40-6,97] para la mortalidad a los 30 y 90 días de la intervención, respectivamente [p=0,683 y 0,724]). El bajo peso preoperatorio presentó una correlación negativa con los desenlaces de los pacientes (OR [IC del 95%]: 0,82/10kg [0,70-0,97]; p=0,019), mientras que la analgesia regional combinada con anestesia general y el ingreso fuera de la UCI presentó una correlación positiva con los desenlaces de los pacientes (OR [IC del 95%]: 0,39 [0,69-0,96]; p=0,006). El ingreso extra en la UCI se correlacionó con malos resultados para el paciente (OR [IC del 95%]: 4,18 [2,23-7,81], p < 0,0001).
ConclusionesEl ingreso posoperatorio en la UCI no demostró asociarse con ningún beneficio significativo en un grupo variado de pacientes con comorbilidades graves sometidos a cirugías programadas.
The new guidelines for intensive care unit (ICU) admission, discharge, and triage published in 2016 recommend that patients with a heightened risk for postoperative instability or decompensation should be closely monitored and managed in a higher-level care unit rather than in the ward during the immediate postoperative period.1 Patients with comorbidities may even become critical during the perioperative phases of general surgeries. In fact, mortality at postoperative days 30 and 90 in patients with an American Society of Anesthesiologists physical status (ASA PS) classification III—i.e., with severe systemic disease—and ASA PS classification IV—i.e., with severe systemic disease that is a constant threat to the patient's life—went up to 0.5–30% after miscellaneous operations.2–4 Therefore, high-risk surgical patients, such as those with ASA PS III and IV, will have better outcomes when they remain in the ICU rather than in regular surgical wards, even during the immediate postoperative period. In daily hospital settings, immediate postoperative care for high-risk patients is frequently provided in an ICU environment. However, elective surgical patients with severe comorbidities are generally noncritical before surgery. They are electively admitted to the ICU when there is a concern that they might become critical postoperatively. This ICU admission monitors their postoperative status rather than providing care or continuing surgical intervention as in cardiac surgery, for which specific risk-adjustment models after ICU admission have been developed, validated, and reported.5,6 However, for other general surgeries that usually do not require a postoperative ICU stay, it remains uncertain whether postoperative ICU admission is beneficial for high-risk surgical patients. This issue should be addressed considering the generally limited intensive care resources.
Our institute has arranged and determined absolute indications for the ICU admission of elective surgical patients. For example, intermediate- to high-risk surgeries7 in comorbid patients (ASA PS≥III) are absolute indications for ICU admission. However, the postoperative ICU admission of these patients is sometimes canceled because of a shortage of ICU resources. Thus, we can investigate whether postoperative vigilance in the ICU contributes to better patient outcomes by comparing the outcomes of postoperative ICU-admitted and non-ICU-admitted surgical patients with comorbidities who underwent intermediate- to high-risk surgeries. This retrospective clinical chart review study aimed to determine whether a short postoperative ICU stay could improve outcomes in surgical patients with a high level of comorbidity, classified as ASA PS III or IV. To reduce selection bias, we compared the outcomes of propensity-matched patient pairs with or without intensive care. Using this technique, we analyzed patients who were equally eligible to be treated postoperatively in the ICU or in general surgical ward. Additionally, we excluded patients if there was a high likelihood of admission to only one of the postoperative care environments.
Patients and methodsThe Ethics Committee of the Nara Medical University Hospital approved this observational cohort study (Kashihara, Japan; study number 1094). The Institutional Review Board waived the need for written informed consent. This study case–control study was performed in accordance with the recommendations of the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) consensus statement.8 We reviewed elective miscellaneous surgical cases performed at Nara Medical University between January 2007 and December 2015 and extracted the records of 1218 consecutive surgical patients with ASA PS III or IV. We investigated the patients’ statuses at postoperative days 30 and 90.
Perioperative patient treatmentNo uniform methods of induction or maintenance of anesthesia were followed. However, general anesthesia was usually induced with intravenous propofol (1–2.5mg/kg) plus either fentanyl (1–2μg/kg) or remifentanil (0.2–0.3μg/kg/min), and neuromuscular blockade was achieved with rocuronium (0.6–0.9mg/kg). All patients received tracheal intubation. Anesthesia was maintained with sevoflurane (1.5–2%) or desflurane (4–6%) in a 40% oxygen and air mixture, or with propofol (6–10mg/kg/h). Nitrous oxide was not used. Fentanyl (1–2μg/kg/h) or remifentanil (0.1–0.2μg/kg/min) was used for analgesia. Rocuronium (0.2–0.3mg/kg/h) was used for neuromuscular blockade. Neostigmine (40μg/kg) plus atropine (20μg/kg) was used until July 2010, whereas sugammadex (2–4mg/kg) was used thereafter for the reversal of the neuromuscular blockade after status evaluation by a nerve stimulator. Fluid management was performed in accordance with the attendant's judgment and the type of surgery performed. Transfusion was provided as necessary. For example, red cell concentrates were transfused when Hb concentrations reached approximately 7g/dl. Occasionally, postoperative analgesia was provided via intravenous fentanyl or epidural ropivacaine combined with fentanyl using a patient-controlled analgesia (PCA) device. An additional low dose of droperidol (1.25–2.5mg/day) was administered while using the PCA device. In addition, flurbiprofen (50mg) or acetaminophen (500–1000mg) was administered as necessary. Following the completion of the surgical procedures, sevoflurane, desflurane, or propofol was discontinued and tracheal extubation was performed in the operating room. Subsequently, the postoperative patients were transferred either to the surgical ward (non-ICU group) or the ICU (ICU group). The patients in the non-ICU group were transferred to the surgical ward after withdrawal of their intra-arterial catheter. The patients admitted to the surgical ward received continuous oxygen administration and ECG monitoring until the following morning. Noninvasive measurements of blood pressure and SpO2 were performed by the nursing staff every 3–4h during the postoperative 12h. In the surgical ward setting, the nurse-to-patient ratio was 1:7; surgical residents were also available. However, early warning and intervention systems, such as a rapid response team to identify clinically deteriorating patients in general wards, were not available. The ICU group received continuous oxygen administration and ECG, invasive arterial blood pressure, and SpO2 monitoring until the following morning. Moreover, an arterial blood gas analysis was performed at least twice. A certified intensivist was readily available and supervised the ICU team, which included intensive care nurses and a surgical or anesthesia resident. The nurse-to-patient ratio was 1:2. The following day, patients without complications were transferred to the surgical ward.
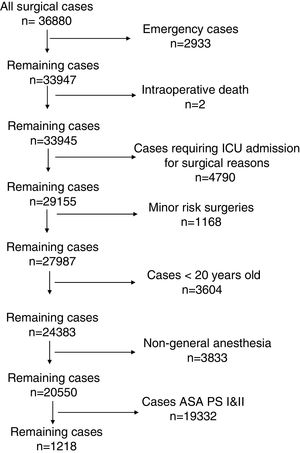
Data handlingData from 36,880 patients who underwent surgery between January 2007 and December 2015 were reviewed. The present study included the following exclusion criteria (Fig. 1): (1) emergency patients; (2) intraoperative death; (3) patients who absolutely required ICU admission for surgical reasons according to our institutional protocol except for the last clause (Table 1); (4) low-risk patients not requiring ICU admission in our institute, such as electronic convulsive therapy, breast surgery, dental surgery, ophthalmic surgery, and bone marrow harvesting; (5) patients aged <20 years; (6) patients who did not undergo general anesthesia; and (7) patients classified as ASA PS I—i.e., normal health—or ASA PS II—i.e., mild systemic disease.2 These were used as one of the exclusion criteria to identify adult intermediate- to high-risk surgical patients with comorbidities, i.e., “borderline” patients who require a postoperative intensive environment. Regarding exclusion criterion (4), other minor risk surgeries, such as those described previously (minor gynecological or orthopedic surgeries, etc.),7 were not excluded because these surgical patients with severe comorbidities were sometimes managed on demand in the ICU after surgery. Consequently, non-major neurological surgeries, including supine surgery, head and neck surgeries, non-major thoracic surgeries, laparotomies, orthopedic surgeries, and superficial surgeries, were included.
Absolute indication for the ICU in elective surgical patient at Nara Medical University Hospital.
| •Post-cardiovascular surgery |
| •Post-major lung surgery |
| •Post-esophageal surgery |
| •Post-microvascular anastomosis requiring immobilization |
| •Post-long surgery (preoperative estimated duration of surgery >10h) |
| •Post-major neurosurgery |
| •Post-intermediate to high risk surgeries in the comorbid patients (ASA PS≧3) |
ASA PS: American Society of Anesthesiologists physical status classification.
Continuous variables are presented as the mean±standard deviation (SD) if normally distributed or the median±interquartile range (IQR) if skewed. Categorical variables are presented as the number of patients and frequency (%). For the primary outcome analysis, a poor outcome was defined as either not being discharged to home or death at postoperative day 90. In the secondary analysis, mortality at postoperative days 30 and 90 were compared. Generally, perioperative mortality is defined as any death within postoperative day 30.9 However, 30 days is not a sufficient period to evaluate the risk of postoperative mortality.10,11 Therefore, we chose the 90-day status to define primary and secondary outcomes. In addition, the rates of emergency ICU admission, defined as a patient who was admitted to the ICU in the non-ICU group, and readmission, defined as a patient who required ICU readmission in the ICU group, were compared. The rates of emergency ICU admission and readmission combined with the rate of postoperative prolonged ICU stay, defined as a case that could not be discharged from the ICU the following day in the ICU group for any reason, usually respiratory or cardiac problems, were also compared. First, the outcomes were analyzed using data from the initial 1218 patients. Fisher's exact test or the Chi-squared test was used to estimate the odds ratios (ORs) and 95% confidence intervals (CIs) of incidence (ICU group vs. non-ICU group). Furthermore, to minimize selection bias, we performed a propensity score analysis of clinical characteristics to generate a set of matched cases (ICU group) and controls (non-ICU group). After the propensity score matching, 726 patients were excluded from the final analysis. A propensity score was generated for each patient from a multivariable logistic regression model based on the covariates using data from the institutional registry as the independent variables and the treatment type (ICU group vs. non-ICU group) as a binary dependent variable. The registered variables, including age, sex, height, weight, ASA PS, surgical site, surgical posture, duration of surgery, surgical risk stratification,7 provision of regional anesthesia, intraoperative adverse events (cardiac arrest, severe hypotension, severe hypoxemia, etc.), massive bleeding (>1500mL),12 and the requirement of transfusion, were included as potential confounders. In addition, the year of surgery was added as a covariate to generate a propensity score to avoid the effects of perioperative and anesthetic advances during the long study period. As suggested in a review of statistical research on propensity score development,13 we used a structured iterative approach to refine this model with the goal of achieving a covariate balance between the matched pairs. Covariate balance was measured using the standardized difference, while an absolute standardized difference of >0.1 was considered a meaningful covariate imbalance.14 We matched patients using a greedy-matching algorithm with a caliper width 0.01 of the estimated propensity score. A 1:1 matching ratio was employed. This procedure yielded 248 ICU-transferred patients who were propensity matched to 248 surgical ward patients. Statistical inference methods were used that account for the matched nature of the samples. The Cochran–Mantel–Haenszel test, stratified on matched pairs, was used to estimate the OR and 95% CI for overall incidence (ICU group vs. non-ICU group).
Additionally, we conducted a multivariate logistic regression analysis on the entire cohort (1218 patients) using the rate of poor outcomes as the dependent variable and other covariates, including the presence or absence of ICU admission, as independent variables. This analysis was performed to assess whether ICU admission was associated with poor outcomes. Univariate analyses were used to identify other factors associated with poor outcomes. Candidate factors that had a significant univariate association (p<0.15) with poor outcomes were entered into a multivariable logistic regression analysis by forced-entry methods. All candidate factors were entered in the initial model and presented as adjusted ORs with 95% CIs. Interactions between variables were systematically searched and collinearity was concluded for a r or ρ of >0.8 using the Pearson or Spearman correlation coefficient matrices, respectively. Discrimination of the final model for poor outcomes was assessed using the likelihood ratio test. The Hosmer–Lemeshow statistic was used to test model calibration.
Sample size calculationFor a post hoc sample size calculation, we assumed a 10% rate of poor outcomes in our population based on previous studies reporting 5–30% mortality at postoperative day 90 among ASA PS III-IV patients undergoing miscellaneous operations.2,3 We estimated that 208 patients in each group were required to provide 90% power to detect a 10% difference in the rate of poor outcomes between the ICU (5%) and non-ICU (15%) groups, with a type I error probability of 0.05. Therefore, our sample size was sufficient to detect a difference in outcomes. The analyses were computed using R (version 3.0.3, R Foundation for Statistical Computing, Vienna, Austria). A value of p<0.05 was considered statistically significant.
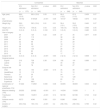
ResultsThe clinical characteristics of the unmatched groups (n=273 for ICU admission and n=945 for non-ICU admission) and the two propensity-matched groups (n=248 each) are presented in Table 2. Before matching, the covariates were not statistically different between groups; however, several variables, including age, ASA PS, surgical risk, surgical site, transfusion requirement, massive bleeding, and duration of surgery, were not balanced adequately, as judged by the absolute standardized difference of >0.1. After matching, the covariates were balanced adequately. Records of intraoperative adverse events assumed to be correlated with poor outcomes showed that four patients in the non-ICU admission group developed transient severe bradycardia close to cardiac arrest, which was easily treated using atropine, but recoded as intraoperative adverse events. These events were not related to poor outcomes.
Baseline patient's characteristics.
| Unmatched | Matched | |||||||
|---|---|---|---|---|---|---|---|---|
| ICU admission | Non ICU admission | p value | ASD | ICU admission | Non ICU admission | p value | ASD | |
| (n=273) | (n=945) | (n=248) | (n=248) | |||||
| Age (year) | 68.6 (12.0) | 66.9 (12.8) | 0.05 | 0.13 | 68.4 (12.2) | 67.6 (11.5) | 0.436 | 0.07 |
| Sex (male/female) | 181/92 | 519/426 | <0.001 | 0.09 | 161/87 | 166/82 | 0.675 | 0.02 |
| Weight (kg) | 58.6 (13.1) | 58.5 (13.6) | 0.91 | 0.01 | 58.4 (13.1) | 59.2 (13.5) | 0.460 | 0.07 |
| Height (cm) | 159 (10) | 159 (9) | 0.54 | 0.04 | 159 (10) | 160 (9) | 0.566 | 0.05 |
| ASA PS | 3 (3–3) | 3 (3–3) | 0.163 | 0.10 | 3 (3–3) | 3 (3–3) | 0.480 | 0.03 |
| Year of surgery | ||||||||
| 2007 | 39 | 125 | 0.22 | 0.09 | 37 | 35 | 0.877 | 0.09 |
| 2008 | 47 | 113 | 38 | 46 | ||||
| 2009 | 35 | 104 | 33 | 39 | ||||
| 2010 | 32 | 99 | 31 | 29 | ||||
| 2011 | 18 | 99 | 18 | 12 | ||||
| 2012 | 28 | 121 | 27 | 22 | ||||
| 2013 | 33 | 133 | 26 | 31 | ||||
| 2014 | 22 | 83 | 21 | 17 | ||||
| 2015 | 19 | 68 | 17 | 17 | ||||
| Surgical risk | 2 (2–2) | 2 (2–2) | <0.001 | 0.63 | 2 (2–2) | 2 (2–2) | 0.908 | 0.01 |
| Surgical posture | ||||||||
| Supine | 216 | 728 | 0.38 | 0.04 | 192 | 191 | 0.989 | 0.01 |
| Lateral | 31 | 139 | 30 | 30 | ||||
| Prone | 26 | 78 | 26 | 27 | ||||
| Surgical site | ||||||||
| CNS | 35 | 150 | <0.001 | 0.16 | 35 | 39 | 0.467 | 0.09 |
| Head&Neck | 15 | 112 | 15 | 10 | ||||
| Thoracic | 27 | 35 | 24 | 17 | ||||
| Abdominal | 128 | 27 | 114 | 129 | ||||
| Extremities | 68 | 128 | 60 | 53 | ||||
| Superficial | 0 | 68/0 | 0 | 0 | ||||
| Combination of regional anesthesia (Y/N) | 51/222 | 114/831 | 0.006 | 0.08 | 43/205 | 48/200 | 0.644 | 0.02 |
| Massive bleeding (Y/N) | 20/253 | 23/922 | <0.001 | 0.11 | 14/234 | 13/235 | 1 | 0 |
| Transfusion (Y/N) | 73/200 | 134/811 | <0.001 | 0.14 | 58/190 | 62/186 | 0.743 | 0.02 |
| Adverse events (Y/N) | 0/273 | 4/941 | 0.581 | 0.01 | 0/248 | 0/248 | 1 | 0 |
| Duration of surgery | 252 (164) | 193 (141) | <0.001 | 0.4 | 236 (153) | 239 (164) | 0.795 | 0.02 |
ICU: intensive care unit; ASD: absolute standardized difference; ASA PS: American Society of Anesthesiologists physical status classification; CNS: minor neurosurgery and spinal surgery; Head&Neck: head and neck surgery; Thoracic: thoracic surgery; Abdominal: abdominal surgery; Extremities: orthopedic surgery; Superficial: plastic surgery.
Patient outcomes are summarized in Table 3. There was no difference in poor outcomes on postoperative day 90 between groups, regardless of whether they were matched (ICU group vs. non-ICU group: 13% vs. 14% before matching, 13% vs. 11% after matching, respectively). Similarly, no group differences in mortality were found at postoperative days 30 and 90, regardless of whether the matching procedure had been performed. Before matching, no group differences were found in the rate of emergency ICU admission or readmission; however, the value combined with the rate of postoperative prolonged ICU stay was significantly higher in the ICU group than in the non-ICU group. Conversely, after matching, the rate of emergency ICU admission or readmission was higher in the non-ICU group than in the ICU group. However, the value combined with the rate of postoperative prolonged ICU stay was not statistically different between the groups.
Patient outcomes.
| Unmatched | ICU admission | Non ICU admission | Measure of effect (95% CI) | p value |
|---|---|---|---|---|
| (n=273) | (n=945) | |||
| Poor outcomes at 90 day (%) | 35 (13) | 134 (14) | OR, 0.89 (0.58–1.34) | 0.62 |
| Mortality at 30 day (%) | 5 (1.8) | 12 (1.3) | OR, 1.45 (0.40–4.47) | 0.556 |
| Mortality at 90 day (%) | 6 (2.2) | 18 (1.9) | OR, 1.16 (0.37–3.08) | 0.805 |
| Emergency ICU admission or readmission (%) | 4 (1.5) | 31 (3.3) | OR, 0.44 (0.11–1.26) | 0.149 |
| Emergency ICU admission or readmission and postoperative prolonged ICU stay (%) | 22 (8.1) | 31 (3.3) | OR, 2.58 (1.40–4.70) | 0.002 |
| Matched | ICU admission | Non ICU admission | Measure of effect (95% CI) | p value |
|---|---|---|---|---|
| (n=248) | (n=248) | |||
| Poor outcomes at 90 day (%) | 33 (13) | 28 (11) | OR, 1.19 (0.71–2.01) | 0.596 |
| Mortality at 30 day (%) | 4 (1.6) | 2 (0.8) | OR, 2.00 (0.37–10.9) | 0.683 |
| Mortality at 90 day (%) | 5 (2.0) | 3 (1.2) | OR, 1.67 (0.40–6.97) | 0.724 |
| Emergency ICU admission or readmission (%) | 2 (0.8) | 14 (5.6) | OR, 0.14 (0.03–0.63) | 0.006 |
| Emergency ICU admission or readmission and postoperative prolonged ICU stay (%) | 18 (7.3) | 14 (5.6) | OR, 1.31 (0.64–2.69) | 0.584 |
OR: odds ratio; 95% CI: 95% confident interval; SD: standard deviation; ICU: intensive care unit.
Poor outcomes were defined as “not discharge to home” or “death” at postoperative 90-day. Emergency ICU admission was a case that was admitted to ICU in the non-ICU group. Readmission was a case that required ICU readmission in the ICU group. Postoperative prolonged ICU stay was a case that could not discharge from ICU the following day in the ICU group.
A multivariable logistic regression analysis by forced-entry methods was performed with the following candidate factors: age, height, weight, duration of surgery, patient posture during surgery, combination of regional anesthesia, emergency ICU admission or readmission, and postoperative prolonged ICU stay (emergency ICU admission or readmission was excluded because ρ=0.806, although this item showed a significant value), and ICU admission. ICU admission was included to assess whether ICU admission was associated with patient outcomes. The results showed that body weight, the provision of regional analgesia combined with general anesthesia, emergency ICU admission or readmission, and postoperative prolonged ICU stay were independently associated with poor outcomes (Table 4). However, ICU admission was not associated with patient outcomes. Using the likelihood ratio test, the discrimination of the final model was significant (p<0.0001). Furthermore, the Hosmer–Lemeshow statistic did not reject a logistic regression model fit (p=0.6473). The explanatory model based on these variables had an area under the receiver operating characteristic curve of 0.654 (95% CI, 0.627–0.681). Post hoc power calculations were performed for this forced-entry multivariate logistic regression model using eight variables. Standard methods were used to estimate the sample size for the multivariate logistic regression analysis, indicating a requirement of at least 10 outcomes for each independent variable.15 With a 13.9% (169/1218) rate of poor outcomes in the study population, 576 patients were required to appropriately perform the multivariate logistic regression analysis. Therefore, the sample sizes used were of sufficiently size to build the multivariate models.
Results of multivariable logistic regression analyses for poor outcomes.
| Odds | 95% CI | p value | |
|---|---|---|---|
| Age per 10 years old | 1.10 | 0.94–1.27 | 0.231 |
| Height per 10cm | 1.02 | 0.82–1.27 | 0.844 |
| Weight per 10kg | 0.82 | 0.69–0.96 | 0.015 |
| Duration of surgery per 10min | 0.99 | 0.98–1.00 | 0.087 |
| ICU admission | 0.79 | 0.50–1.25 | 0.320 |
| Combination of regional anesthesia | 0.39 | 0.20–0.77 | 0.007 |
| Patient posture during surgery | |||
| Lateral positiona | 1.30 | 0.82–2.04 | 0.260 |
| Prone positiona | 1.42 | 0.82–2.45 | 0.160 |
| Emergency ICU admission or readmission and postoperative prolonged ICU stay | 7.30 | 2.72–19.6 | 0.0001 |
ICU: intensive care unit. Emergency ICU admission or readmission and postoperative prolonged ICU stay was a case that was admitted to ICU in the non-ICU group, a case that required ICU readmission in the ICU group, or a case that could not discharge from ICU the following day in the ICU group.
The present study investigated whether a brief ICU stay could improve outcomes in patients with severe comorbidities after miscellaneous elective surgeries. Based on the retrospective analysis of patient data, postoperative ICU admission failed to affect any meaningful outcomes. The rate of emergency ICU admission or readmission was significantly higher in the non-ICU group than in the ICU group after matching. However, this difference disappeared when the rate of postoperative prolonged ICU stay in the ICU group was combined with the rate of readmission. This study was conducted retrospectively. Therefore, it is difficult to judge whether prolonged ICU stay prevented ICU readmission or whether some of the elective ICU patients deteriorated during the first ICU day as much as non-ICU patients who would be emergently admitted to the ICU. Interestingly, the additional multivariate logistic regression analysis of the entire cohort confirmed that postoperative ICU admission was not associated with improved patient outcomes; however, this analysis showed that low preoperative body weight was negatively associated with patient outcomes, while the provision of regional analgesia combined with general anesthesia was positively associated with patient outcomes. In addition, emergency ICU admission, readmission, or postoperative prolonged ICU stay was also strongly associated with poor outcomes.
As noted earlier, apart from postcardiac surgery, ICUs that are staffed with critical care caregivers are known to improve patient outcomes. For example, Dimick et al. have shown that intensive care provided by ICU physicians was associated with shorter durations of stay, lower hospital costs, and a decreased frequency of postoperative complications following esophageal resection.16 Varelas et al. reported that a neurointensivist-led team model showed a relative reduction in in-hospital mortality and improved Glasgow Coma Scale scores in head injury patients.17 Additionally, Tang et al. observed a worse-than-predicted survival in the absence of ICUs for critically ill patients who received mechanical ventilation in general wards.18 Overall, ICUs that are staffed with critical care caregivers are considered beneficial for patients in specific situations and for the critically ill. Conversely, it has been suggested that specific procedures in elective surgical patients, such as a craniotomy, do not require postoperative intensive care.19,20 Moreover, among ICU admissions monitored only on their first ICU day, more than 90% of low-risk patients did not receive subsequent active life-supporting treatment.21 Recently, Kahan et al. conducted an international cohort study and reported that they did not identify any survival benefits from critical care admission following elective surgery.22 Although they provided several critical care treatments for a wide range of postoperative patients, post hoc sensitivity analyses exploring the effect of postoperative critical care admission within a high-risk subgroup of patients and according to university hospital status also failed to show a protective effect of critical care in either of these analyses.
Because surgical patients with severe comorbidities are generally not in critical condition before elective surgery, they may be too healthy to benefit from ICU admission for mere monitoring to prevent them from becoming critical postoperatively. The increasing use of less invasive techniques in modern surgery and advances in anesthesia contribute to improved patient outcomes, which may mask the benefits of ICU admission for miscellaneous elective surgical patients with severe comorbidities. As stated previously, the postoperative ICU admission of these patients is sometimes canceled even though they display an absolute indication for the ICU as elective surgical patients in our institute because of a shortage of ICU resources. The occasional cancelation may be due to the underlying argument that it remains uncertain whether postoperative ICU admission is beneficial for such high-risk surgical patients. We believe that this study helps answer this clinical question. However, it remains unknown whether postoperative ICU would be beneficial in patients with a high likelihood of ICU admission because of missing comparison targets.
Moreover, the results showed that a low preoperative body weight was negatively associated with patient outcomes, the provision of regional analgesia combined with general anesthesia was positively associated with patient outcomes, and emergency ICU admission, readmission, or postoperative prolonged ICU stay was also strongly associated with poor outcomes. We would like to comment on these findings. Preoperative frailty has displayed an association with significant morbidity and mortality.23 Low body weight is an important marker of frailty.24 Therefore, it is plausible that a low preoperative body weight was associated with poor outcomes in our population. Although it remains unclear whether regional analgesia combined with general anesthesia reduces mortality, it may have contributed to improved postoperative pain management and enhanced postoperative mobility, rehabilitation, and discharge destination.25,26 Therefore, it is also reasonable to conclude that regional analgesia combined with general anesthesia was associated with better outcomes in the present study. A preoperative exercise and nutritional support program may help reduce sarcopenia and improve postoperative outcomes in elderly sarcopenic patients with gastric cancer.27 These reports imply that rather than strict ICU vigilance for patients such as those in our study, the preoperative treatment of frailty and early postoperative mobilization might contribute to better patient outcomes. Interestingly, Levy et al.’s study demonstrating the negative effect of intensivists suggested that some of the routine practices and procedures in critical care may not be optimally beneficial and that the cumulative use of additional interventions may negatively affect patient outcome.28 This suggestion is relevant for the present study because our ICU patients wore extra-monitoring devices, such as an invasive arterial blood pressure monitor, which may have limited patient mobilization. However, recently, early mobilization has been observed in the ICU even in patients with mechanical ventilation or other devices such as ECMO.29 Therefore, this concern might be overcome in the future. Furthermore, emergency ICU admission and readmission and prolonged ICU stay may be indications poor clinical progress. Therefore, it is not unusual that poor progress was directly associated with poor outcomes.
The present study contains several limitations. To minimize the effect of selection bias on outcomes, propensity score matching for clinical characteristics was used to reduce distortion by confounding factors. However, in this retrospective study, several unmeasured or irretrievable variables might still have confounded patient outcomes. Second, mortality was not used as a primary outcome because the mortalities at postoperative days 30 and 90 were too small to perform meaningful analyses. Therefore, using mortality as a primary outcome measure would have required us to recruit many more patients to increase the statistical power. Another limitation was the study location of Japan, where the length of hospitalization is relatively long.30 Therefore, “not discharged to home” as an index of patient outcome cannot be adequately used for a direct comparison with other countries. The Japanese universal public insurance system is relatively generous in its support of hospitalization. Therefore, both a patient's medical status and his or her social background usually determine hospitalization in Japan. However, even in Japan, more than 90 days of hospitalization is considered quite long. Therefore, it is not unreasonable that “not discharged to home” at postoperative day 90 was taken as an outcome indicator, although the results cannot be generalized to countries with generally shorter hospitalizations. However, similarly, it is unlikely that “not discharged to home” after 30 days can serve as an appropriate outcome indicator. In addition, it is not possible to extrapolate the Japanese postoperative surgical ward setting to other countries and environments. The current findings might be irrelevant in much lower quality settings. Finally, many patients were excluded from this study based on the exclusion criteria. However, the exclusion procedures were necessary to appropriately select high-risk surgical patients who underwent elective miscellaneous surgeries that usually do not require a postoperative intensive care environment.
In conclusion, we investigated whether a brief ICU stay following miscellaneous elective surgeries could improve outcomes in patients with severe comorbidities. However, postoperative ICU admission failed to demonstrate any meaningful benefits. Because a low preoperative body weight was associated with poor outcomes and the provision of regional analgesia combined with general anesthesia was associated with better outcomes, other preoperative and postoperative interventions rather than intensive care monitoring might contribute to better patient outcomes in elective surgical patients with severe comorbidities.
Authors’ contributionsTeppei Ogawa collected and analyzed all data. Satoki Inoue designed the study, collected and analyzed all data, interpreted the results, and wrote the manuscript. Michiyo Inada helped to collect and analyze the data. Masahiko Kawaguchi reviewed and helped modify the design of the study. All authors have read and approved the final manuscript.
FundingThis work was supported by departmental sources.
Conflict of interestsThe authors declare that they have no conflict of interests.