Edited by: Federico Gordo - Medicina Intensiva del Hospital Universitario del Henares (Coslada-Madrid)
Last update: December 2023
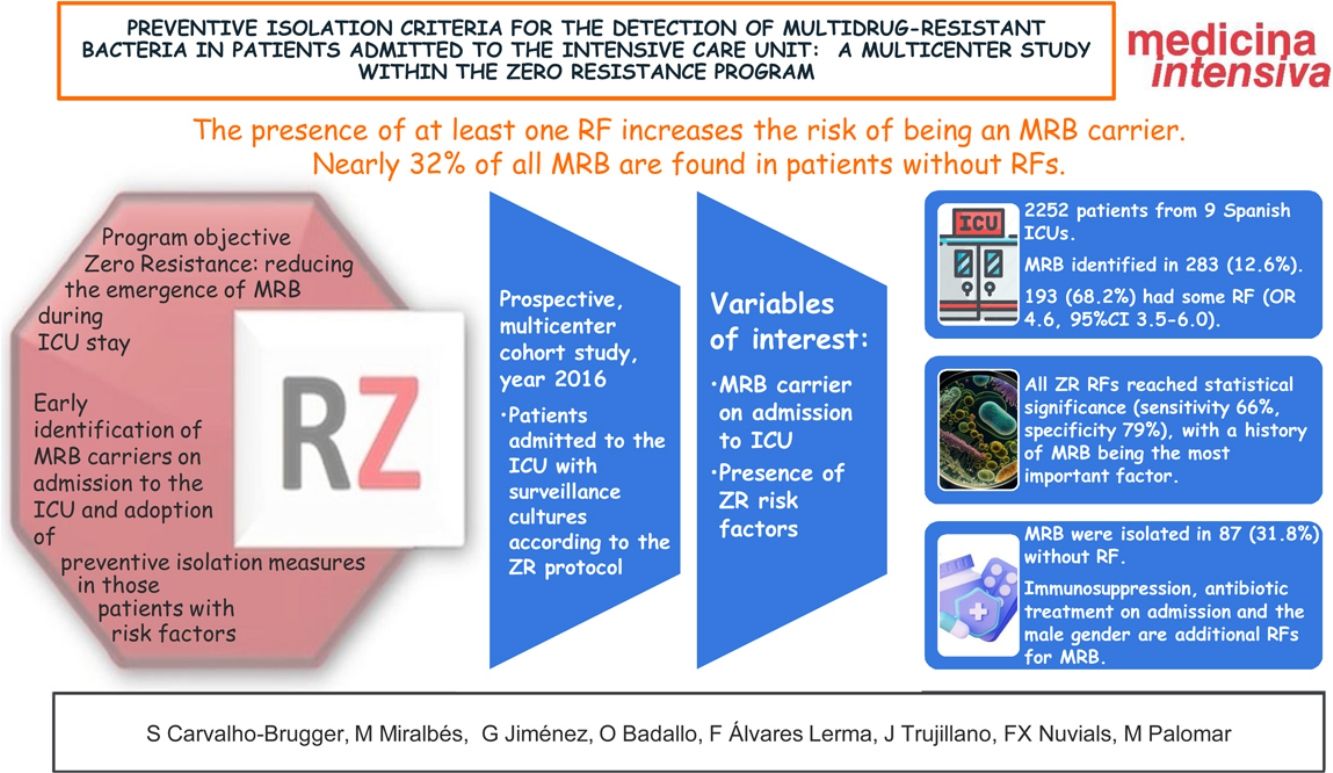
More infoTo verify the validity of a checklist of risk factors (RFs) proposed by the Spanish “Zero Resistance” project (ZR) in the detection of multidrug-resistant bacteria (MRB), and to identify other possible RFs for colonization and infection by MRB on admission to the Intensive Care Unit (ICU).
DesignA prospective cohort study, conducted in 2016.
SettingMulticenter study, patients requiring admission to adult ICUs that applied the ZR protocol and accepted the invitation for participating in the study.
Patients or participantsConsecutive sample of patients admitted to the ICU and who underwent surveillance (nasal, pharyngeal, axillary and rectal) or clinical cultures.
InterventionsAnalysis of the RFs of the ZR project, in addition to other comorbidities, included in the ENVIN registry. A univariate and multivariate analysis was performed, with binary logistic regression methodology (significance considered for p < 0.05). Sensitivity and specificity analyses were performed for each of the selected factors.
Main variables of interestCarrier of MRB on admission to the ICU, RFs (previous MRB colonization/infection, hospital admission in the previous 3 months, antibiotic use in the past month, institutionalization, dialysis, and other chronic conditions) and comorbidities.
ResultsA total of 2270 patients from 9 Spanish ICUs were included. We identified MRB in 288 (12.6% of the total patients admitted). In turn, 193 (68.2%) had some RF (OR 4.6, 95%CI: 3.5–6.0). All 6 RFs from the checklist reached statistical significance in the univariate analysis (sensitivity 66%, specificity 79%). Immunosuppression, antibiotic use on admission to the ICU and the male gender were additional RFs for MRB. MRB were isolated in 87 patients without RF (31.8%).
ConclusionsPatients with at least one RF had an increased risk of being carriers of MRB. However, almost 32% of the MRB were isolated in patients without RFs. Other comorbidities such as immunosuppression, antibiotic use on admission to the ICU and the male gender could be considered as additional RFs.
Conocer el rendimiento de los criterios de aislamiento preventivo del programa Resistencia Zero (RZ) e identificar factores que pudieran mejorar su rendimiento.
DiseñoEstudio de cohorte prospectivo, multicéntrico.
ÁmbitoUnidades de cuidados críticos que aplicaban el protocolo RZ y que aceptaron la invitación al estudio.
Pacientes o participantesPacientes a los que se les realizaron cultivos de vigilancia (nasal, faríngeo, axilar y rectal) y/o diagnósticos al ingreso en UCI.
IntervencionesAnálisis de los factores de riesgo (FR) RZ y otras variables del registro ENVIN. Se realizó un estudio univariable y multivariable con metodología de regresión logística binaria (significación con p < 0.05). Se realizó análisis de sensibilidad y especificidad para cada uno de los factores seleccionados.
Variables de interés principalesPortador de bacteria multirresistente (BMR) al ingreso en UCI, FR (antecedente de colonización/infección por BMR, ingreso hospitalario en los 3 meses previos, uso de antibiótico el mes previo, estar institucionalizado, diálisis y otras condiciones crónicas) y comorbilidades.
ResultadosParticiparon 2252 pacientes de 9 UCIs españolas. Fueron identificados BMR en 283 (12,6%). 193 (68,2%) presentaban algún FR (OR 4,6, IC 95% 3,5–6,0). Todos los FR RZ alcanzaron significación estadística (sensibilidad 66%, especificidad 79%), siendo el antecedente de BMR el factor con más peso. Inmunodepresión, tratamiento antibiótico al ingreso y sexo masculino son FR adicionales para BMR. Se aislaron BMR en 87 (31,8%) sin FR.
ConclusionesLa presencia de al menos un FR aumenta el riesgo de ser portador de BMR, siendo el más importante el antecedente de colonización/infección por BMR. Casi el 32% de las BMR se encuentran en pacientes sin FR. Inmunodepresión, tratamiento antibiótico al ingreso y sexo masculino podrían ser añadidos al algoritmo de FR para decidir el aislamiento preventivo.
The emergence of multidrug-resistant bacteria (MRB) is a natural biological phenomenon growing worldwide due to the widespread and inadequate use of antibiotics.1
This phenomenon increases the healthcare costs, treatment failures and mortality in both the hospital setting and in the community.2,3
Patients admitted to Intensive Care Units (ICUs) are particularly susceptible to acquiring MRB in the form of either colonization or infection.
In the case of infections due to MRB, the management options are limited, with an increased risk of inadequate empirical treatment and delays in starting correct therapy — thereby aggravating the disease, prolonging the ICU stay and increasing the costs and patient morbidity–mortality.4,5
Mortality among patients with MRB is greater than in patients with bacteria sensitive to the commonly used antibiotics.4–6
Even in situations of colonization without concomitant infection, the mortality risk has been shown to increase, along with the duration of hospital stay and costs.2,7
In Spain, the Spanish Society of Intensive and Critical Care Medicine and Coronary Units (Sociedad Española de Medicina Intensiva, Crítica y Unidades Coronarias [SEMICYUC]) has led the implementation of the “Zero Tolerance” projects sponsored by the Ministry of Health to improve the safety of critical patients and of reducing infections related to the use of medical devices,2 with good results.
The Zero Resistance (ZR) project was introduced in 2014 with the purpose of reducing the appearance of MRB during ICU stay.8
The proposed recommendations include the early identification of MRB carriers on admission to the ICU and the adoption of preventive isolation measures in those patients with MRB carrier risk factors (RFs).
The objectives of the present study were to determine the performance of the preventive isolation criteria proposed by the ZR project used as RFs for MRB carrier status among critical patients on admission to the ICU, and to identify factors which when added to these criteria might improve their performance.
Patients and methodsStudy designA prospective, multicenter observational study was carried out.
SettingThe study was conceived within the Infection and Sepsis Working Group (Grupo de Trabajo de Infecciones y Sepsis [GTEIS]) of the SEMICYUC.
An invitation to participate was sent to all the ICUs in Spain through the diffusion media of the Society.
Finally, 9 Spanish ICUs that had participated in the ZR project from January to December 2016 agreed to participate in the study.
PatientsThe study included those patients admitted to the ICU in which smear sample cultures of different mucous membranes (nasal, pharyngeal, axillary, rectal) were made during the first 48 h in an active search for MRB, according to the recommendations of the ZR project and the protocol of each hospital. Diagnostic clinical sample cultures according to medical criterion were also made.
Patients under 15 years of age and those in which sampling was not carried out were excluded from the study.
MethodCompliance with the ZR project includes the completion of a checklist documenting RFs associated to MRB: hospital admission during more than 5 days in the last three months; institutionalized patients; a history of MRB carrier status; antibiotherapy for more than 7 days in the month before admission; patients subjected to hemodialysis or peritoneal dialysis; and chronic infection with a high incidence of MRB colonization/infection (cystic fibrosis, bronchiectasis, chronic ulcers, etc.).
The patients meeting any of the criteria were subjected to preventive contact isolation measures including mandatory universal protocol hygiene of the hands and the wearing of single-use gowns and gloves.
The maintenance or suppression of these measures was decided based on the microbiological results obtained.
No collection of samples additional to those already obtained on a routine basis in the participating ICUs proved necessary for the purposes of this study.
We analyzed demographic variables (age, gender and the APACHE II [Acute Physiology and Chronic Health Evaluation] score as a severity measure on admission to the ICU), stay, mortality and other disease and comorbidity variables included in the ENVIN-HELICS registry (accessible on http://hws.vhebron.net/envin-helics/)9 (diabetes mellitus [DM], acute or chronic renal failure, immunosuppression, previous neoplastic disease, liver cirrhosis, chronic obstructive pulmonary disease [COPD], malnutrition and solid organ transplantation).
Likewise, we recorded the origin of the patients (community, nursing home or other institution, hospital ward or other ICU) and the reason for admission (medical patient, elective surgery, urgent surgery, traumatologic or coronary patient), and whether antibiotic (ATB) treatment was prescribed on admission to the ICU.
The data were initially entered on a paper-format case report form that was subsequently transferred to an electronic spreadsheet (MS Access).
The patients and/or relatives received information on the microbiological procedures and on the preventive isolation policy.
Approval was obtained from the Ethics Committee of Hospital del Mar, Hospital Doce de Octubre and Burgos (Spain) for the ENVIN registry.
DefinitionsPatient MRB carrier status on admission was considered when one of the surveillance cultures and/or clinical samples (blood culture, urine culture, sputum culture, tracheal or bronchoalveolar aspirate, surgical wound smear, etc.) obtained in the first 48 h of ICU stay proved positive for MRB.
The included MRB were:
Methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus spp. (VRE), extended spectrum betalactamase (ESBL) and carbapenemase producing enterobacteria, Pseudomonas aeruginosa resistant to three or more families of antibiotics to which the organism is normally sensitive, and carbapenem-resistant Acinetobacter baumannii.
Those MRB not included in the above list (gramnegative Enterobacterales resistant to three or more families of antibiotics or with other resistance mechanisms such as AMP-C) were classified as “others”.
Statistical analysisThe data were reported as median (interquartile range [IQR]) or percentage, depending on the type of variable involved.
Comparisons between groups were made using the Mann–Whitney U-test or chi-squared test, depending on the variable, with statistical significance being accepted for p < 0.05.
The analysis was carried out in two phases: a first phase including all the hospitals with data on the ZR isolation criteria, and a second phase involving the RFs and which excluded two hospitals that had not collected this information.
Two multivariate binary logistic regression models were generated with MRB positivity as the outcome variable.
The first model, adjusted for age and gender, included the factors defined by the ZR program.10
The second model included the variables that proved significant in the bivariate analysis, added to those of the ZR isolation criteria (≥1 criterion), using a stepwise variable screening system.
A sensitivity analysis was made of the second model, obtaining values corresponding to sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV), with 95% confidence intervals (95%CIs).
The SPSS version 23 statistical package was used throughout.
ResultsNine ICUs from 6 Spanish regions (Autonomous Communities) participated in the study:
Aragón, Castilla y León, Catalonia, Galicia, Madrid and the Basque Country.
A total of 2252 records were included.
Of the 9 participating ICUs, only 7 collected surveillance samples of all patients on admission to the Unit, while the other two ICUs limited sample collection to those patients presenting RFs for MRB carrier status according to the ZR checklist.
For this reason the study was divided into two phases: a first phase including the 9 hospitals with data on patients with isolation criteria and the MRB found; and a second phase with data from the 7 hospitals that also registered those patients without ZR isolation criteria, used for the study of risk factors.
Phase 1Differences according to hospitals and MRBA total of 2252 patients were included in this phase.
Table 1 analyzes the demographic characteristics of the patients, degree of severity, surveillance samples obtained, and MRB identified for each of the participating ICUs.
Differential characteristics of the 9 hospitals included in the study (n = 2252).
| Variable | H1 | H2 | H3 | H4 | H5 | H6 | H7 | H8* | H9* |
|---|---|---|---|---|---|---|---|---|---|
| N = 899 | N = 432 | N = 278 | N = 208 | N = 146 | N = 140 | N = 82 | N = 55 | N = 22 | |
| MRB | 77 (8.7) | 94 (21.8) | 27 (9.7) | 22 (11.2) | 21 (14.0) | 20 (14.1) | 3 (3.6) | 10 (18.2) | 9 (40.9) |
| Age (years) | 62 (50−72) | 65 (51−74) | 66 (54−76) | 67 (55−74) | 70 (61−79) | 57 (45−67) | 61 (49−68) | 71 (63−78) | 69 (52−81) |
| Male gender | 562 (63.2) | 274 (63.4) | 171 (61.5) | 139 (66.8) | 101 (69.2) | 87 (62.1) | 50 (61.0) | 41 (74.5) | 17 (77.3) |
| APACHE II score | 13 (7−20) | 20 (13−26) | 18 (12−24) | 13 (8−20) | 15 (11−20) | 19 (14−25) | 8 (5−12) | 21 (16−25) | 18 (13−25) |
| In-hospital mortality | 102 (11.5) | 45 (10.4) | 30 (10.8) | 29 (13.9) | 31 (21.2) | 27 (19.3) | 11 (13.4) | 4 (7.3) | 7 (31.8) |
| Surveillance cultures performed on admission | |||||||||
| Nasal | 873 (98.2) | 427 (98.8) | 254 (91.4) | 194 (93.5) | 144 (98.3) | 127 (90.9) | 81 (98.7) | 47 (85.9) | 4 (20.4) |
| Pharyngeal | 695 (78.2) | 411 (95.2) | 248 (89.2) | 166 (79.9) | 143 (98.2) | 121 (86.4) | 43 (52.7) | 0.0 | 15 (66.6) |
| Axillary | 871 (98.6) | 0.0 | 0.0 | 1 (0.5) | 142 (97.8) | 6 (4.5) | 0.0 | 0.0 | 1 (0.7) |
| Rectal | 889 (100.0) | 406 (94.0) | 254 (91.4) | 189 (91.1) | 143 (98.3) | 121 (86.4) | 72 (88.0) | 48 (88.0) | 15 (69.1) |
| MRB isolated on admission (n = 283) | |||||||||
| MRSA | 33 (42.9) | 18 (19.1) | 9 (33.3) | 10 (45.5) | 5 (23.8) | 5 (25.0) | 0.0 | 4 (40.0) | 3 (33.3) |
| Pseudomonas aeruginosa | 3 (3.9) | 27 (28.7) | 1 (3.7) | 0.0 | 2 (9.5) | 7 (35.0) | 0.0 | 1 (10.0) | 1 (11.1) |
| ESBL-producing Enterobacterales | 35 (45.5) | 58 (61.7) | 16 (59.3) | 13 (59.1) | 13 (61.9) | 10 (50.0) | 3 (100.0) | 5 (50.0) | 4 (44.4) |
| Acinetobacter baumannii | 2 (2.6) | 3 (3.2) | 1 (3.7) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Others | 8 (10.4) | 17 (18.1) | 1 (3.7) | 0.0 | 2 (9.5) | 4 (20.0) | 0.0 | 0.0 | 1 (11.1) |
Values expressed as n (percentage) or median (interquartile range). MRB: multidrug-resistant bacteria; MRSA: methicillin-resistant Staphylococcus aureus; ESBL: extended spectrum betalactamase. (*) Data collection was limited to those patients with isolation criteria; these patients (3.4% of the total) were removed for the calculation of risk factors for MRB.
Differences were observed in the percentage of MRB (ranging from 3.6% to 21.8%), in the use of surveillance samples (many hospitals did not perform axillary sampling, and in one of them only rectal samples were collected) and in the identified MRB.
One or more MRB were identified in 283 patients (12.6% of the total admissions), of which 193 (68.2%) presented one or more RFs according to the ZR checklist.
Table 2 shows the characteristics of these patients with MRB, classified according to the microorganism involved.
Differential characteristics according to isolated multidrug-resistant bacteria (n = 283).
| Variable | TOTAL | ESBL-producing Enterobacterales | MRSA | Pseudomonas aeruginosa | Acinetobacter baumannii | Others |
|---|---|---|---|---|---|---|
| N = 283* | N = 157 | N = 87 | N = 42 | N = 6 | N = 33 | |
| Age (years) | 66 (56−75) | 68 (58−76) | 63 (53−76) | 65 (59−74) | 60 (52−76) | 62 (49−69) |
| Male gender | 204 (72.1) | 105 (66.9) | 65 (74.7) | 30 (71.4) | 5 (83.3) | 28 (84.8) |
| APACHE II (score) | 19 (14−25) | 18 (13−25) | 19 (13−26) | 23 (16−27) | 25 (15−35) | 23 (15−27) |
| In-hospital mortality | 45 (15.9) | 25 (15.9) | 12 (13.8) | 7 (16.7) | 1 (16.7) | 5 (15.2) |
| Presence of RF | 193 (68.2) | 108 (68.8) | 57 (65.5) | 40 (95.2) | 5 (83.3) | 22 (66.7) |
| Isolation criteria ZR | ||||||
| Previous hospital admission | 138 (48.8) | 74 (47.1) | 39 (44.8) | 31 (73.8) | 3 (50.0) | 17 (51.5) |
| Institutionalized patient | 20 (7.1) | 15 (9.6) | 10 (11.5) | 2 (4.8) | 1 (16.7) | 1 (3.0) |
| Previous colonization | 97 (34.3) | 54 (34.4) | 30 (34.5) | 33 (78.6) | 4 (66.7) | 10 (30.3) |
| Previous antibiotic treatment | 109 (38.5) | 62 (39.5) | 28 (32.2) | 28 (66.7) | 3 (50.0) | 15 (45.5) |
| Renal failure subjected to dialysis | 10 (3.5) | 5 (3.2) | 6 (6.9) | 1 (2.4) | 0.0 | 1 (3.0) |
| Chronic patient+ | 16 (5.7) | 7 (4.5) | 4 (4.6) | 5 (11.9) | 0.0 | 1 (3.0) |
| Culture positivity on admission to ICU | ||||||
| Nasal | 69 (24.4) | 15 (9.6) | 59 (67.8) | 2 (4.8) | 2 (33.3) | 4 (12.1) |
| Pharyngeal | 62 (21.9) | 25 (15.9) | 30 (34.5) | 14 (33.3) | 2 (33.3) | 7 (21.2) |
| Axillary | 1(0.4) | 1 (0.6) | 0.0 | 0.0 | 0.0 | 0.0 |
| Rectal | 157 (55.5) | 128 (81.5) | 16 (18.4) | 22 (52.4) | 4 (66.7) | 15 (45.5) |
| Blood culture | 42 (14.8) | 25 (15.9) | 9 (10.3) | 9 (21.4) | 3 (50.0) | 4 (12.1) |
| Urine culture | 51 (18.0) | 32 (20.4) | 10 (11.5) | 11 (26.2) | 2 (33.3) | 11 (33.3) |
| Respiratory | 52 (18.3) | 24 (15.3) | 15 (17.2) | 13 (31.0) | 2 (33.3) | 8 (24.2) |
| Wound | 25 (8.8) | 13 (8.3) | 9 (10.3) | 9 (21.4) | 2 (33.3) | 6 (18.2) |
Values expressed as n (percentage) or median (interquartile range). (*) Some patients have more than one isolated MRB. (+) Chronic patient at high risk of MRB colonization; includes bronchiectasis, cystic fibrosis and skin ulcers. ZR: Zero Resistance; RF: risk factor; MRSA: methicillin-resistant Staphylococcus aureus; ESBL: extended spectrum betalactamase.
Differences were observed in the presence of ZR isolation criteria, with Pseudomonas aeruginosa being the MRB with the most RFs.
Variability was recorded in the use and performance of the cultures in detecting the different MRB, with a predominance of nasal sample cultures in MRSA and rectal sample cultures in ESBLs and Acinetobacter spp.
Phase 2Study of MRB carrier risk factorsA total of 2175 patients were included in this phase, corresponding to 7 hospitals with 264 MRB isolations in the cultures performed.
Table 3 shows their demographic characteristics, comorbidities, severity, origin, isolation criteria and evolution referred to the total group and classified according to the presence of MRB.
Demographic characteristics of the patients admitted to the ICU (n = 2175), according to detection of multidrug-resistant bacteria (MRB) carriers.
| Variable | Total | NO MRB | MRB | p-Value |
|---|---|---|---|---|
| N = 2175 | N = 1911 | N = 264 | ||
| Age (years) | 64 (51−73) | 63 (51−73) | 65 (56−75) | 0.022 |
| Age (groups) | 0.299 | |||
| <50 | 486 (22.3) | 438 (22.9) | 48 (18.2) | |
| 50–65 | 692 (31.8) | 607 (31.8) | 85 (32.2) | |
| 66–75 | 557 (25.6) | 487 (25.5) | 70 (26.5) | |
| >75 | 440 (20.2) | 379 (19.8) | 61 (23.1) | |
| Male gender | 1384 (63.6) | 1194 (62.5) | 190 (72.0) | 0.003 |
| Disease history | ||||
| DM | 559 (25.7) | 468 (24.5) | 91 (34.5) | 0.001 |
| COPD | 334 (15.4) | 285 (14.9) | 49 (18.6) | 0.123 |
| Renal failure | 559 (25.7) | 449 (23.5) | 110 (41.7) | <0.001 |
| Neutropenia | 33 (1.5) | 27 (1.4) | 6 (2.3) | 0.284 |
| Immunosuppression | 220 (10.1) | 169 (8.8) | 51 (19.3) | <0.001 |
| Neoplasm | 530 (24.4) | 470 (24.6) | 60 (22.7) | 0.508 |
| Cirrhosis | 105 (4.8) | 86 (4.5) | 19 (7.2) | 0.055 |
| Transplantation | 41 (1.9) | 25 (1.3) | 16 (6.1) | <0.001 |
| Origin | <0.001 | |||
| Emergency | 1553 (71.4) | 1420 (74.3) | 133 (50.4) | |
| Nursing home | 25 (1.1) | 21 (1.1) | 4 (1.5) | |
| Ward | 569 (26.2) | 446 (23.3) | 123 (46.6) | |
| Other ICU | 28 (1.3) | 24 (1.3) | 4 (1.5) | |
| Diagnosis | <0.001 | |||
| Medical | 1238 (56.9) | 1053 (55.1) | 185 (70.1) | |
| Elective surgery | 428 (19.7) | 395 (20.7) | 33 (12.5) | |
| Urgent surgery | 205 (9.4) | 179 (9.4) | 26 (9.8) | |
| Traumatism | 169 (7.8) | 161 (8.4) | 8 (3.0) | |
| Coronary | 135 (6.2) | 123 (6.4) | 12 (4.5) | |
| Presence of risk factors ZR | 737 (33.9) | 563 (29.5) | 174 (65.9) | <0.001 |
| Isolation criteria ZR | ||||
| Previous hospital admission | 591 (27.2) | 463 (24.2) | 128 (48.5) | <0.001 |
| Institutionalized | 44 (2.0) | 28 (1.5) | 16 (6.1) | <0.001 |
| Previous colonization | 123 (5.7) | 31 (1.6) | 92 (34.8) | <0.001 |
| Previous antibiotic treatment | 332 (15.3) | 231 (12.1) | 101 (38.3) | <0.001 |
| Renal failure subjected to dialysis | 30 (1.4) | 20 (1.0) | 10 (3.8) | <0.001 |
| Chronic patient+ | 56 (2.6) | 42 (2.2) | 14 (5.3) | 0.003 |
| ATB on admission to ICU | 1217 (56.0) | 1024 (53.6) | 193 (73.1) | <0.001 |
| APACHE II (score) | 15 (9−22) | 15 (9−22) | 19 (14−25) | 0.008 |
| ICU stay (days) | 3 (2−8) | 3 (2−7) | 5 (2−9) | 0.001 |
| In-hospital mortality | 275 (12.6) | 233 (12.2) | 42 (15.9) | 0.089 |
Values expressed as n (percentage) or median (interquartile range). MRB: multidrug-resistant bacteria; DM: diabetes mellitus; COPD: chronic obstructive pulmonary disease; ATB: antibiotic; RF: risk factor; ZR: Zero Resistance. (+) Chronic patient at high risk of MRB colonization; includes bronchiectasis, cystic fibrosis and skin ulcers. p-Value: chi-square test or Mann–Whitney U-test.
The incidence of MRB was 6.4% in the population without ZR risk factors (1438 patients) versus 21.6% in the presence of such RFs.
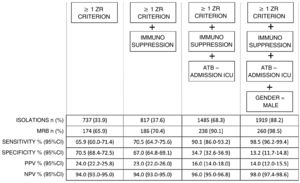
A total of 737 patients presented ZR risk factors (33.9% of the total admissions), and of these 174 presented MRB (sensitivity 65.9% and specificity 79.5%).
A progressive increase in the number of ZR criteria was seen to be associated with a greater percentage detection of MRB: 16% in those with 1 RF (407 patients), 20% in those with 2 RFs (240 patients), 64% in those with 3 RFs (75 patients), 81.8% in those with 4 RFs (11 patients), and 100% in those with 5 RFs (4 patients) according to the ZR checklist.
On analyzing the ZR isolation criteria (Table 4), the criterion “previous colonization” was seen to yield the highest odds ratio (OR).
Multivariate logistic regression analysis of the different preventive isolation factors included in the ZR project.
| Variable | OR (95%CI) | p-Value |
|---|---|---|
| Previous hospital admission (n = 591) | 1.46 (1.10–2.14) | 0.043 |
| Institutionalized (n = 44) | 2.98 (1.38–6.44) | 0.005 |
| Previous colonization (n = 123) | 23.74 (14.80–38.01) | <0.001 |
| Previous antibiotic treatment (n = 332) | 1.81 (1.18–2.79) | 0.007 |
| CRF subjected to dialysis (n = 30) | 1.59 (0.83–5.01) | 0.132 |
| Chronic patient+ (n = 56) | 1.61 (1.01–5.08) | 0.048 |
OR: odds ratio; CI: confidence interval; CRF: chronic renal failure. Model adjusted for age and gender. (+) Chronic patient at high risk of MRB colonization; includes bronchiectasis, cystic fibrosis and skin ulcers.
The criterion “renal failure” was the only criterion not reaching statistical significance in the multivariate analysis, though it proved significant in the univariate analysis.
In the study of new RFs (Table 5), three factors were seen to reach statistical significance in the multivariate analysis and were added to the ZR isolation criteria: immunosuppression (10.1%), the prescription of antibiotic treatment on admission to the ICU (56.0%), and the male gender (63.6%).
Multivariate logistic regression model of factors influencing the presence of MRB.
| Variable | OR (95%CI) | p-Value |
|---|---|---|
| Isolation criteria ZR | 3.98 (3.01–5.27) | <0.001 |
| Immunosuppression | 1.45 (1.01–2.10) | 0.046 |
| Antibiotic treatment on admission | 1.82 (1.35–2.46) | <0.001 |
| Gender (male) | 1.52 (1.13–2.05) | 0.005 |
MRB: multidrug-resistant bacteria; ZR: Zero Resistance; OR: odds ratio; CI: confidence interval.
The analysis of the patients with a prescription of antibiotic treatment on admission to the ICU showed them to be different in terms of diagnosis, with a greater presence of medical problems (72.5% versus 37.2% with surgical, coronary or traumatic conditions; p < 0.001), a greater presence of ZR criteria (40.8% versus 25.1%; p < 0.001), greater severity with an APACHE II score of 19 (13-25) versus 11 (617) (p < 0.001), and greater in-hospital mortality (17.3% versus 6.7%; p < 0.001).
In turn, males presented a greater history of chronic obstructive pulmonary disease (COPD) (19.2% versus 8.6%; p < 0.001) and differences in diagnosis - with a lesser percentage of elective surgery (17.8% versus 23.0%; p < 0.001) and more trauma cases (6.9% versus 4.9%; p = 0.04).
Fig. 1 shows that the progressive inclusion of identified RFs increases the sensitivity in detecting MRB.
On analyzing performance, we found that in order for this increase in sensitivity to occur, an increase in preventive isolations is required (e.g., for a sensitivity of 90% we must perform isolation in 68.3% of the patients on admission).
DiscussionOne of the main findings of the present study was that 12% of the critical patients were MRB carriers on admission to the ICU. This is consistent with the data of the annual report of the European Center for Disease Prevention and Control (ECDC).3
This percentage increased to 21.6% in the presence of one of the risk factors included in the ZR project.
In Spain, Abella et al.10 recorded similar figures in patients with ZR isolation criteria (about 30%), with the presence of MRB in approximately 14% of all the patients admitted to the ICU, versus in 7% of the patients without RFs.
In our series, although the mortality rate was higher in the group with MRB (16% versus 12%), the difference failed to reach statistical significance.
Screening for MRB on admission to the ICU is one of the recommendations of the ZR project.
The study evidences that not all the ICUs participating in the ZR project perform surveillance cultures in all patients on admission: some collect routine samples only in those individuals presenting RFs on the checklist.
The literature11 describes such screening as offering little benefit when the prevalence of MRB is low (<5%), though it is useful as a tool for the control of nosocomial transmission when the prevalence is higher.
Variability was also observed in the cultures performed in the different participating hospital centers.
A recent study12 has found that in the presence of an incidence of about 4.5%, over one-third of the cases of MRSA are identified in cultures of samples different from nasal samples, while other authors find pharyngeal sampling to increase the detection of MRB by up to 10%.13
In our study, involving over 2000 patients, the clinical criteria included in the ZR checklist as RFs for MRB carrier status were seen to be useful (only renal failure did not reach statistical significance, due to the limited number of cases involved), with identification of 68% of the patients with MRB.
In coincidence with other publications, the most important factor was found to be a history of MRB carrier status.10
The simultaneous presence of more than one RF increases the probability of MRB positivity.14
Menéndez et al.15 obtained similar results evaluating RFs for MRB in patients with bronchiectasis requiring hospital admission due to infectious exacerbation. They identified hospitalization in the year before admission, chronic renal failure, and a history of MRB carrier status as independent RFs.
However, the strategy followed in the ZR project has weaknesses, since MRB appeared in up to 31.8% of the patients without RFs included on the checklist, and needless isolation was performed in 31.5% of the patients, with no evidence of the presence of MRB.
The ZR risk factors alone have a sensitivity of 66% and a specificity of close to 80%, that could be improvable.
Callejo-Torre et al.16 proposed the creation of a diagnostic tool involving the combination of RFs with rapid laboratory tests such as real time PCR, which is relatively easy to use and is cost-effective.
In the past, other authors17 have advocated the use of clinical algorithms including rapid diagnostic tests with a high negative predictive value — though no universal model in terms of microorganisms and geographical setting has yet been developed.
As a contribution to improve the prediction of MRB in critical patients, our study generated a risk model to improve the criteria proposed by the ZR project, adding other variables identified in the multivariate logistic regression analysis, i.e., immunosuppression, antibiotic treatment on admission to the ICU, and the male gender.
The first of the aforementioned factors reinforces the concept of patients with chronic disease by incorporating immunosuppressed individuals, and improves sensitivity by 5 points, with the need to isolate 5% more of the total patients.
In order to improve sensitivity to 90%, the factor we found was the prescription of antibiotic treatment on admission to the ICU.
This factor can be explained by the fact that those patients who do not receive antibiotics are admitted due to non-infectious conditions (e.g., coronary disease, elective surgery or trauma), presenting fewer RFs, and with a lesser use of microbiological tests.
In contrast, those who do receive antibiotics are characterized by their medical diagnosis, emergency admission and increased severity.
The addition of this criterion requires preventive isolation in two-thirds of the total admissions.
The last identified factor was the male gender.
This could be explained by the fact that males have a greater history of chronic obstructive pulmonary disease and a lesser percentage of elective admissions.
By adding this factor to the model, sensitivity increased to almost 99%, but requiring isolation in almost 90% of the patients admitted to the ICU.
Other publications have also identified the male gender as a RF.14
Further work is needed in search of models able to improve this performance, with the incorporation of rapid MRB identification techniques.16,17
The search for a balance between the number of preventive isolations and performance in the detection of MRB may be conditioned by the particular situation of each ICU. In this regard, Units with a greater MRB problem could benefit from an increase in the number of preventive isolations.
Mention also must be made of the problems associated with patient isolation, with management difficulties and an increased workload.18
Our study has several limitations. On one hand, a larger sample is always desirable, including more MRB isolations and allowing a specific analysis according to each microorganism. External validation of the model is another pending issue.
We did not detect vancomycin-resistant Enterococcus spp.; it therefore was not possible to validate the RFs for this organism.
Another limitation has been the lack of standardization in sampling for the surveillance cultures. Some centers use nasal and rectal samples, while others also include axillary samples. Despite the recommendations of the ZR project, this situation depends on the policy applied in each hospital, and on the available resources in the microbiology laboratories.
On the other hand, a strong point of our study is the fact that there are very few multicenter studies addressing all MRB groups in patients in the ICU. In this respect, our series is the largest found to date.
In sum, we have described the independent RFs for MRB carrier status on admission to the ICU, corroborating part of the ZR project checklist, and also identifying other potential RFs.
The isolation criteria based on the ZR project afford acceptable performance, but fail to identify one-third of the cases of MRB.
The most important criterion is a history of MRB carrier status.
It seems clear that the accumulation of RFs implies a high probability of predicting the presence of MRB.
The model could be improved upon by adding immunosuppression as a criterion.
In order to reach a sensitivity of 90%, the model must include the group of patients requiring antibiotic treatment on admission to the ICU, and this implies preventive isolation in two-thirds of all individuals admitted to the ICU.
The participating hospitals show geographical and epidemiological differences referred to both the incidence and type of MRB, but also in the use of diagnostic means.
Our series evidences low performance of axillary sample cultures.
Based on the evidence available to date, each ICU should create its own contact isolation protocols based on the clinical-demographic variables of the patients on admission, combined with the local epidemiology, and promoting the efficient and rapid use of microbiological tests.
Author’s contributionsStudy concept and design: Sulamita Carvalho-Brugger and Mercedes Palomar. All the authors have participated in data compilation. Statistical analysis and data interpretation: Sulamita Carvalho-Brugger and Javier Trujillano. Writing of the manuscript: Sulamita Carvalho-Brugger, Javier Trujillano, Francisco Álvarez Lerma and Mercedes Palomar. All the authors have participated in critical review of the final manuscript.
FundingThe present study has received no funding from public or private sources.
Conflicts of interestAll the authors declare that they have no conflicts of interest in relation to the present study.
Thanks are due to Ana Ezpeleta, Cristina Climent, Manuel Solsona, Mercedes Catalán, Montserrat Ortiz, Javier Blanco, Francisco Javier González de Molina, Pedro Olaechea and Naia Más for their contribution to data compilation.