To evaluate the clinical outcomes of patients with severe acute respiratory distress syndrome (ARDS) subjected to prone positioning before extracorporeal membrane oxygenation (ECMO).
DesignA retrospective analysis of a multicenter cohort was carried out.
SettingPatients admitted to the Intensive Care Units of 11 hospitals in Korea.
PatientsPatients were divided into those who underwent prone positioning before ECMO (n=28) and those who did not (n=34).
InterventionsNone.
Variables of interestThirty-day mortality, ECMO weaning failure rate, mechanical ventilation weaning success rate, mechanical ventilation-free days at day 60.
ResultsThe prone group had lower median peak inspiratory pressure and lower median dynamic driving pressure before ECMO. Thirty-day mortality was 21% in the prone group and 41% in the non-prone group (p=0.098). The prone group also showed a lower ECMO weaning failure rate, and a higher mechanical ventilation weaning success rate and more mechanical ventilation-free days at day 60. In the non-prone group, median dynamic compliance marginally decreased shortly after ECMO, but no significant change was observed in the prone group.
ConclusionsProne positioning before ECMO was not associated to increased mortality and tended to exert a protective effect.
Evaluar los resultados clínicos de pacientes con síndrome de dificultad respiratoria aguda (SDRA) quienes fueron colocados en decúbito prono previo a la oxigenación con membrana extracorpórea (ECMO).
DiseñoAnálisis retrospectivo de una cohorte multicéntrico.
EscenarioPacientes admitidos en las unidades de cuidado intensivo de 11 hospitales en Corea.
PacientesLos pacientes fueron divididos en aquellos que fueron colocados en decúbito prono antes de la ECMO (n=28) y aquellos que no fueron colocados en decúbito prono antes de la ECMO (n=34).
IntervencionesNinguna.
Variables de interés principalesMortalidad a los 30 días, tasa de fracaso de retirada gradual de la ECMO, tasa de éxito de retirada gradual de la ventilación mecánica, días sin ventilación mecánica a los 60 días.
ResultadosEl grupo prono tuvo una mediana más baja de la presión inspiratoria máxima y una mediana más baja de la presión de conducción dinámica antes de la ECMO. La mortalidad a los 30 días fue 21% en el grupo prono y 41% en el grupo no prono (P = 0.098). El grupo prono también mostró un valor numérico menor de tasa de fracaso de retirada progresiva de la ECMO, y valores más altos de tasa de éxito de destete de la ventilación mecánica y días sin ventilación mecánica a los 60 días. En el grupo no prono, la mediana del cumplimiento dinámico descendió marginalmente, poco después de ECMO, pero no se observó un cambio significativo en el grupo prono.
ConclusionesLa colocación en decúbito prono antes de la ECMO no se asoció con un incremento en mortalidad y tendió a ser de protección.
Acute respiratory distress syndrome (ARDS) is lung injury caused by either direct or indirect insults, and leads to severe respiratory failure that is refractory to conventional oxygen therapy.1,2 Hospital mortality in patients with severe ARDS ranges from 45% to 60%.2,3 Lung protective ventilation strategies using low tidal volume and higher levels of positive end-expiratory pressure (PEEP) are widely accepted approaches,4,5 although rescue therapies may still be required in refractory cases.
Prone positioning improves oxygenation by increasing lung homogeneity and counteracting gravitational forces.6 Prolonged prone positioning (≥16h) in ARDS patients with PaO2/FiO2 ratio ≤150 showed positive results.7 Meanwhile, extracorporeal membrane oxygenation (ECMO) can provide adequate blood carbon dioxide removal and oxygenation, allowing a reduction in mechanical ventilation (MV) and minimization of ventilator-induced lung injury. Recent clinical trials in severe ARDS have shown positive results of venovenous-ECMO,8,9 although there are no absolute criteria for ECMO implementation timing in ARDS. Given the lack of definite evidence for ECMO and promising favorable trials of prone positioning, prone positioning may be considered before ECMO implementation in patients with severe ARDS. Thus, knowing the outcome of prone positioning then ECMO would provide guidance to clinicians on which rescue therapy to choose first in severe ARDS patients. However, to date, there is little information on the use of prone positioning before ECMO.
In this study, we analyzed the clinical outcomes of patients with severe ARDS who underwent prone positioning before ECMO and compared them with those who did not undergo prone positioning before ECMO.
Patients and methodsStudy design and patient selectionThis study was a retrospective analysis of critically ill adult patients admitted to one of the intensive care units of the 11 participating tertiary or referral hospitals of Korea from January 2014 to December 2015 with a severe ARDS requiring ECMO. The exclusion criteria were: received lung transplantation (either bridge to transplant or destination therapy), cardiopulmonary resuscitation before ECMO, ECMO transferred from other hospital, venoarterial-ECMO, acute respiratory diagnosis other than ARDS, and incomplete data for analysis. The patients were divided into groups who were treated with prone positioning (prone group) and those who were not (no prone group) before ECMO implementation. The primary study outcome was 30-day mortality. Secondary outcomes included ECMO duration, ECMO weaning failure rate, MV weaning success rate, MV-free days at day 60, and 60- and 90-day mortality. We also analyzed factors associated with the time to 30- and 60-day mortality, including prone positioning. The local institutional review board or independent ethics committee of each hospital approved the study protocol (Institutional Review Board of Asan Medical Center, No. 2016-0269). Written informed consent was waived due to the observational nature of the study.
Data collection and definitionsBaseline demographic and clinical characteristics included age, sex, body mass index, immune status, etiologies of ARDS, severity of illness, and treatment before ECMO. The severity of illness was assessed using the Sequential Organ Failure Assessment (SOFA)10 scores at the time of intensive care unit admission and ECMO implementation. The severity of ARDS before ECMO implementation was assessed by the Respiratory ECMO Survival Prediction (RESP) score11 as previously described. The pre-ECMO variables included arterial blood gas and ventilator settings, which included PEEP, peak inspiratory pressure, dynamic driving pressure (the difference between peak inspiratory pressure and PEEP),12 tidal volume, dynamic compliance (tidal volume/dynamic driving pressure), respiratory rate, and FiO2. Arterial blood gas and ventilator settings were determined at baseline (before ECMO application) and at 4h and 24h after ECMO implementation. Severe ARDS was diagnosed by a consensus definition.2 An immunocompromised status was diagnosed if there was an underlying disease or condition that affected the immune system (human immunodeficiency virus infection, malignancy, or severe neutropenia) or if immunosupressive therapy was being administered. Vasopressors used included norepinephrine, epinephrine, dopamine, dobutamine, and vasopressin. Steroid use was defined as corticosteroid administration within 14 days of ECMO implementation. ECMO weaning failure was defined as death on ECMO or weaning from ECMO support followed by death within 48h. MV weaning success was defined as the ability of the patients to maintain breathing for 48h without any form of ventilator support.
Statistical analysisContinuous variables are presented as median and interquartile range or as mean±standard deviation, whereas categorical variables are presented as percentages. Continuous variables were compared using a Mann–Whitney U test or Student's t test. Categorical variables were compared using chi-square or Fisher's exact test. A Cox proportional hazards regression model was used to identify factors associated with time to 30- and 60-day mortality. The variables with P values <0.20 in the univariate analysis were included in the multivariate analysis by using stepwise backward selection procedures. To prevent multicollinearity, variables with high correlation between each other were controlled. Mortality after ECMO implementation was determined by Kaplan–Meier survival curve, and the date of the outcome or last data collection was censored. Paired Wilcoxon signed-rank test was carried out for intra-group (before and after ECMO) comparison. All tests of significance were two-tailed, and P values < 0.05 were considered statistically significant. All analyses were performed using SPSS version 18.0 for Windows (SPSS Inc., Chicago, IL, USA).
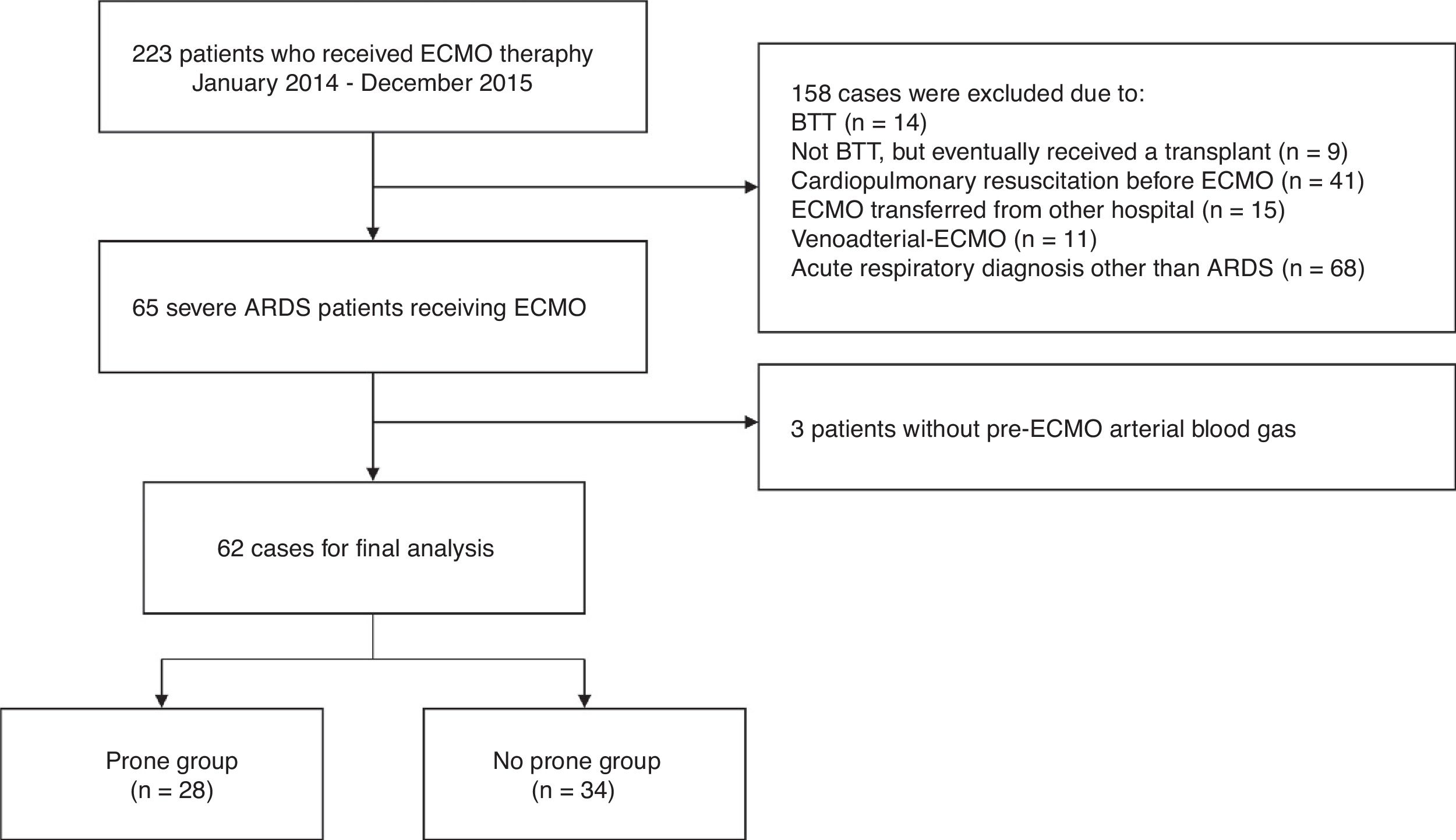
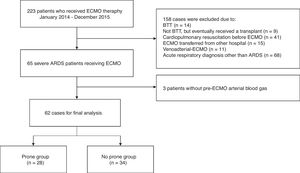
ResultsOf the 223 patients in the initial cohort, 161 were not included for the following reasons: met specific exclusion criteria (n=90); ECMO provided for an acute respiratory diagnosis other than ARDS (n=68); and pre-ECMO arterial blood gas unavailable for analysis (n=3) (Fig. 1). Thus, our study group consisted of 62 severe ARDS patients who received ECMO as a rescue therapy. There were 28 patients (45%) in the prone group and 34 (55%) in the no prone group.
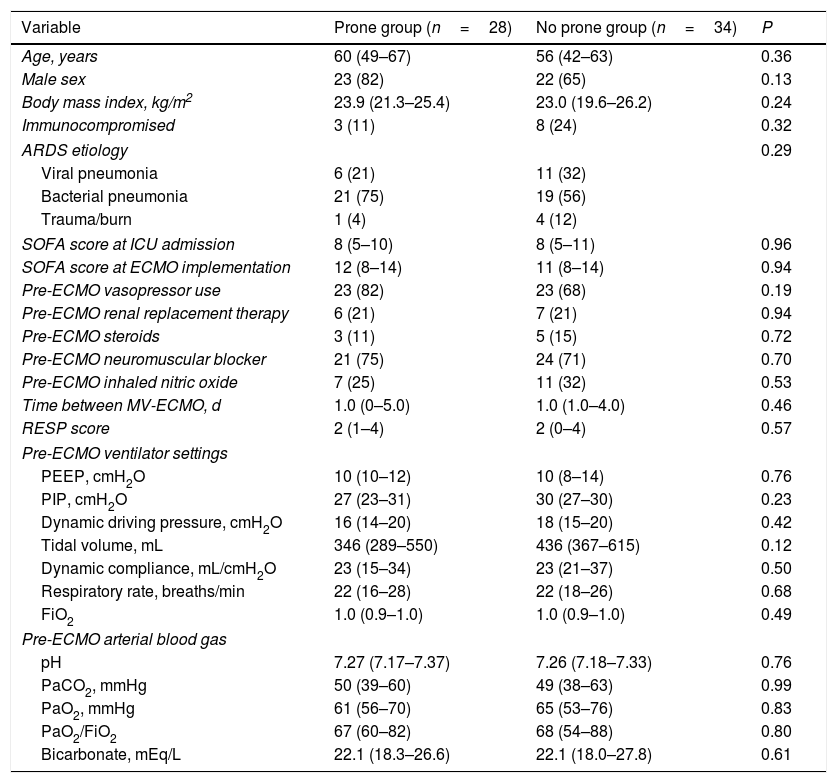
There were no significant differences between the two groups in terms of age, sex, body mass index, or immune status. In both groups, the main etiology of ARDS was bacterial pneumonia, followed by viral pneumonia. The baseline SOFA score, treatment before ECMO, and RESP score were also similar between the two groups. The median time from MV to ECMO cannulation was 1.0 (0–5.0) days for the prone group and 1.0 (1.0–4.0) days for the no prone group (P=0.46). Compared with the no prone group, the prone group had numerically lower median peak inspiratory pressure [27 (23–31) cmH2O vs. 30 (27–30) cmH2O] and lower median dynamic driving pressure [16 (14–20) cmH2O vs. 18 (15–20) cmH2O] before ECMO implementation. Other ventilator settings and arterial blood gas before ECMO support were not significantly different between the groups. Baseline characteristics of the study patients are summarized in Table 1.
Baseline characteristics of the study patients.
| Variable | Prone group (n=28) | No prone group (n=34) | P |
|---|---|---|---|
| Age, years | 60 (49–67) | 56 (42–63) | 0.36 |
| Male sex | 23 (82) | 22 (65) | 0.13 |
| Body mass index, kg/m2 | 23.9 (21.3–25.4) | 23.0 (19.6–26.2) | 0.24 |
| Immunocompromised | 3 (11) | 8 (24) | 0.32 |
| ARDS etiology | 0.29 | ||
| Viral pneumonia | 6 (21) | 11 (32) | |
| Bacterial pneumonia | 21 (75) | 19 (56) | |
| Trauma/burn | 1 (4) | 4 (12) | |
| SOFA score at ICU admission | 8 (5–10) | 8 (5–11) | 0.96 |
| SOFA score at ECMO implementation | 12 (8–14) | 11 (8–14) | 0.94 |
| Pre-ECMO vasopressor use | 23 (82) | 23 (68) | 0.19 |
| Pre-ECMO renal replacement therapy | 6 (21) | 7 (21) | 0.94 |
| Pre-ECMO steroids | 3 (11) | 5 (15) | 0.72 |
| Pre-ECMO neuromuscular blocker | 21 (75) | 24 (71) | 0.70 |
| Pre-ECMO inhaled nitric oxide | 7 (25) | 11 (32) | 0.53 |
| Time between MV-ECMO, d | 1.0 (0–5.0) | 1.0 (1.0–4.0) | 0.46 |
| RESP score | 2 (1–4) | 2 (0–4) | 0.57 |
| Pre-ECMO ventilator settings | |||
| PEEP, cmH2O | 10 (10–12) | 10 (8–14) | 0.76 |
| PIP, cmH2O | 27 (23–31) | 30 (27–30) | 0.23 |
| Dynamic driving pressure, cmH2O | 16 (14–20) | 18 (15–20) | 0.42 |
| Tidal volume, mL | 346 (289–550) | 436 (367–615) | 0.12 |
| Dynamic compliance, mL/cmH2O | 23 (15–34) | 23 (21–37) | 0.50 |
| Respiratory rate, breaths/min | 22 (16–28) | 22 (18–26) | 0.68 |
| FiO2 | 1.0 (0.9–1.0) | 1.0 (0.9–1.0) | 0.49 |
| Pre-ECMO arterial blood gas | |||
| pH | 7.27 (7.17–7.37) | 7.26 (7.18–7.33) | 0.76 |
| PaCO2, mmHg | 50 (39–60) | 49 (38–63) | 0.99 |
| PaO2, mmHg | 61 (56–70) | 65 (53–76) | 0.83 |
| PaO2/FiO2 | 67 (60–82) | 68 (54–88) | 0.80 |
| Bicarbonate, mEq/L | 22.1 (18.3–26.6) | 22.1 (18.0–27.8) | 0.61 |
Data are presented as median (interquartile range) or number (percentage) of patients. ARDS: acute respiratory distress syndrome; SOFA: Sequential Organ Failure Assessment; ICU: intensive care unit; ECMO: extracorporeal membrane oxygenation; MV: mechanical ventilation; RESP: Respiratory ECMO Survival Prediction; PEEP: positive end-expiratory pressure; PIP: peak inspiratory pressure; FiO2: fraction of inspired oxygen; PaCO2: arterial partial pressure of carbon dioxide; PaO2: arterial partial pressure of oxygen.
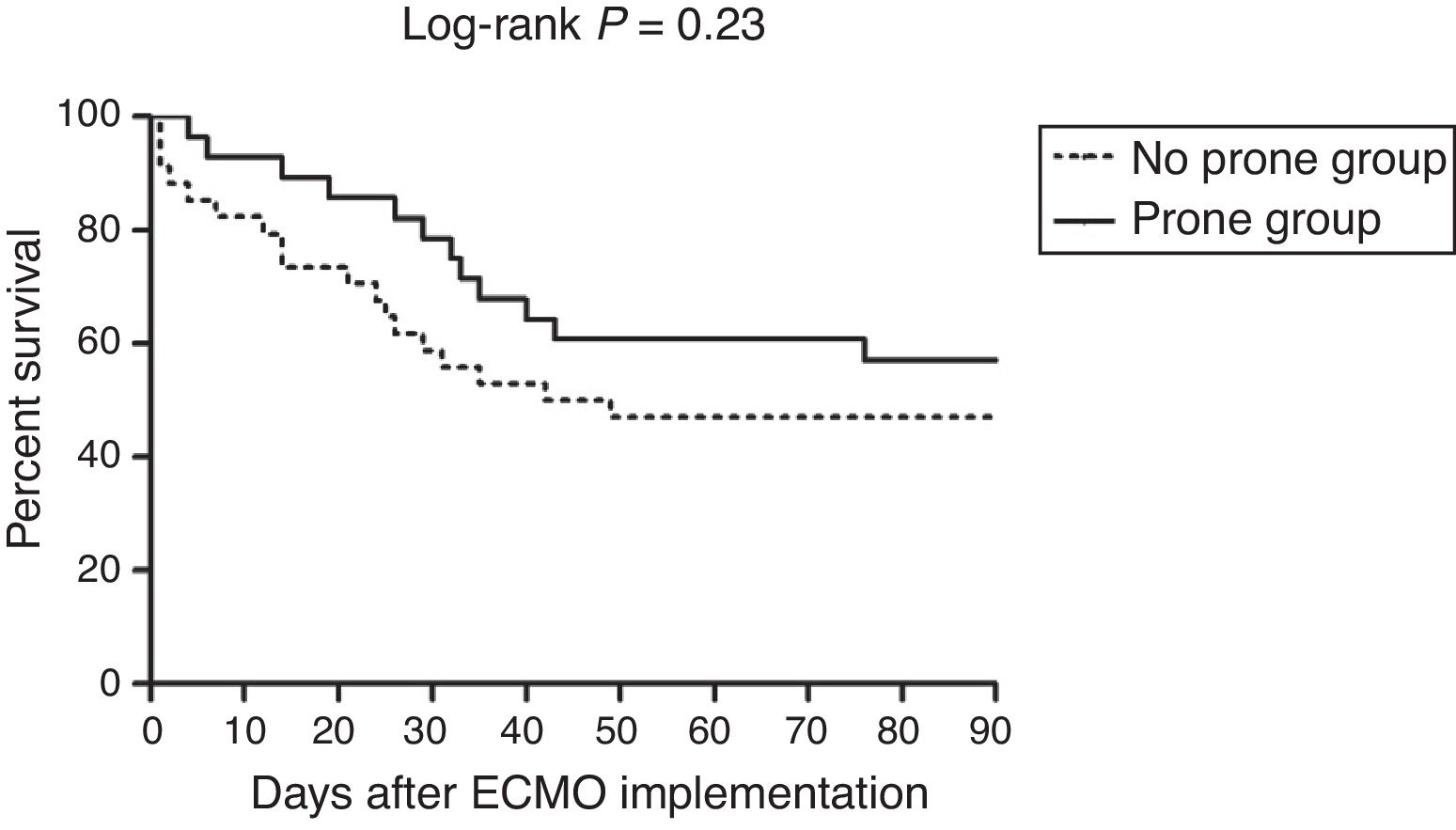
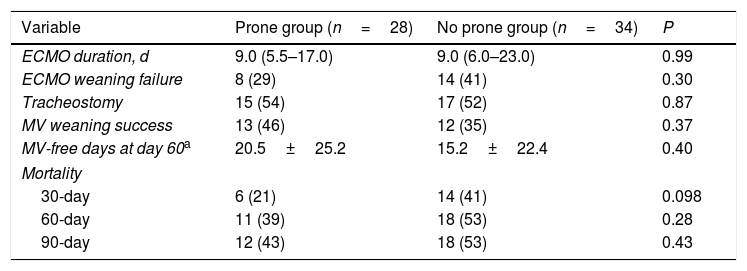
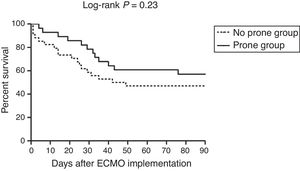
The primary outcome, 30-day mortality, was observed in 6 of the 28 patients (21%) in the prone group and 14 of the 34 (41%) in the no prone group (P=0.098) (Table 2). There was a trend toward lower 30- and 60-day mortality rates in the prone group, but not to a statistically significant degree. Moreover, ECMO weaning failure rate was numerically lower, and MV weaning success rate and MV-free days at day 60 were numerically higher in the prone group than in the no prone group, although the results were not statistically significant. Fig. 2 shows the Kaplan–Meier survival curves of the study patients.
Clinical outcomes of the study patients.
| Variable | Prone group (n=28) | No prone group (n=34) | P |
|---|---|---|---|
| ECMO duration, d | 9.0 (5.5–17.0) | 9.0 (6.0–23.0) | 0.99 |
| ECMO weaning failure | 8 (29) | 14 (41) | 0.30 |
| Tracheostomy | 15 (54) | 17 (52) | 0.87 |
| MV weaning success | 13 (46) | 12 (35) | 0.37 |
| MV-free days at day 60a | 20.5±25.2 | 15.2±22.4 | 0.40 |
| Mortality | |||
| 30-day | 6 (21) | 14 (41) | 0.098 |
| 60-day | 11 (39) | 18 (53) | 0.28 |
| 90-day | 12 (43) | 18 (53) | 0.43 |
Data are presented as median (interquartile range) or number (percentage) of patients. ECMO: extracorporeal membrane oxygenation; MV: mechanical ventilation.
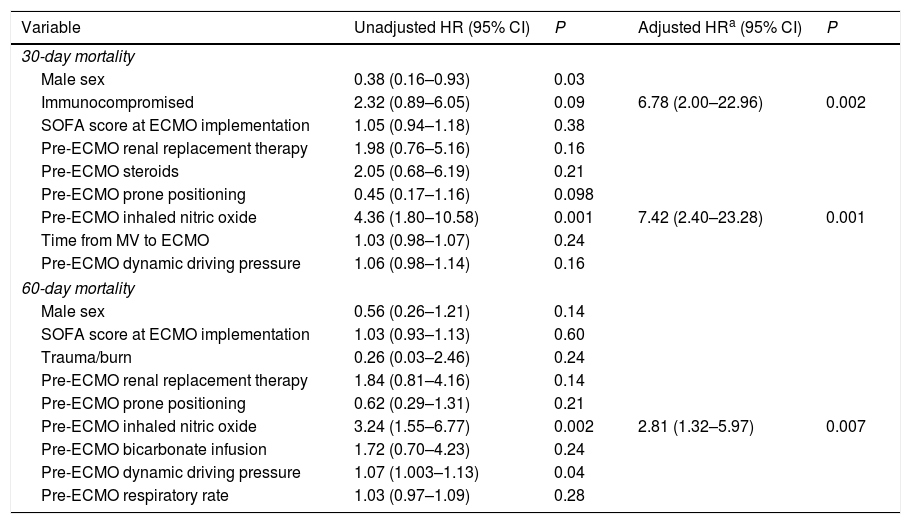
Table 3 shows the results of Cox proportional hazards regression model of risk factors associated with 30- and 60-day mortality. Multivariate analysis showed that immunosuppression and pre-ECMO inhaled nitric oxide (NO) were significantly associated with a shorter time to 30-day mortality. Pre-ECMO inhaled NO was also a significant prognostic factor for time to 60-day mortality. Meanwhile, prone positioning tended to be associated with a longer time to 30- and 60-day mortality only in univariate analysis.
Cox proportional hazards regression model with 30- and 60-day mortality as outcome.
| Variable | Unadjusted HR (95% CI) | P | Adjusted HRa (95% CI) | P |
|---|---|---|---|---|
| 30-day mortality | ||||
| Male sex | 0.38 (0.16–0.93) | 0.03 | ||
| Immunocompromised | 2.32 (0.89–6.05) | 0.09 | 6.78 (2.00–22.96) | 0.002 |
| SOFA score at ECMO implementation | 1.05 (0.94–1.18) | 0.38 | ||
| Pre-ECMO renal replacement therapy | 1.98 (0.76–5.16) | 0.16 | ||
| Pre-ECMO steroids | 2.05 (0.68–6.19) | 0.21 | ||
| Pre-ECMO prone positioning | 0.45 (0.17–1.16) | 0.098 | ||
| Pre-ECMO inhaled nitric oxide | 4.36 (1.80–10.58) | 0.001 | 7.42 (2.40–23.28) | 0.001 |
| Time from MV to ECMO | 1.03 (0.98–1.07) | 0.24 | ||
| Pre-ECMO dynamic driving pressure | 1.06 (0.98–1.14) | 0.16 | ||
| 60-day mortality | ||||
| Male sex | 0.56 (0.26–1.21) | 0.14 | ||
| SOFA score at ECMO implementation | 1.03 (0.93–1.13) | 0.60 | ||
| Trauma/burn | 0.26 (0.03–2.46) | 0.24 | ||
| Pre-ECMO renal replacement therapy | 1.84 (0.81–4.16) | 0.14 | ||
| Pre-ECMO prone positioning | 0.62 (0.29–1.31) | 0.21 | ||
| Pre-ECMO inhaled nitric oxide | 3.24 (1.55–6.77) | 0.002 | 2.81 (1.32–5.97) | 0.007 |
| Pre-ECMO bicarbonate infusion | 1.72 (0.70–4.23) | 0.24 | ||
| Pre-ECMO dynamic driving pressure | 1.07 (1.003–1.13) | 0.04 | ||
| Pre-ECMO respiratory rate | 1.03 (0.97–1.09) | 0.28 | ||
HR: hazard ratio; CI: confidence interval; ECMO: extracorporeal membrane oxygenation; MV: mechanical ventilation; SOFA: Sequential Organ Failure Assessment.
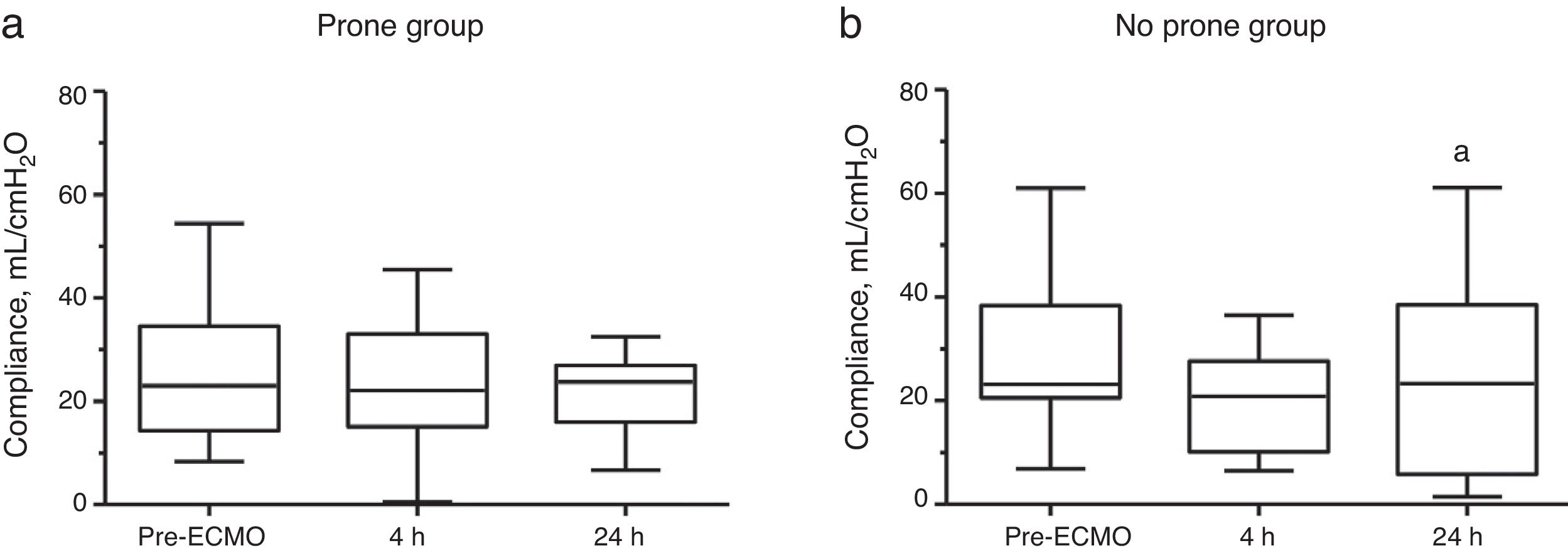
Fig. 3 shows the changes in the dynamic compliance between the prone group and the no prone group during a 24-h study period. No change in median dynamic compliance was observed in the prone group. In the no prone group, median dynamic compliance was 23 (range: 21–37)mL/cmH2O before ECMO and marginally decreased to 21 (range: 10–26) mL/cmH2O after 4h (P=0.15 vs. pre-ECMO) and 23 (range: 6–38)mL/cmH2O after 24h (P=0.06 vs. pre-ECMO). Supplementary Fig. 1 shows the changes in dynamic compliance between the study groups stratified according to survival status. In both groups, compared with survivors, non-survivors exhibited a lower median dynamic compliance until 24h.
DiscussionThe main findings of the present study are that (i) the use of prone positioning before ECMO was not associated with increased mortality and (ii) prone positioning before ECMO tended to be protective. The 47% 60-day mortality in our study patients (where the median RESP score at ECMO implementation was 2) is comparable with the mortality rates reported in previous studies of similar ECMO-treated ARDS patients.11,13 To the best of our knowledge, this is one of the few studies to evaluate the use of prone positioning before ECMO implementation in patients with severe ARDS.
Prone positioning may prevent lung injury by recruiting the dorsal regions of lung, improving respiratory mechanics, and clearing pulmonary secretions. Increasing the chest wall elastance, offloading the weight of the heart, and minimizing the weight of the abdominal contents on the diaphragm may enhance respiratory mechanics and increase recruitable lung units.6,14,15 Recent meta-analysis and randomized controlled trial showed that prone positioning can improve survival when applied early in the course of severe ARDS.7,16 Moreover, the use of prone positioning has been shown to significantly reduce the need for ECMO in severe ARDS patients.7 Meanwhile, early application of ECMO in patients with less severe ARDS may be beneficial by further reducing ventilator-induced lung injury,17 reducing hypoxic pulmonary vasoconstriction and unloading the right ventricle,18 and facilitating early rehabilitation.19 Failure of prone positioning may be associated with a delay in starting early ECMO. Therefore, there is controversy over which rescue therapy to choose first in severe ARDS patients. Although our study was based on a limited number of selected patients, it shows that the treatment outcome of the prone group was comparable or better to that of the no prone group, suggesting that prone positioning may be considered before ECMO implementation in patients with severe ARDS.
Several studies have evaluated the impact of prone positioning before or during ECMO in ARDS patients.13,20–23 Prone positioning before ECMO was shown to be a protective factor in two studies.13,20 In the study conducted by Schmidt et al.,13 refractory hypoxemia occurred despite prone positioning in approximately two-thirds of severe ARDS patients, although prone-positioned patients had significantly lower plateau pressures and driving pressures before ECMO. In our study, the prone group tended to have lower peak inspiratory pressure and dynamic driving pressure before ECMO implementation. In addition, pre-ECMO peak inspiratory pressure and dynamic driving pressure were lower in survivors compared with non-survivors in the prone group (data not shown). These findings are in line with the results of Schmidt et al.’s study, supporting the notion that prone positioning before ECMO might have the advantage of protecting lungs from further MV-induced lung injuries. The combination of prone positioning and ECMO has been recently studied in three trials.21–23 These studies demonstrated a clear benefit in terms of oxygenation and lung compliance and the absence of serious adverse events. Using ultra-protective ventilation with low plateau pressures during ECMO could develop the formation of poorly aerated areas in dependent lung regions.24 The aforementioned findings suggest that by recruiting the dorsal regions of the lungs, prone positioning could exert beneficial effects during ECMO. Meanwhile, we found that dynamic compliance decreased shortly after ECMO implementation in the no prone group, but compliance was kept constant in the prone group. Based on our findings, prone positioning might also be helpful in the pre-ECMO setting for preventing lung de-recruitment caused by ultra-protective ventilation. Albeit preliminary, our results do warrant further studies to confirm its applicability to clinical settings.
Inhaled NO is a selective pulmonary vasodilator that acts by dilating pulmonary vascular beds in those areas of the lungs where ventilation is preserved, thereby leading to a reduction in ventilation/perfusion mismatch and pulmonary vascular pressures. Despite a transient improvement in oxygenation in adults with ARDS, the recent meta-analysis has demonstrated no survival benefit associated with NO use regardless of severity.25 Moreover, it was even associated with a higher mortality within the RESP score.11 The present results, in which pre-ECMO inhaled NO was significantly associated with 30- and 60-day mortality, are consistent with those of previous studies. The underlying mechanism for this phenomenon is not clear, although the use of NO appears to increase the incidence of renal failure.25
This multicenter study had several limitations. First, its non-randomized design was prone to selection bias, and the low power due to relatively small sample sizes of our study are likely to be responsible for some of the non-significant results. Second, the data prior to prone positioning were not collected in this study. We do not know what prompted physicians to use prone positioning before ECMO for patients in the prone group. Third, we were not able to specify the timing and the duration of prone positioning before ECMO in our study, although the median time from intubation to ECMO was about 1 day in both groups. It is likely that prone positioning sessions were performed insufficiently before ECMO, which may have affected the treatment outcomes. Moreover, we were unable to ascertain whether such findings were due to excessive severity of patients, or because other rescue therapies had been used instead of prone positioning. Fourth, criteria for assessing ECMO for ARDS, MV on ECMO, and weaning from ECMO were not standardized among the participating centers. Thus, it is possible that the two groups were not treated with the exact same modalities across institutions. Lastly, we used the difference between the peak inspiratory pressure and PEEP to calculate the “dynamic” driving pressure12 because most patients were on pressure-controlled ventilation during the evaluation of ventilator settings. However, the ventilation variable that best stratifies risk in ARDS patients is driving pressure, calculated as the end-inspiratory pressure minus PEEP.26
ConclusionsIn conclusion, our results indicate that prone positioning before ECMO was not associated with increased mortality in patients with severe ARDS. Prone positioning before ECMO tended to be protective, even if it failed. These findings suggest that prone positioning may be applied as a first line therapy in patients with severe hypoxemia, and also highlight the need to understand when to use other potential rescue therapies before ECMO implementation. Further studies are required to clearly demonstrate the effect of prone positioning prior to ECMO implementation.
Contribution of the authorsWYK participated in the literature search, analysis of data, and manuscript preparation. BJK participated in the data collection and analysis of data. CRC, SHP, JYO, SYP, WHC, YSS, YJC, SP, and JHK participated in the data collection and helped to review the manuscript for important intellectual content. SBH participated in the study design and helped to review the manuscript for important intellectual content. All authors read and approved the final manuscript.
FundingThis research was supported by a grant of the Korean Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HC15C1507). The funders had no role in study design, data collection and analysis, preparation of the manuscript, or decision to publish.
Conflicts of interestThe authors declare no conflict of interest.
We thank Moon Seong Baek for querying data and Hwa Jung Kim for professional statistics review.