Activated protein C is associated with a risk of bleeding and its effects on survival in septic shock patients are questionable. Protein C zymogen has no risk of bleeding and improves the outcome of patients with septic shock. We hereby describe the largest published case series of adult patients receiving protein C zymogen.
Design, setting and participantsA prospective study on 23 adult patients with severe sepsis or septic shock, two or more organ failures and at high risk for bleeding, treated with protein C zymogen (50IU/kg bolus followed by continuous infusion of 3IU/kg/h for 72h).
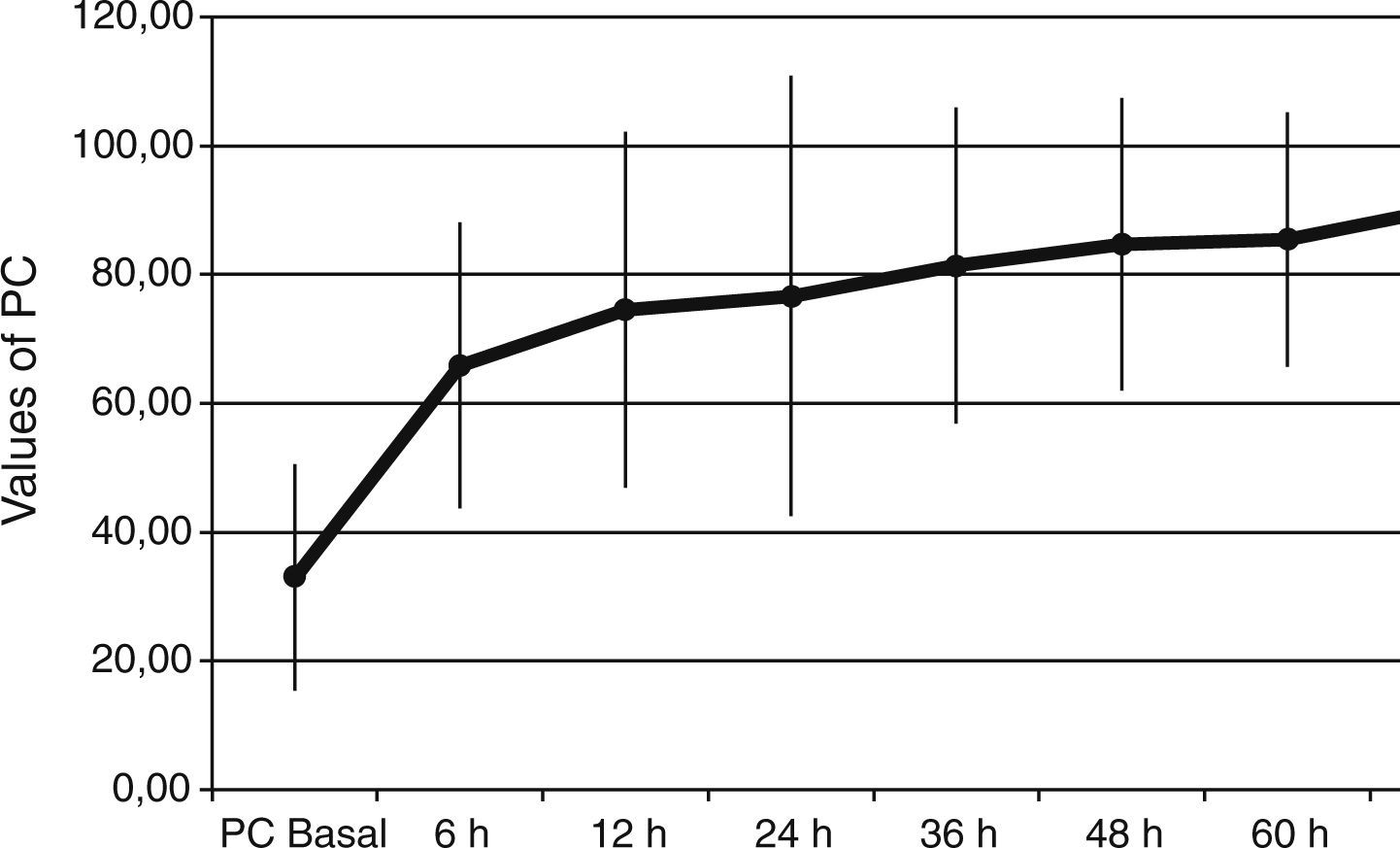
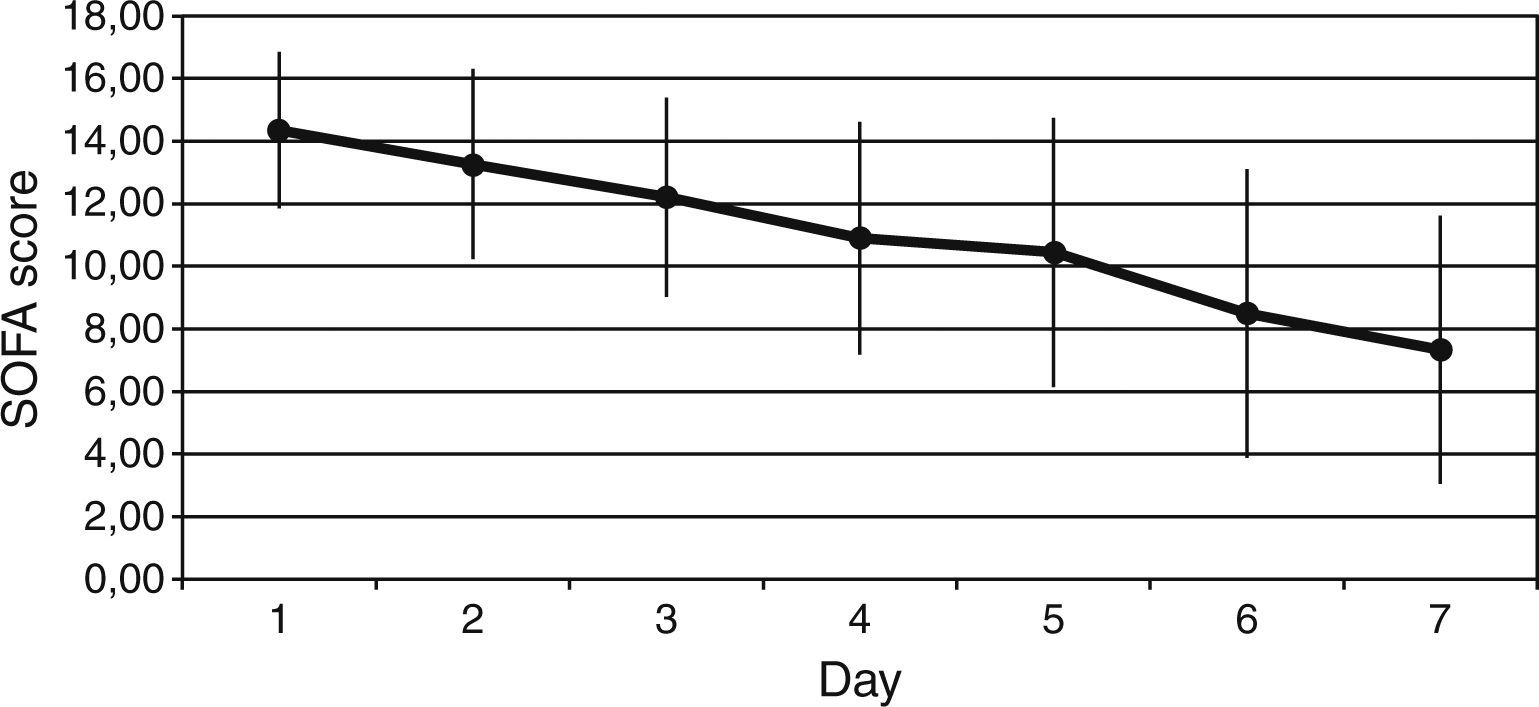
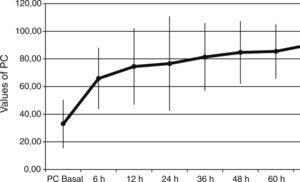
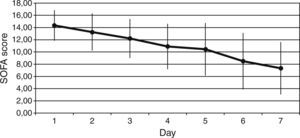
ResultsThe Z-test evidenced a significant reduction between the expected mortality (53%) and the observed mortality 30% (Z value=1.99, p=0.046) in our sample population. Protein C levels increased from 34±18% to 66±22% at 6h after PC bolus (p<0.001), and kept on increasing during 72h of administration (p<0.001 to baseline). Sequential Organ Failure Assessment (SOFA), score of organ dysfunction, decreased from baseline to 7 days after administration of protein C from 14±2 to 7±4 (p<0.001). No adverse event drug related was noted.
ConclusionProtein C zymogen administration is safe and its use in septic patients should be investigated through a randomized controlled trial.
La proteína C activada se asocia a un elevado riesgo de hemorragia, y sus efectos sobre la supervivencia en los pacientes con choque séptico son cuestionables. El zimógeno de proteína C no presenta ningún riesgo de hemorragia, y mejora los resultados en los pacientes con choque séptico. Describimos la serie de casos más amplia publicada de pacientes adultos tratados con zimógeno de proteína C.
Diseño, ámbito y participantesSe ha llevado a cabo un estudio prospectivo en el que han participado 23 adultos con sepsis grave o choque séptico, 2 o más fallos orgánicos, y un elevado riesgo de hemorragia, tratados con zimógeno de proteína C (dosis en bolo de 50UI/kg seguida de una infusión continua de 3UI/kg/h durante 72h).
ResultadosLa prueba Z puso de manifiesto una disminución significativa entre la mortalidad prevista (53%), y la mortalidad observada 30% (valor Z=1,99; p=0,046) en nuestra serie. Las concentraciones de proteína C incrementaron de 34±18% a 66±22% a las 6h de la dosis en bolo (p<0,001), y siguieron incrementando durante las 72h siguientes a la administración (p<0,001 respecto a la situación basal). La puntuación en la evaluación secuencial del fallo orgánico (SOFA) disminuyó entre la situación basal, y 7 días después de la administración de proteína C de 14±2 a 7±4 (p<0,001). No se registraron reacciones farmacológicas adversas.
ConclusiónEl zimógeno de proteína Z debería investigarse su utilización en los pacientes con sepsis mediante un estudio aleatorizado y controlado.
Severe sepsis and septic shock are life-threatening medical emergencies and are among the most significant challenges in critical care. Mortality rates approach 30–50%, and can be as high as 90% when multiple organ dysfunctions ensue.1,2 Improvement in survival rates was achieved through early broad-spectrum antibiotic administration, organ supportive therapy, and, until recently, through Recombinant Human Activated Protein C (rhAPC) in selected patients at low risk of bleeding. A recent randomized study showed that rhAPC did not significantly reduce mortality at 28 or 90 days, as compared with placebo, in patients with septic shock.3
Protein C (PC) is the vitamin K-dependent zymogen of a serine protease with antithrombotic, anti-inflammatory, and profibrinolytic properties.4 Due to its action on the coagulation pathway, rhAPC, the active form of drug, exposes treated patients to a serious hemorrhagic risk5–8 and its administration was subject to careful evaluation of the risk-to-benefit ratio. Attempts were made to use protein C zymogen, its “inactive” precursor, endowed with anti-inflammatory activity but devoid of anticoagulant properties. Among its advantages, PC is activated “on demand” in sites of major thrombin formation, and this is expected to limit or eliminate unwanted bleeding.
Few case series have been published on adult septic patients receiving protein C zymogen. We hereby describe the largest case series of adult patients with severe sepsis or septic shock receiving PC in a single center.
Patients and methods, setting and study populationAfter ethical committee approval and with patients’ written consent, we collected data from 23 adult patients with severe sepsis or septic shock admitted to two intensive care units (ICU) of San Raffaele Scientific Institute over a 2-year period. Eleven of these 23 patients were already reported in other publications.9,10 Inclusion criteria were represented by age >18 years, diagnosis of severe sepsis (acute organ dysfunction secondary to documented or suspected infection) or septic shock (severe sepsis plus hypotension not reversed with fluid resuscitation) and two or more organ failures due to sepsis of recent onset (less than 48h); contraindication to receive rhAPC (recent major surgery in most patients); being admitted in the ICU. Exclusion criteria were represented by known allergy to the study product and inclusion in other studies.
In addition to current standard-of-care therapies for severe sepsis and septic shock, patients received PC concentrates (Ceprotin®, Baxter, Wien) administered as a starting bolus (50IU/kg) plus a 3IU/kg/h continuous infusion over 72h.
We measured plasma PC activity, prothrombin time (PT), activated partial thromboplastin time (aPTT), Platelets (PLTs), C-reactive protein (CRP), white cell count (WBC), D-dimer and fibrinogen (FG) values at baseline, at 6 and 12h after PC concentrate administration, then every 12h for 60h. The sequential organ assessment failure (SOFA) score, the Acute Physiology and Chronic Health Evaluation (APACHE II) score, and the simplified acute physiology score (SAPS II) were recorded at baseline (when patient received PC zymogen), daily for 7 days.
Laboratory methodsSerial venous samples (4.5ml) were collected in siliconized Vacutainer tubes (Becton-Dickinson, Plymouth, UK) containing (0.5ml) tri-sodium citrate (0.129M) and in tubes containing 0.5ml of a mixture of tri-sodium citrate and benzamidine–HCl (200mM) at the following times: before the bolus dose, 6h after bolus and every 12h thereafter up to 72h. Within 1h from collection, platelet poor plasma was obtained by centrifugation for 10min at 2000×g at room temperature. PT (Hemoliance Recombiplastin, Instrumentation Laboratory, Lexington, MA), aPTT (STA aPTT Kaolin, Diagnostica Stago, Asnier sur Seine, France), FG (clotting assay, STA Fibrinogen, Stago), and D-dimer (STA Liatest D-D, Stago) determinations were performed on fresh citrated plasma samples with an automated coagulometer (STA, Stago). Plasma aliquots were snap-frozen with methanol and dry ice and stored at −70°C for additional measurements in citrated plasma of PC anticoagulant activity (STA Protein C, Stago), and antithrombin (amidolytic activity, STA Antithrombin, Stago). Blood samples collected in tri-sodium citrate and benzamidine–HCl were also centrifuged as described above with plasma aliquots snap-frozen and stored at −70°C. Within one month, prothrombin fragment 1+2 (F1+2, Enzygnost F1+2, Dade-Behring, Marburg) and thrombin-antithrombin III complex (TAT Enzygnost TAT micro, Dade-Behring, Marburg) were measured with commercially available ELISA kits.
StatisticsCollected data were analyzed through repeated measure ANOVA, chi-square (χ2), and Friedman's test using SPSS® 13 statistical package (Chicago, Illinois). The Z-test for two proportions was employed to compare expected versus observed mortality. A p-value <0.05 was considered statistically significant.
ResultsWe enrolled 23 consecutive patients with severe sepsis or septic shock. Twenty-one (91%) of them were surgical patients (18 cardiac surgery and one each for thoracic, vascular, urologic and abdominal surgery) and 1 patient presented to the emergency department with septic shock. Mean patient age was 63±12 years (range 25–77) and 4 (17%) were female.
All patients showed signs of sepsis-induced multiorgan dysfunction syndrome: 22 patients had respiratory failure, 16 had acute renal failure requiring renal replacement therapy and 16 had pharmacological sedation. The average APACHE II was 25±5, mean SAPS II was 58±14, and mean SOFA score was 14±2. The timing from surgery (or ICU admission) to the first cultural sample was 5±4.7 days while the timing from surgery (or ICU admission) to PC administration was 7±5.1 days. Seventeen patients had single or multiple cultural examination (in 4 patients the germs were identified in multiple sites and in 6 patients more than one germ was identified). Blood culture (8 patients), bronchoalveolar lavage (10 patients) and urine culture (2 patients) were positive for the following germs: Staphylococcus spp. (7 patients), Escherichia coli (6 patients), Acinetobacter spp. (2 patients), Proteus, Serratia marcescens, Streptococcus spp., Enterobacter cloacae, Klebsiella pneumonia, Citrobacter and Pseudomonas aeruginosa.
Baseline plasma PC activity was 33±18% [normal values 65–140%], and increased to 66±22% at 6h after PC bolus (p<0.001). Afterwards, it remained constantly within normal range values during PC concentrate continuous infusion and was 90±25% at 72h (p<0.001 to baseline) (Fig. 1).
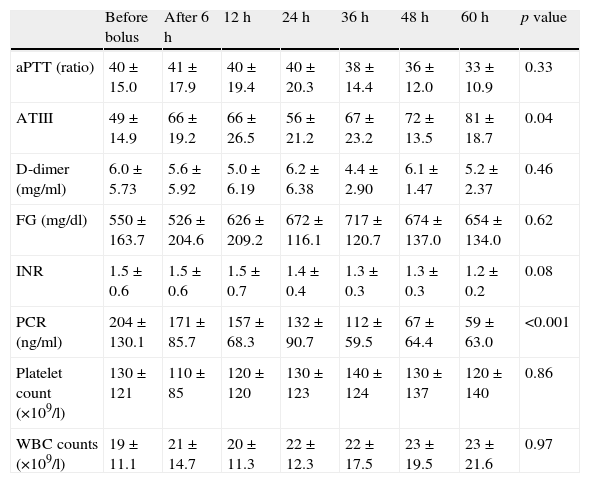
Changes in laboratory variables after protein C concentrate administration showed in Table 1. A significantly increasing of ATIII values from 49±15 to 81±19 was observed at 60h (p=0.04), FG and fibrin D-dimer showed a trend toward reduction and PLT count tended to increase from 130 (×109/l)±121 (×109/l) to 120 (×109/l)±140 (×109/l) during the 60h period.
Changes in laboratory variables throughout protein C concentrate administration.
| Before bolus | After 6h | 12h | 24h | 36h | 48h | 60h | p value | |
| aPTT (ratio) | 40±15.0 | 41±17.9 | 40±19.4 | 40±20.3 | 38±14.4 | 36±12.0 | 33±10.9 | 0.33 |
| ATIII | 49±14.9 | 66±19.2 | 66±26.5 | 56±21.2 | 67±23.2 | 72±13.5 | 81±18.7 | 0.04 |
| D-dimer (mg/ml) | 6.0±5.73 | 5.6±5.92 | 5.0±6.19 | 6.2±6.38 | 4.4±2.90 | 6.1±1.47 | 5.2±2.37 | 0.46 |
| FG (mg/dl) | 550±163.7 | 526±204.6 | 626±209.2 | 672±116.1 | 717±120.7 | 674±137.0 | 654±134.0 | 0.62 |
| INR | 1.5±0.6 | 1.5±0.6 | 1.5±0.7 | 1.4±0.4 | 1.3±0.3 | 1.3±0.3 | 1.2±0.2 | 0.08 |
| PCR (ng/ml) | 204±130.1 | 171±85.7 | 157±68.3 | 132±90.7 | 112±59.5 | 67±64.4 | 59±63.0 | <0.001 |
| Platelet count (×109/l) | 130±121 | 110±85 | 120±120 | 130±123 | 140±124 | 130±137 | 120±140 | 0.86 |
| WBC counts (×109/l) | 19±11.1 | 21±14.7 | 20±11.3 | 22±12.3 | 22±17.5 | 23±19.5 | 23±21.6 | 0.97 |
On the inflammatory viewpoint PCR values significantly decreased from 204±130.1 to 59±63.0 during 60h (p<0.01).
We observed a rapid reduction of the SOFA (from 14±2 to 7±4, p<0.001) (Fig. 2), the SAPS II (from 58±14 to 37±12, p<0.001), and the APACHE II (from 25±5 to 14±6, p<0.001) scores at 7 days.
Septic shock mortality rates in the literature approach 60% and the expected mortality in our sample population was 53±17%. The Z-test for two proportions evidenced a significant reduction between the expected mortality (53%) and the observed mortality 30% (Z value=1.99, p=0.046). In fact, 7 patients of our sample died (30%), all of them by refractory shock. A post hoc analysis showed that the best results in terms of crude mortality were observed in the subgroup of 11 patients with a cardiac index ≥2.5L/min/m2. In that group, only 1 patient (9%) died (Z value=3.5, p=0.001).
We observed two cases of hemorrhagic cystitis, respectively three days and two weeks after PC concentrate interruption. One case of bilateral jugular vein thrombosis was recorded as well. These phenomena could not be attributed to the drug administration. No bleeding complication was reported.
DiscussionTo the best of our knowledge this is the largest case series ever reported on the use of PC zymogen. In this study we showed a favorable effects on coagulation, multiorgan function and survival in patients that received PC zymogen.
PC levels increased from 33±18% to 66±22% [normal values 65–140%], at 6h after PC bolus (p<0.001), and kept on increasing during the 72h of administration (p<0.001 to baseline). Interestingly, all our patients had low baseline levels of PC. This finding is important because a low PC value is a strong predictor of unfavorable outcome11–14 and septic patients are associated with increased morbidity and mortality. Despite this baseline data we achieved a survival rate of 70%. Given the expected poor outcome on the basis of the score risks and our patients’ PC activity, the 70% survival rate may indicate that perhaps a beneficial drug effect on survival is present.
In our patients PC levels increased at 6h after PC bolus and remained constant thereafter, furthermore ATIII levels significantly increased after drug administration.
We also reported clinical benefit as documented by the significant reduction in the indices of organ dysfunction (the SOFA). Finally patient mortality was 30% versus the expected 53% and this difference was even more evident if only septic patients with cardiac index ≥2.5L/min/m2 were considered (mortality was 9%).
Consistently with the findings of the largest adult case series (20 patients) published so far15 we achieved normalization of coagulation parameters as a increasing of ATIII, as indicated by prompt raise of plasma PC activity within normal values, and a decreasing of PCR levels; an improvement of indices of organ dysfunction and a beneficial effect on patient survival. Similar findings were noted in our previously published case series that included 9 of these 23 patients.9 A recent systematic review on all the published case reports and case series of adult septic patients receiving PC suggested that mortality rates are low when receiving this drug.10
One interesting finding of our case series is that the beneficial effects on survival were more important in the patients with high cardiac output. The results of our study are important especially in view of the paucity of drugs, techniques or strategies that might reduce perioperative mortality in critically ill patients.16
LimitationsAlthough this is the largest case series of adult patients receiving PC ever published in literature, the small sample size and the non randomized study design do not allow us to draw definitive conclusions on the beneficial effect of PC administration in severe sepsis or septic shock. Furthermore, we measured only PCR and not other inflammatory markers such as procalcitonin or interleukine-6. On top of this, we acknowledge that the diagnosis of sepsis after major surgery might be challenging and that SIRS without infection is to be taken into consideration. Lastly, in half of our patients sepsis was diagnosed in patients with ongoing LCOS, a condition that is frequent after cardiac surgery and that can further confound the clinical picture of these patients.
ConclusionsThe favorable effects on coagulation, multiorgan function and survival suggest potential beneficial effects of PC concentrate on restoring homeostasis, at least as coagulation is concerned, and should raise interest in confirming our and others’ promising results through a randomized clinical trial or at least case match studies.
Conflict of interestThe authors declare no conflicts of interest.