The diagnosis of influenza A/H1N1 is mainly clinical, particularly during peak or seasonal flu outbreaks. A diagnostic test should be performed in all patients with fever and flu symptoms that require hospitalization. The respiratory sample (nasal or pharyngeal exudate or deeper sample in intubated patients) should be obtained as soon as possible, with the immediate start of empirical antiviral treatment.
Molecular methods based on nucleic acid amplification techniques (RT-PCR) are the gold standard for the diagnosis of influenza A/H1N1. Immunochromatographic methods have low sensitivity; a negative result therefore does not rule out active infection. Classical culture is slow and has low sensitivity. Direct immunofluorescence offers a sensitivity of 90%, but requires a sample of high quality. Indirect methods for detecting antibodies are only of epidemiological interest.
Patients with A/H1N1 flu may have relative leukopenia and elevated serum levels of LDH, CPK and CRP, but none of these variables are independently associated to the prognosis. However, plasma LDH>1500IU/L, and the presence of thrombocytopenia <150×109/L, could define a patient population at risk of suffering serious complications.
Antiviral administration (oseltamivir) should start early (<48h from the onset of symptoms), with a dose of 75mg every 12h, and with a duration of at least 7 days or until clinical improvement is observed. Early antiviral administration is associated to improved survival in critically ill patients. New antiviral drugs, especially those formulated for intravenous administration, may be the best choice in future epidemics.
Patients with a high suspicion of influenza A/H1N1 infection must continue with antiviral treatment, regardless of the negative results of initial tests, unless an alternative diagnosis can be established or clinical criteria suggest a low probability of influenza.
In patients with influenza A/H1N1 pneumonia, empirical antibiotic therapy should be provided due to the possibility of bacterial coinfection. A beta-lactam plus a macrolide should be administered as soon as possible. The microbiological findings and clinical or laboratory test variables may decide withdrawal or non-withdrawal of antibiotic treatment. Pneumococcal vaccination is recommended as a preventive measure in the population at risk of suffering severe complications.
Although the use of moderate- or low-dose corticosteroids has been proposed for the treatment of influenza A/H1N1 pneumonia, the existing scientific evidence is not sufficient to recommend the use of corticosteroids in these patients.
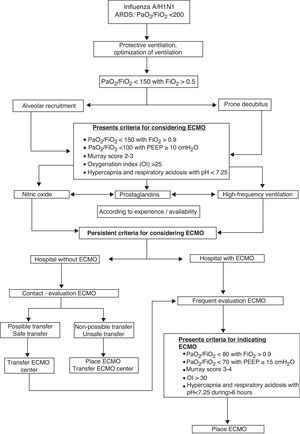
The treatment of acute respiratory distress syndrome in patients with influenza A/H1N1 must be based on the use of a protective ventilatory strategy (tidal volume <10ml/kg and plateau pressure <35mmHg) and positive end-expiratory pressure set to high patient lung mechanics, combined with the use of prone ventilation, muscle relaxation and recruitment maneuvers. Noninvasive mechanical ventilation cannot be considered a technique of choice in patients with acute respiratory distress syndrome, though it may be useful in experienced centers and in cases of respiratory failure associated with chronic obstructive pulmonary disease exacerbation or heart failure.
Extracorporeal membrane oxygenation is a rescue technique in refractory acute respiratory distress syndrome due to influenza A/H1N1 infection. The scientific evidence is weak, however, and extracorporeal membrane oxygenation is not the technique of choice. Extracorporeal membrane oxygenation will be advisable if all other options have failed to improve oxygenation. The centralization of extracorporeal membrane oxygenation in referral hospitals is recommended. Clinical findings show 50–60% survival rates in patients treated with this technique.
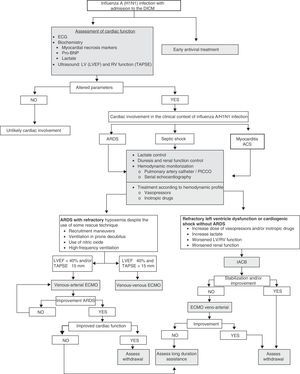
Cardiovascular complications of influenza A/H1N1 are common. Such problems may appear due to the deterioration of pre-existing cardiomyopathy, myocarditis, ischemic heart disease and right ventricular dysfunction. Early diagnosis and adequate monitoring allow the start of effective treatment, and in severe cases help decide the use of circulatory support systems.
Influenza vaccination is recommended for all patients at risk. This indication in turn could be extended to all subjects over 6 months of age, unless contraindicated. Children should receive two doses (one per month). Immunocompromised patients and the population at risk should receive one dose and another dose annually. The frequency of adverse effects of the vaccine against A/H1N1 flu is similar to that of seasonal flu. Chemoprophylaxis must always be considered a supplement to vaccination, and is indicated in people at high risk of complications, as well as in healthcare personnel who have been exposed.
El diagnóstico de gripe A/H1N1 es fundamentalmente clínico sobre todo durante los picos de la gripe estacional o en brotes epidémicos. Se recomienda realizar un test diagnóstico a todos los pacientes con fiebre y cuadro gripal que requieran hospitalización. La muestra respiratoria (exudado nasal, faríngeo o muestra profunda en pacientes intubados) se debe obtener lo antes posible e iniciar inmediatamente tratamiento antiviral empírico.
Los métodos moleculares basados en técnicas de amplificación de ácidos nucleicos (rt-PCR) son el «gold standard» para el diagnóstico de la gripe A/H1N1. Los métodos inmunocromatográficos son poco sensibles, por lo cual un resultado negativo no excluye la infección activa. El cultivo clásico en células es poco sensible y lento. La inmunofluorescencia directa tiene una sensibilidad del 90%, pero requiere una muestra de calidad. Los métodos indirectos de detección de anticuerpos tienen solo interés epidemiológico.
Los pacientes afectados de gripe A/H1N1 pueden presentar leucopenia relativa, con elevación de LDH, CPK y PCR, aunque estas variables del laboratorio no se asocian de forma independiente con el pronóstico. Sin embargo, niveles plasmáticos de LDH >1.500U/L y la presencia de plaquetopenia <150×109/L podrían definir una población de pacientes con riesgo de complicaciones graves.
La administración del antiviral (oseltamivir) debe ser precoz (<48h desde el inicio de los síntomas), en una dosis de 75mg cada 12h, con una duración de al menos 7 días o hasta la mejoría clínica evidente. La administración precoz se asocia a mejor superviviencia en pacientes críticos. Nuevos antivirales, en especial aquellos formulados para administración intravenosa, podrían ser los de elección en futuras epidemias.
Los pacientes con alta sospecha de gripe A/H1N1 deben continuar con tratamiento, independientemente de los resultados negativos de las pruebas iniciales, a menos que se pueda establecer un diagnóstico alternativo o los criterios clínicos sugieran una baja probabilidad de influenza.
En pacientes con neumonía por gripe A/H1N1 y dada la posibilidad de coinfección bacteriana, se recomienda cobertura antibiótica empírica (asociando un betalactámico con un macrólido) administrada lo antes posible. Los resultados de los cultivos y las variables clínicas o de laboratorio decidirán la retirada o no de los antibióticos. Como medida de prevención se recomienda la vacunación antineumocócica en la población de riesgo.
A pesar de que se ha propuesto el uso de corticosteroides en dosis moderadas-bajas para el tratamiento de la neumonía por gripe A/H1N, con la finalidad de mejorar la lesión pulmonar aguda, hasta el presente no existe evidencia científica suficiente que permita recomendar el uso de esteroides en estos pacientes.
El tratamiento del síndrome de distrés respiratorio agudo en pacientes con gripe A/H1N1 debe basarse en el empleo de estrategias ventilatorias protectoras del pulmón (volumen tidal <10ml/kg y presión plateau <35mmHg) y utilización de presión positiva al final de la espiración alta ajustada a la mecánica pulmonar del paciente, combinadas con el empleo de ventilación en decúbito prono, relajación muscular y maniobras de reclutamiento. La ventilación mecánica no invasiva no puede ser considerada una técnica de elección en los pacientes con síndrome de distrés respiratorio agudo, aunque podría ser útil en centros de gran experiencia y en casos de insuficiencia respiratoria asociados a reagudización de enfermedad pulmonar obstructiva crónica o insuficiencia cardiaca.
La oxigenación por membrana extracorpórea es una técnica de rescate en la gripe A/H1N1 con síndrome de distrés respiratorio agudo refractario. La evidencia científica es débil y no es la técnica de primera elección. Se instaurará si todas las otras medidas para mejorar la oxigenación han fracasado. Es recomendable la centralización de la técnica en hospitales de referencia. Los resultados clínicos muestran una supervivencias entre el 50-60% de los pacientes.
La afectación cardiovascular de la gripe A/H1N1 es frecuente y secundaria a la inestabilización de miocardiopatías preexistentes, miocarditis, cardiopatía isquémica y disfunción del ventrículo derecho. El diagnóstico precoz y la monitorización adecuada permiten iniciar un tratamiento efectivo y valorar, en los casos más graves, la necesidad de instaurar sistemas de soporte circulatorio.
Se recomienda la vacunación antigripal a todos los pacientes con riesgo, aunque podría ser necesario ampliar esta indicación a todos los mayores de 6 meses, salvo contraindicaciones. Los niños deben recibir 2 dosis con 1 mes de diferencia. Los inmunodeprimidos y la población con riesgo han de recibir una dosis con revacunación anual. La frecuencia de efectos adversos de la vacuna contra la gripe A/H1N1 es similar a la de la gripe estacional. La quimioprofilaxis siempre ha de ser considerada un complemento de la vacunación y está indicada en personas con alto riesgo de complicaciones así como en el personal sanitario que ha sufrido exposición.
During the summer and first months of the autumn of 2009, the Spanish Society of Intensive and Critical Care Medicine and Coronary Units (SEMICYUC), in collaboration with its Infectious Diseases Work Group (GTEI) and Acute Respiratory Failure and Organization and Management Work Groups, carried out an exhaustive review of the literature on influenza A/H1N1, and organized a scientific meeting in the month of October of that same year with the purpose of knowing the experience of other investigators in other countries (Canada and South America), and of presenting a project for the collection of information on this new disease through its network of hospitals. In that period, and in collaboration with the Spanish Ministry of Health, a series of documents were developed as a reference for Spanish intensivists in dealing with the serious complications that could develop in the context of the influenza A/H1N1 pandemic expected for the winter of that same year—taking as basis the body of scientific information available at the time. This was the first time that the influenza pandemic manifested in our setting with the real possibility of providing answers and solutions from the Intensive Care Units (ICUs), after more than 30 years of organized development of these Units in our country.
On the other hand, the availability of real time information on the evolution of patients with severe forms of influenza A/H1N1, fundamental on the generation of a large database, was seen to be of vital importance for defining the clinical course this new disease and for adopting modifications in prevention, diagnostic and treatment practices capable of exerting a positive influence upon patient prognosis—particularly in reference to severe respiratory failure. In this context, the GTEI established a network of hospitals and investigators (the Spanish Severe Influenza A Work Group [GETGAG]) that contributed their cases to the global national registry. In the epidemic period 2009/2010, this initiative made it possible to document 965 cases of severe influenza A/H1N1 in 148 hospitals, with another 517 patients in 58 hospitals in the seasonal period 2010/2011. This latter period involved a broader protocol with the collection of a larger number of variables and body of epidemiological, prognostic and therapeutic data.
In addition, and for the first time within the SEMICYUC, this project facilitated multidiscipline cooperation with other important national research groups such as the Network-based Biomedical Research Center of Respiratory Diseases (CIBERes) and the Spanish Research Network in Infectious Diseases (REIPI), with which collaborative study agreements were established for the clinical, etiological and molecular characterization of pandemic influenza A/H1N1 in Spain.
This important initiative, coordinated and directed by the GTEI, has made it possible to obtain key information on the severe forms of influenza A/H1N1, with the publication of over 20 articles in different national and international journals. In turn, it has allowed the activation of a research network in record time, affording an adequate response in all senses—organizational, media and scientific—to a pandemic situation that has generated considerable social alarm.
The present consensus document summarizes the broad experience commented above, affording a more concrete and detailed account of the recommendations for the treatment of influenza A/H1N1 in seriously ill adults admitted to the ICU. The document has been developed by many authors and covers a range of different aspects, including diagnostic and therapeutic considerations (use of antiviral drugs and the management of severe viral pneumonia, the associated distress, and cardiovascular complications), the influence of risk factors, and prognostic parameters. In addition, the document contains a specific section referred to prevention, with reference to vaccination, particularly in risk groups. The recommendations summarized in the first pages are a clear and concise reflection of all that has been learned from the management of this new disease, condensed in a few lines, and that can serve as a reference or guide for future epidemic and pandemic crises. Learning from experience is useful, and it is particularly important to make such experience known—as through the present consensus document—with a view to improving knowledge among the healthcare professionals that treat seriously ill patients.
From the SEMICYUC, we wish to thank the work and dedication of hundreds of intensivists who during the epidemic and seasonal periods of this disease have contributed to improve our knowledge of the characteristics and evolution of influenza A/H1N1, particularly in reference to its more severe manifestations.
General objectives and methodology of the recommendationsThe general objective of this consensus document is to offer all those healthcare professionals who treat patients with influenza A/H1N1 a series of clear guidelines for the early diagnosis and treatment of this disease. From the GTEI/SEMICYUC and the Infections in Critically Ill Patients Study Group/Spanish Society of Infectious Diseases and Clinical Microbiology (GEIPC/SEIMC), we have developed this consensus document with the purpose of offering healthcare professionals concise information on different aspects—many of which remain subject to controversy—referred to the diagnosis and treatment of seriously ill patients with influenza A/H1N1, in anticipation of the coming winter season and probable recirculation of the pandemic H1N1 virus.
The present consensus document has been drafted by representatives of each of the participating groups, with no interfering conflicts of interest. The work groups were composed of specialists with acknowledged experience in the field, selected by the coordinators of the GTEI and GEIPC. Each work group in turn comprised four members who developed a document containing a series of recommendations referred to each topic. For the definition and review of these recommendations, each group made use of the published literature and clinical guides, and of experience related to the management of patients with influenza A/H1N1 in Spain. The drafted documents in turn were forwarded to the consensus document secretary, who prepared the final manuscript, incorporating and homogenizing each of the documents submitted by the different work groups. The final manuscript was posteriorly submitted to all the participants for comments and considerations. Following incorporation of all the comments, the final manuscript was approved on a consensus basis by all the participants and submitted for evaluation and publication.
Epidemiology—when should we start to evaluate the presence of influenza A/H1N1 infection in critical patients with respiratory failure?The influenza A/H1N1 outbreak in the pandemic of 2009 represented a challenge for Intensive Care, due to the frequent appearance of severe complications, particularly of a respiratory nature, with the need for mechanical ventilation. Although most of the patients with influenza A/H1N1 presented a benign clinical course, with no need for hospitalization, a significant group of subjects required admission to hospital (0.3–0.5%) and eventual admission to the ICU (10–30%)—with a global hospital mortality rate of 4.5%.1 In turn, of the registered fatalities, 30–50% corresponded to patients without previous comorbidities.
The diagnosis of influenza is fundamentally clinical in most cases, particularly during the seasonal influenza peaks and in epidemic outbreaks of the disease. The combination of fever and cough in the 48h before onset of the rest of the respiratory manifestations has a positive predictive value (PPV) of 79%, with good correlation to the reverse transcription, real-time polymerase chain reaction (RT-PCR) test findings.2,3 Even in these periods, atypical presentations are commonly seen (elderly subjects, small children, immune compromised individuals, and also patients with previous chronic conditions of some kind), characterized by a possible absence of fever and by the predominance of gastrointestinal symptoms, or a lack of respiratory manifestations.
Outside the period of seasonal influenza or epidemic outbreaks, clinical criteria alone are unlikely to be able to distinguish influenza from other respiratory viral infections. Although as has been commented above the diagnosis of influenza can be clinical, in some cases it is very important to use laboratory tests to establish the diagnosis, for example in: (a) hospitalized patients with a presumed diagnosis of influenza; and (b) patients in whom confirmation of the diagnosis would imply changes in clinical management (such as the decision to use antibiotics and/or antiviral agents), or influence the use of other diagnostic techniques, the providing of recommendations regarding cohabitation with other high risk individuals, and the establishment of in-hospital infection control measures.
In the guides of the Infectious Diseases Society of America of 2009,4 and in reference to the influenza season, the performance of diagnostic tests is advised in all patients (of any age, whether immunocompetent or immunocompromised) with fever and respiratory symptoms requiring admission to hospital—including those with a diagnosis of community-acquired pneumonia, and independently of the time since development of the disease.
Likewise, it must be remembered that influenza can be transmitted to patients within the hospital through other patients, relatives or the healthcare workers, via inhalation as well as contact with hands contaminated with respiratory secretions. In the analysis of the first 131 deaths due to influenza A/H1N1 infection recorded in the Departments of Intensive Care Medicine in Spain, corresponding to the pandemic of 2009–2010, 6.1% were cases of nosocomial infection.5 In the same period, in the United Kingdom, 2% of all the cases of influenza admitted to hospital were likewise of nosocomial origin, with a mean stay from admission to the appearance of symptoms of 11 days. It is a source of concern that one-third of these patients did not receive antiviral therapy, and that of the rest, only one half received such treatment in the first 48h after onset of the symptoms.6 Consequently, in the influenza season, the appropriate diagnostic tests should be performed in all patients admitted to hospital and who develop fever and respiratory symptoms.
A recent study7 in patients admitted to the ICU has recommended the conduction of diagnostic tests and the start of empirical antiviral treatment, with the adoption of infection control measures, in those patients admitted during the seasonal influenza period with a diagnosis of pneumonia or respiratory infection, and in those presenting fever or who are admitted during the weeks of greatest incidence of influenza cases. In addition, at any time of the year, tests should be performed among healthcare workers, residents or visiting people with febrile respiratory disease related to an institution in which an influenza outbreak has occurred, and in people epidemiologically linked to an influenza outbreak.
When and what samples should be collected?Samples should be collected once the suspected clinical diagnosis of influenza infection has been established in patients requiring hospitalization or admission to the ICU. Respiratory samples should be obtained as soon as possible after the onset of symptoms (ideally in the first 48–72h), in order to maximize the sensitivity of the techniques. In hospitalized patients, the samples can be collected from different points of the upper (UA) or lower airway (LA). In ventilated patients we should obtain respiratory samples from the upper tract, in the form of smears or nasopharyngeal aspirates, and also from the lower tract (bronchial aspirate [BAS], bronchoalveolar lavage [BAL] or miniBAL). Viruses can be detected in LA samples in up to 20% (1 out of every 6 in the Spanish series) of all patients with viral pneumonia who present negative UA samples.8,9 Consequently, BAS or BAL should be performed whenever possible in those seriously ill patients with suspected viral pneumonia10,11 and, if the tests prove negative, they should be repeated in the subsequent 48–72h. Occasionally, some patients require more than three samples to yield a positive result.9
Monitorization of viral clearanceIdentification of the viral elimination period can help us establish the duration of the adopted infection control measures, thereby avoiding potential in-hospital outbreaks,12 particularly in the ICU, where such problems can prove fatal. Infection isolation and control measures are essential for controlling the transmission of influenza, and must be continued until the diagnostic test results prove negative (i.e., until negative conversion). The influenza virus persists in the secretions of immunocompetent patients for 5–7 days on average, though the elimination or clearance period may be prolonged particularly in elderly people, small children, patients with chronic diseases, immunocompromised individuals, more seriously ill patients, and in those who develop acute respiratory distress syndrome (ARDS).13,14
The early use of oseltamivir can shorten the viral clearance period.15 We therefore consider it necessary to collect new respiratory samples from 7 to 10 days after the start of symptoms, to confirm RT-PCR test negativity and be able to suspend the infection isolation and control measures—facilitating the vigilance and care of critical patients subjected to mechanical ventilation in isolation wards. No studies have assessed the risk of influenza transmission in those patients in which the PCR test results or culture findings remain positive during prolonged periods of time, though in theory these individuals would be able to transmit the infection. Based on these premises, individual evaluations will be needed in order to define the duration of the isolation measures.
ConclusionThe diagnosis of influenza A/H1N1 is fundamentally clinical, particularly during the seasonal influenza peaks or in epidemic outbreaks. Diagnostic tests are advised in all patients with fever and flu symptoms who require admission to hospital. Respiratory samples (nasal or pharyngeal exudate or deep samples in intubated patients) are to be obtained as soon as possible, with the immediate start of empirical antiviral treatment.
Risk factors—what patients should be considered at risk, and what mortality should we expect?In the last 100 years there have been four influenza pandemics: H1N1 in 1918, H2N2 in 1957, H3N2 in 1962, and H1N1 in 2009. Following the pandemics of 1918, 1957 and 1962, the hospital admission and mortality rates associated to influenza decreased, though with year-to-year variations. The most serious cases correspond to the youngest individuals, people over 65 years of age, pregnant women, and patients with previous disease.14 In the case of the influenza A/H1N1 2009 virus, the pattern seems to have changed, however—most of the severe cases caused by the pandemic virus corresponding to children and young adults, and about 90% of all the deaths affected patients under 65 years of age.8
The risk factors for complications are: age under 5 years, pregnancy, morbid obesity and chronic disease. While elderly subjects over 65 years of age have a lower infection risk, once they become infected, the severity of the illness is comparatively greater. In almost 50% of all patients with severe disease it has not been possible to identify any risk factor.16
The hospital admission rate varies greatly from one country to another, and is generally higher in patients under 5 years of age, particularly in young infants under one year of age. Between 9 and 31% of the hospitalized patients require admission to the ICU, and between 14 and 46% of those admitted to the ICU die of the disease.16–18 As regards comorbidities, 46% of the patients admitted to the ICU suffer no associated comorbidities. Likewise, no comorbid conditions are observed in 42.1% of the patients who die of the disease.19
In the Spanish registries, the mortality rate among patients admitted to the ICU is 22–25%,20,21 and this percentage in turn increases with age (12.8% in patients under 15 years of age, 22.3% between 15 and 64 years of age, and 32.3% in patients over 64 years of age). According to the Spanish Registry of the Health Alerts and Emergencies Coordination Center (CCAES) of the Ministry of Health (MSPS), 76.3% of the patients admitted to the ICU presented some underlying risk factor.20 Of note is the observation that among the patients over 64 years of age, 93.9% recovered, and all of those who died presented some background disorder. In the patients under 15 years of age, 13% of those who died presented no risk condition for the complication of influenza.
The global mortality rate of influenza A/H1N1 has been less than 0.5%, with a broad range of estimation (0.0004–1.47%).22,23 In the United States the mortality rate reached 0.048%,24 vs 0.026% in the United Kingdom.25 In Spain during the 2009–2010 pandemic the estimated global mortality rate was 0.43 deaths per 1000 cases. The lowest mortality rate corresponded to the group between 5 and 14 years of age (0.05 per 1000 cases), which was the group with the highest confirmed pandemic virus incidence, while the highest mortality rate corresponded to patients over 64 years of age, with four deaths per 1000 cases.26 An observation of note on comparing the age distribution of the deaths during the pandemic season vs that of previous seasonal influenza periods is the existence of a different mortality pattern in the case of influenza A/H1N1. In effect, while most of the influenza deaths corresponded to people over 60 years of age in the seasonal influenza period (98%), in the pandemic season only 28% of the recorded deaths corresponded to this same age group.26
Regarding mortality by risk groups, respiratory disease (asthma, chronic obstructive pulmonary disease [COPD] and other lung diseases) is the disorder most often reported among patients admitted to Spanish ICUs with influenza A/H1N1, according to the CCAES20 registry, with a mortality rate of 16.8% in the series published by Martin-Loeches et al.5 Among patients hospitalized due to influenza A/H1N1, between 24 and 50% of the children presented antecedents of asthma, while 36% of the adults had a history of COPD.10
Obesity is associated to a proinflammatory state and to insulin resistance that can increase the morbidity–mortality in patients infected with influenza A/H1N1. Furthermore, obesity is often associated to other chronic diseases. In the first published series of patients infected with influenza A/H1N1, the individuals with a body mass index (BMI) of ≥30kg/m2 accounted for a large proportion of the patients requiring admission to hospital and to the ICU, and also represented an important percentage of the fatalities.11,17,27 In later series, and following the advance of the pandemic, obesity remains the most frequently described risk factor, and is the second most common factor in adult patients admitted to the ICU—with a significantly higher prevalence among the deceased cases (27.6%) in the Spanish CCAES registry.20 However, other studies have found no association between mortality and obesity.28–30
An increase in morbidity–mortality has been documented in pregnant women during the influenza pandemics.31 The immune changes that occur during pregnancy, the increased ventilatory demands, the decrease in residual functional capacity and oncotic pressure make pregnant and puerperal women more vulnerable to serious lung disease caused by the influenza virus.32 Its association to mortality is subject to controversy. In previous pandemics, mortality among pregnant women proved higher than in the general population; during the influenza pandemic of 1918, the mortality rate among pregnant women was over 27%, and in the epidemic of 1957, a full 50% of the women of child bearing potential who died were pregnant.33,34 In the recent influenza A/H1N1 pandemic, pregnant women represented about 5.4% of the patients admitted to the ICU in Spain.35 Similar figures in turn were reported in Australia and New Zealand (9.1%)17 or Canada (7.7%).11 The mortality rate among pregnant women infected with influenza A/H1N1 varies among the different studies, and while some publications report figures of up to 20%, the percentage in Spain is approximately 14%—which is similar to the mortality data reported by other registries such as the ANZIC34 or the study carried out by Louie in California.36 The mortality rate in pregnant women has been associated to increased severity upon admission, as assessed by the Acute Physiology and Chronic Health Evaluation (APACHE) score, and obesity.34
Immune deficiencies and cancer are variables that have been independently associated to mortality among patients admitted to Spanish ICUs due to influenza A/H1N1 infection.20,37,38 In some studies, diabetes has been correlated to the severity of the infection.39,40 Other risk factors are cardiovascular diseases, chronic liver disease, hemoglobin disorders, chronic renal failure, asplenia, neuromuscular disease, cognitive dysfunction and diseases characterized by seizures. In children, the most frequently reported disorders are respiratory diseases (mainly asthma), cognitive dysfunction and seizures.20 In this context, cognitive dysfunction and seizures have been associated to severity in children with serious pandemic infection in the United States.3Table 1 describes the incidence of comorbidities associated to influenza A in series of critical patients. Regarding the complications among patients admitted to the ICU who died, the most frequently described problems according to the CCAES registry were ARDS, shock, sepsis, multiorgan failure and acute renal failure.
Demographic characteristics and comorbidities and/or risk factors in patients with influenza A/H1N1 infection according to different authors.
| Age | Total cases | No comorb | Death | Lung disease | Obesity | Immune suppression | Diabetes | Pregnancy postpartum | |
| Kumar11 | 32.3±21.4 | 168 | 3/168 (1%) | 29/168 (17%) | 69/168 (41%) | 56/168 (33%) | 39/168 (23%) | 35/168 (20%) | 13/168 (7%) |
| Miller41 | 34 (15–62) | 47 | 5/47 (10%) | 8/47 (17%) | NA | 35/47 (74%) | NA | 8/47 (17%) | 4/47 (8%) |
| Tabarsi42 | 36.9 (21–66) | 20 | 11/20 (55%) | 6/20 (30%) | 5/20 (25%) | NA | 3/20 (15%) | 0 | NA |
| Estenssoro43 | 47±17 | 337 | 121/336 (36%) | 156/337 (46%) | 79/337 (23%) | 80/337 (23%) | 50/332 (15%) | 41/337 (12%) | 22/337 (6%) |
| Santa-Olalla Peralta20 | 40 (0–90) | 1048 | 208/932 (22%) | 246/1048 (23%) | 71/886 (8%) | 164/872 (18%) | 120/916 (13%) | 141/916 (15%) | 50/239 (20%) |
| Domínguez-Cherit44 | 44.0 (10–83) | 58 | 9/58 (15%) | 24/58 (41%) | 4/58 (6%) | 21/58 (36%) | 2/58 (3%) | 10 /58 (17%) | NA |
| González-Vélez39 | 46 (1–72) | 19 | 4/19 (21%) | 2/19 (10%) | 4/19 (21%) | 2/19 (10%) | 3/19 (15%) | 8/19 (42%) | NA |
| Rello27 | 36 (31–52) | 32 | 15/32 (46%) | 8/32 (25%) | 9/32 (28%) | 10/32 (31%) | 2/32 (6%) | 1/32 (3%) | 2/32 (6%) |
| ANZIC17 | 40 (26–54) | 722 | 229/722 (31%) | 103/722 (14%) | 231/707 (32%) | 172/601 (28%) | NA | 112/700 (16%) | 66/722 (9%) |
| Nin45 | 45±14 | 96 | 17/96 (17%) | 48/96 (50%) | 30/96 (31%) | 37/96 (38%) | 8/96 (8%) | 14/96 (14%) | 6/96 (6%) |
| Liu46 | 40 (18–75) | 62 | 28/62 (45%) | 4/62 (6%) | 3/62 (4.8%) | 14/62 (22%) | 2/62 (3%) | 3/62 (4%) | 3/62 (4%) |
| Sertogullarindan47 | 36 (15–72) | 20 | 3/20 (15%) | 9/20 (45%) | 3/20 (15%) | 1/20 (5%) | 2/20 (10%) | NA | 2/20 (10%) |
| Chacko48 | 35 (28.2–42.8) | 31 | 11/31 (35%) | 6/31 (19%) | 3/31 (9%) | 9/31 (29%) | 1/31 (3%) | 3/31 (9%) | 3/31 (9%) |
| Teke49 | 41.52±15.7 | 61 | 11/61 (18%) | 31/61 (50%) | 14/61 (22%) | 17/61 (27%) | 10/61 (16%) | 8/61 (13%) | 3/61 (4%) |
NA: not available.
Immune suppression: cancer+immune suppression+human immunodeficiency virus (HIV).
Obesity: body mass index >30kg/m2.
Previous respiratory disease: asthma+chronic obstructive pulmonary disease+others.
Age expressed as mean±standard deviation or median (percentiles 25–75).
In Spain, the risk factors associated to the development of serious disease and to admission to the ICU in the recent influenza A/H1N1 pandemic were respiratory disorders and morbid obesity, while the most frequently documented complications were respiratory distress related to the development of primary viral pneumonia, hemodynamic instability, acute renal failure and multiorgan failure. The mortality rate among the patients admitted to Spanish ICUs was 22–25%, with a global mortality rate of 0.43 deaths per 1000cases.
Virological diagnosis—how and when should it be established?The emergence and global diffusion of the new influenza A/H1N1 virus during the second half of 2009 has led to the development of new molecular methods for the diagnosis of infection produced by influenza viruses, based on genomic amplification techniques. In addition, exhaustive evaluations have been made of the diagnostic efficacy of those rapid microbiological diagnostic procedures that were available before eclosion of the pandemic virus, together with the introduction of diagnostic algorithms applicable to influenza in most third-level hospital centers in Spain. A summarized account is provided below of the information obtained in this respect during the new influenza A/H1N1 pandemic.
Diagnostic techniques- (a)
The rapid and early detection of infection caused by the new pandemic virus, particularly in patients with criteria for admission to the ICU, allows us to optimize treatment of the disease, and is decisive in the adoption of effective epidemiological control and prevention measures.50,51
- (b)
The molecular methods based on nucleic acid amplification techniques have become the gold standard in the diagnosis of the influenza A/H1N1 infection, at the expense of classical cell cultures,50,51 and have been included by the United States Centers for Disease Control (CDC) in the definition of “confirmed” cases of the disease. The extreme sensitivity and specificity of these methods allow an early, precise and rapid diagnosis (within a few hours) of the infection. A range of RT-PCR protocols have been developed and clinically evaluated, and some of them have been marketed and approved by the United States Food and Drug Administration (FDA) for diagnostic use. These tests detect genic sequences specific of the pandemic virus and pertaining to the genes that encode for hemagglutinin (most tests), neuraminidase or M protein. Likewise, PCR multiplex procedures have been developed in which the targets are detected by means of hybridization techniques (ResPlex II, Luminex X-TAG, among others), and which allow us to simultaneously examine the presence of multiple respiratory pathogens, as well as several subtypes of influenza viruses.52
- (c)
The immunochromatographic methods that were commercially available before eclosion of the pandemic virus generally lack sensitivity in diagnosing the new influenza A/H1N1 virus (11–80%), and do not allow us to differentiate among the different subtypes of the virus.53 The analytical limit of detection of these tests is approximately 1.0×105TCID50/ml.54–56 Their sensitivity is critically dependent on the type and quality of the sample (number of epithelial cells present), the age of the patient (being more useful in children than in adults, particularly when sampling is performed in the first 24–48h after infection, since children eliminate a larger number of viral particles from the upper airway), the delay in obtaining the sample after infection, the rapidity of sample transport, and the care with which the sample is processed.53 Consequently, a negative result of these tests does not rule out the possibility of active infection. New immunochromatographic methods have been developed and marketed that allow the specific diagnosis of infection caused by the pandemic virus, offering a sensitivity of about 30% in nasopharyngeal exudates, and with a limit of detection of between 2.6×104 and 1.0×105TCID50/ml57,58—though they generally have a high positive predictive value.
- (d)
Classical culture in MDCK cells is slow and of low sensitivity. The appearance of cytopathic effects may take 2–14 days. The combined use of a shell vial method (with A549 cells and Mv1Lu cells) and a pool of monoclonal antibodies against different subtypes of influenza viruses (and other respiratory viruses) offers a diagnosis within 24–48h, with a sensitivity comparable to that of classical culture.59,60
- (e)
Direct immunofluorescence with samples obtained from the UA or LA is an adequate alternative to the immunochromatographic tests, with the added advantage of allowing us to assess the quality of the sample obtained. Its sensitivity is approximately 90% compared with the molecular methods when the sample is of optimum quality (>30 columnar epithelial cells per well) and the slides are prepared in centrifuge, allowing the exclusion of inadequate preparations.59,60
- (f)
The indirect specific antibody detection methods, which allow us to establish a confirmative diagnosis after initially elevated levels or the confirmation of seroconversion against the pandemic virus, are performed with the different patient serum samples, which are submitted and stored for processing in parallel—the true interest of these techniques being confined to epidemiological studies.
The viral load in the UA reaches its peak during the first two days after onset of the symptoms, and quickly decreases over time, in direct relation to the increase in local and systemic neutralizing antibodies, which can be quantified using seroneutralization procedures. In uncomplicated cases we usually do not detect viral RNA in the UA at the end of the first week after symptoms onset.50,51 However, children and immune depressed patients can excrete infective virus for a longer period of time. Taking these variables into account, respiratory sampling should be carried out as soon as possible after appearance of the symptoms, without excluding samples at later timepoints, depending on the type of patient involved (e.g., complicated cases with severe respiratory symptoms in patients admitted to the ICU).
Which are the most appropriate samples and how are they obtained?Nasopharyngeal aspirate offers a greater diagnostic yield than nasopharyngeal, oropharyngeal or nasal exudate,50,51 though a recent study has shown that the combined use of nasal and pharyngeal exudates affords the same diagnostic yield as nasopharyngeal aspirate.61 According to the recommended method, nylon flocked swabs are preferable to conventional swabs, since they are more effective in collecting epithelial cells.62 In the case of intubated patients in the ICU, it may be useful to process specimens from the LA (due to the greater viral load involved),27 in all cases taking the necessary precautions against aerosols in BAL or BAS. Even in the event of impossible intubation due to the severity of the patient condition in admissions to the ICU we could combine the initial nasopharyngeal exudate or aspirate yielding negative results with the processing of tracheal aspirate samples, thereby establishing a confirmatory diagnosis.63 However, the diagnostic efficacy of the molecular and immunochromatographic tests applied to these LA samples has not been sufficiently evaluated to date.
What patients are amenable to diagnosis?In the event of clinical suspicion, the collection of respiratory samples for establishing an etiological diagnosis of influenza A/H1N1 should be carried out following the methodology described above—it being generally advisable to limit sampling and microbiological processing to those cases of infection caused by the pandemic virus in patients who require hospitalization. The necessary diagnostic tests should be made in the Department of Microbiology, and the ultimate choice of test depends both on the characteristics and intervention possibilities defined by the type of patient and/or sample involved, and on the availability of the different techniques in each hospital center. Where available, the RT-PCR molecular methods are the recommended first choice, and the results should be available within 24h after sample reception. Due to its rapidity, high positive predictive value and easy performance, an alternative first choice could be an immunochromatographic technique capable of specifically detecting the pandemic virus. However, if this option is chosen, we must remember that because of its lesser sensitivity, a negative result in this case does not necessarily imply the absence of active infection. Consequently, the samples must be kept under conditions suitable for processing with the molecular nucleic acid amplification techniques, which can ultimately confirm the diagnosis. Clearly, in the case of initially negative microbiological test results (including RT-PCR) in seriously ill patients admitted to the ICU with strong clinical suspicion, we should attempt to establish an etiological diagnosis through processing of the samples, including LA samples where possible. In any case, the clinical picture and patient course will ultimately guide the management decisions.27,63
Lastly, in 2009 the prevalence of influenza A/H1N1 strains resistant to oseltamivir64 (H275Y mutation) was less than 1%. Genotyping techniques for the detection of resistance to oseltamivir are to be preferred over functional viral neuraminidase inhibition (phenotypical) methods, which are only available in specialized centers. Accordingly, it is not advisable to include resistance studies in the routine microbiological diagnostic protocol applied to processes of this kind.
ConclusionMolecular methods based on nucleic acid amplification techniques (RT-PCR) are the gold standard for diagnosing influenza A/H1N1 infection. The immunochromatographic techniques are of limited sensitivity, as a result of which negative readings do not discard the possibility of active infection. Classical cell cultures both lack sensitivity and are slow. Direct immunofluorescence offers a sensitivity of 90%, but requires a good quality sample. The indirect antibody detection methods are only of epidemiological interest.
Usefulness of the laboratory test findings in assessing severity and prognosisThe influenza A/H1N1 pandemic has represented an important challenge for healthcare professionals. The initial clinical picture of influenza A/H1N1 is very similar to that of seasonal influenza or even nonspecific (influenza-like) viral processes.65,66 Its rapid evolution, with serious respiratory complications, made it necessary among other aspects to investigate which laboratory variables could help quickly identify those patients likely to suffer a poorer clinical course.67–69 The first studies made in this sense found increased levels of lactate dehydrogenase (LDH) and creatine phosphokinase (CPK) to be more frequent in patients with confirmed influenza A/H1N1 than in those with a negative diagnosis despite the presence of similar clinical manifestations. Important elevations in C-reactive protein (CRP),67 as well as relative leukopenia,68 have also been related to the presence of influenza A/H1N1 disease. However, there is little information on the potential impact of these alterations upon the assessment of critical patient severity and prognosis.
Different studies (Table 2) have described the laboratory test profile of patients with influenza A/H1N1. Chan et al.68 studied 50 patients with influenza A/H1N1, and found most of them (82%) to have leukocyte counts within normal limits—only three patients showing counts above 11.1×109/L. The authors underscored that although lymphopenia (≤21% of the total leukocytes) was a common finding, it was not severe. On the other hand, thrombocytopenia was infrequent, since only four patients (8%) presented platelet counts of under 150×109/L. However, a communication69 referred to 25 patients described a substantially higher frequency of relative lymphopenia in patients with influenza A/H1N1, affecting 92% (23/25) of the patients despite the fact that there were no subjects with leukopenia under 3.9×109/L.
Laboratory test values in patients with influenza A/H1N1 infection according to different authors.
| Variables | GETGAG | Chan68 | Zarogoulidis65 | Shlomai70 | Cao71 | Kumar11 | Pérez-Padilla72 | Domínguez-Cherit44 |
| Leukocytes (×109/L) | 8.3 (5.2) | 7.1 (5.2–8.8) | 7.1 (4.7) | 14.9 (10.5–22.0) | 3.4 (0.2) | 9.4 (10.0) | 6.0 (3.1–22.2) | 9.9 (5.9) |
| Platelets (×109/L) | 190.0 (19.0) | 239.0 (186–284) | NI | 458 (262–724) | 201.2 (59.0) | 189.0 (87.0) | NI | 222.0 (112.0) |
| LDH (U/L) | 870 (1200) | NI | NI | 833 (607–1096) | 184.8 (47.7) | NI | 1226 (594–3874) | NI |
| CPK (U/L) | 483 (660) | NI | NI | 922 (90–1945) | NI | 243 (99–922) | 366 (58–2156) | NI |
| Creatinine (mg/dL) | 1.15 (1.73) | 0.89 (0.73–1.05) | NI | NI | 0.75 (0.22) | 0.73 (0.5–1.1) | NI | 1.0 (0.8–1.8) |
| CRP (mg/dL) | 29.3 (32.2) | 17.1 (6.4–18.1) | 5.7 (4.5) | 16.5 (10.5–19.6) | NI | NI | NI |
CPK: creatine phosphokinase; GETGAG: Spanish Severe Influenza A Work Group; LDH: lactate dehydrogenase; NI: not informed; CRP: C-reactive protein.
Values expressed as mean and standard deviation, or with the 25–75% interquartile range.
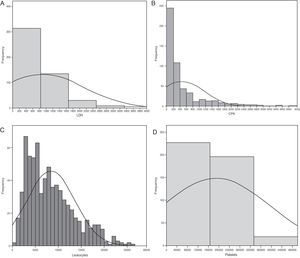
The Spanish experience during the 2009 influenza A/H1N1 pandemic and posterior 2010–2011 epidemic is a little different (unpublished data obtained from the database of the GETGAG/SEMICYUC). In over 1400 included patients, and despite the serious lung conditions observed in most of them, 72% presented LDH levels of under 1000U/L (Fig. 1A). Only 4% of the patients showed LDH>1500U/L, and in these subjects the mortality rate was 33.3% – this being significantly higher than in the patients with LDH upon admission <1500U/L (15.3%; p=0.001; odds ratio [OR]=2.76; 95% confidence interval [95%CI] 1.45–5.20). On the other hand, 28% of the patients had plasma CPK levels of over 500U/L (Fig. 1B), but only 3% reached values of over 1000U/L. The mortality rate did not differ on taking this concentration as cutoff point (19.5% vs 16.8%; p=0.15). Leukopenia, defined as a leukocyte count ≤3000×109/L, was present in only 14% of the patients (Fig. 1C). The mortality rate among the patients with leukopenia (24.8%) was higher than in those without leukopenia (16.4%), but the difference only bordered upon statistical significance (p=0.058; OR=1.67; 95%CI 0.98–2.83). Lastly, 10% of the patients had platelet counts of under 100×109/L (Fig. 1D). The mortality rate in these patients (31.9%) was significantly higher than in those without thrombocytopenia (14.8; p=0.003; OR=2.70; 95%CI 1.30–5.54). In the study published by Pérez-Padilla et al.,72 11 of the 18 patients (61%) suffered lymphopenia (<1.0×109/L). In this small group of patients the authors moreover detected very high levels of LDH (>1000U/L) in 10 patients (62.5%), and of CPK in 5 subjects (31.2%). It is possible that the data of this study were strongly influenced by the small sample size involved (possibly comprising the more seriously ill patients, since they represented the first hospitalized cases). The results therefore must be interpreted with caution. Moreover, all the studies evaluated the global patients, including those with hematological diseases or immune depression – a fact that likewise makes adequate interpretation of the findings difficult. A recent study73 in children suggests that while lymphopenia appears to be more frequent in adults, the fact that children with influenza A/H1N1 infection present leukopenia with relative frequency, and that when leukopenia is present it is moreover intense (<2.5×109/L), may help differentiate those children at a high risk of infection, with a sensitivity of over 80%. The multivariate analysis made in the Spanish population of critical patients with influenza A/H1N1 in an attempt to establish which biomarkers are independently related to mortality found none of the parameters to be associated to increased mortality (data not published) (Table 3).
Histograms showing the frequency of patients with influenza A/H1N1 infection, and the different plasma levels of the biomarkers considered in the analysis. (A) Lactate dehydrogenase; (B) creatine phosphokinase; (C) leukocytes; (D) platelets. CPK: creatine phosphokinase; LDH: lactate dehydrogenase. All values are considered upon admission to the Intensive Care Unit.
Variables independently associated to mortality in the 758 non-immune compromised critical patients with influenza A/H1N1 infection included in the study.
| Variable | OR | 95%CI | p-Value |
| APACHE II upon admission (per point) | 1.08 | 1.01–1.15 | 0.023 |
| SOFA upon admission (per point) | 1.20 | 1.06–1.37 | 0.005 |
| Age (per year) | 0.99 | 0.96–1.02 | 0.81 |
| Basal LDL | 1.00 | 1.00–1.001 | 0.19 |
| Basal platelets | 1.00 | 1.00–1.00 | 0.81 |
| Basal creatinine | 0.64 | 0.38–1.06 | 0.08 |
APACHE II: Acute Physiology and Chronic Health Evaluation; CI: 95% confidence interval; LDH: lactate dehydrogenase; OR: odds ratio.
From the existing data we can conclude that although patients with influenza A/H1N1 infection can present relative leukopenia, with elevations of LDH, CPK and PCR, these laboratory test parameters are not independently related to the patient prognosis. However, plasma LDH>1500U/L and the presence of thrombocytopenia could define a population of patients at risk of suffering serious complications.
Antiviral treatment: when, which, what dose and what impact upon mortality?Most of the available information on the efficacy of the antiviral drugs that inhibit neuraminidase (NA) have been obtained from studies of patients with mild or moderate disease.70–80 In this population, the administration of oseltamivir was associated to a reduction in time to elimination of the virus,78 with a lesser duration of the disease and of the fever,79 and fewer secondary complications.80 However, the true impact of antiviral treatment in critical patients is difficult to establish. In view of the lack of randomized studies in these patients, the data obtained from observational studies during the influenza A/H1N1 pandemic offer the best available evidence to date. These recommendations only refer to patients admitted to the ICU, excluding those admitted to other hospital areas and the pediatric population.
When should we administer oseltamivir?Different health organizations4,10,81 coincide that antiviral treatment should be started as soon as possible in all “possible” or “confirmed” cases of influenza A/H1N1 requiring hospitalization, in patients with severe progressive disease, or in the presence of complications, and that this should be done independently of the previous patient health condition or antecedents of vaccination. However, in Spain the mean time to administration of the antiviral agent from the onset of symptoms was 4.3 days during the influenza A/H1N1 pandemic,82 with no differences between the patients who survived (4.1 days) and those who died (4 days). This time interval was a little shorter than that reported by Domínguez-Cherit et al.44 in Mexico (6 days), but longer than that recorded by Jain et al.83 in the United States (3 days) and by Cao et al.71 in China (1 day). Surprisingly, during the influenza epidemic of the winter of 2010, the mean delay in starting antiviral therapy was even longer (5 days on average).37
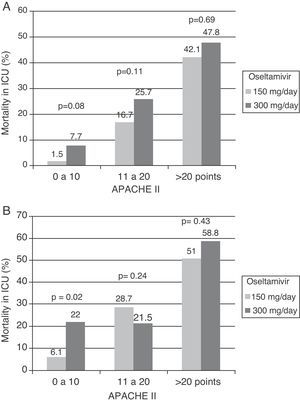
Which antiviral drug, at what dose and for how long?According to the available data referred to the epidemiological vigilance and control of resistances in recent years, oseltamivir and zanamivir remain the antiviral drugs recommended for the treatment of influenza A/H1N1 infection, since over 99% of the currently circulating viruses are sensitive to these agents.84,85 Amantadine and rimantadine are other antivirals that have been associated to treatment with oseltamivir during the pandemic but should not be used, due to the high resistance rates against these agents found among the circulating influenza A/H1N1 strains. Oseltamivir is a neuraminidase (NA) inhibitor which after absorption in the gastrointestinal tract is quickly converted to its active form, oseltamivir carboxylate (OC), with a bioavailability of over 80% and important penetration of the lung tissues.86 The recommended adults dose is 75mg twice a day, via the oral or enteral route. Since OC is mainly excreted through the kidneys, the dosage must be adjusted to the renal function of the patient. In practical terms, in patients with a creatinine clearance of between 10 and 30ml/min, the dose should be only 75mg/day. There is limited experience with the effectiveness of oseltamivir administered via the enteral route in critical patients, which may suffer alterations in gastric motility, and lesser intestinal absorption of the drug. This situation in turn can be worsened by the increase in distribution volume observed in such patients, particularly when mechanical ventilation is required—giving rise to higher plasma drug levels and therefore to lesser antiviral effectiveness. Some authors87,88 have suggested the need to maintain high plasma concentrations or to secure areas under the concentration–time curve (AUC) equivalent to over 50% of the maximum inhibitory concentration (MIC) for the influenza virus, in order to ensure optimum suppression of viral replication. Following the recommendations of the WHO,89 over 70% of the patients admitted to Spanish ICUs during the influenza A/H1N1 pandemic received high-dose oseltamivir (300mg/day). However, the administration of higher oseltamivir doses was not associated to lesser mortality82 (Fig. 2A and B). These findings are consistent with those of a recent study that analyzed the enteric absorption of oseltamivir in critical patients.90 The authors found that the usual doses of 150mg/day resulted in similar plasma concentrations in both critical patients and outpatients. They therefore concluded that the standard dose is sufficient for reaching the desired concentrations in critical patients. However, some patients showing a lack of treatment response to oseltamivir or who cannot receive the medication via the enteral route because of intolerance can be candidates for treatment with an alternative antiviral agent. In this context, zanamivir is the other NA inhibitor that has been approved for the treatment of influenza A/H1N1. In contrast to oseltamivir, it is not absorbed via the enteral route, and therefore must be administered as a powder for inhalation. While suitable for patients with less serious disease, this drug formulation cannot be used in ventilated critical patients. Only 10–20% of the inhaled dose is absorbed, and the extrapulmonary distribution of the drug is minimal.86 The adult dose is 10mg every 12h, and although there are no clear data, it should be lowered in patients with severely impaired kidney function. Zanamivir cannot be nebulized in intubated patients, since it can cause obstruction of the respirator circuits, severe bronchospasm and death.91 Zanamivir via the intravenous route has been suggested as an alternative in patients who fail to respond to oseltamivir or who have developed resistance. Because of the medical emergency posed by the influenza A/H1N1 virus, this formulation has received special treatment, and has been used on a compassionate basis. Few conclusions can be drawn from the limited published cases, since in all instances the drug was used as rescue therapy. A recent report on 6216 cases documented and hospitalized during the pandemic in the United States found only 8patients to have received zanamivir via the intravenous route. In Spain, the use of zanamivir via the intravenous route was anecdotal during the pandemic (0.7%), but increased to 6.4% (33 patients) during the post-pandemic epidemic of 2010. This increase in use does not imply the appearance of resistances during this phase; rather, it reflects the data generated by a safety study on zanamivir conducted during that period. Until further information becomes available, zanamivir via the intravenous route would only be indicated in patients with strongly suspected or confirmed influenza A/H1N1 infection resistant to oseltamivir. Lastly, peramivir, an investigational antiviral drug that received exceptional approval during the pandemic period, has been used with apparently good results in the United States92 and China.93 Developed for intravenous administration, this drug can be used in critical patients without the potential complications of the enteral route. A daily dose of 300–600mg has been found to be safe and lessens the symptoms of the disease, though its impact upon mortality is still difficult to define. Theoretically, and if supported by new results, this would be the antiviral drug of choice in critical patients, due to the possibility of intravenous administration.
The duration of antiviral treatment in critical patients is another controversial issue. In patients with mild or moderate disease, the recommended duration is no more than 5 days.4,81,84,85 This is related to the time to viral clearance from the airways (about 4–5 days from onset of the symptoms). However, in immune depressed patients or subjects with major comorbidities, as well as in critical patients, this viral elimination period can be significantly extended. Leekha et al.94 found over one half (54%) of the patients with influenza A to remain positive for the virus after 7 days of treatment, as determined by PCR. No studies allow us to conclude when to suspend antiviral treatment in seriously ill patients, though in the Spanish ICUs the patients received a median of 10 days of treatment.44,71,82,83,95 Recent studies have described much longer PCR positivity for the virus in critical patients, particularly in those with pneumonia. Ling et al.15 found the mean elimination time to be 6 days, though the virus was still detected in 37% of the patients by day 7, with no apparent relationship to early therapy. In turn, Meschi et al.96 found the presence of pneumonia to be associated with a longer viral clearance time. The authors showed PCR testing to remain positive in 100% of the patients with pneumonia on day 9 of treatment, and in 67% of the patients after 10 days. Although the persistence of viral elimination does not necessarily imply infectious activity, from the health safety perspective we suggest extending treatment at least 10 days in critical patients who evolve favorably, or until negative conversion of the PCR findings. In the case of slowly evolving cases with persistently positive PCR findings, a respiratory sample culture should be obtained to confirm non-viability of the virus, with the suspension or not of treatment, and also to discard the appearance of resistance during treatment.
Impact of antiviral treatment upon mortality and other evolutive parametersAs has been mentioned, few studies have examined the impact of antiviral treatment upon patient mortality during the pandemic of 2009. The use of antiviral agents during the pandemic was based on the cumulative experience gained with seasonal influenza (1970–2008), where early treatment with NA inhibitors (i.e., administered within 48h of symptoms onset) was seen to reduce the severity and duration of the disease and possibly also the risk of complications.79,80 However, in contrast to seasonal influenza, the severe cases of influenza A/H1N1 show a different behavior, not only because this virus affects younger people, but also because it has been shown to be able to produce rapid and serious lung problems – including the development of ARDS and death secondary to refractory respiratory failure. In reference to this special context it is difficult to extrapolate the impact of antiviral treatment from studies in which the patients suffer only mild or moderate influenza infection.
A recent review95 of 11 published studies found that the complications of influenza A/H1N1 can be lessened by the use of antivirals in both high and low risk patients—though the reviewed series involved important methodological limitations. In turn, Jain et al.83 found the only variable independently associated to mortality to be the administration of oseltamivir within the first 48h, while Domínguez-Cherit et al.44 observed that those patients who received antiviral drugs had a greater probability of survival (OR=7.4). Recently, three retrospective studies97–99 have evaluated the impact of treatment during the pandemic. Hiba et al.97 studied 506 hospitalized patients and found early treatment to be associated with a lesser incidence of complications and with lesser in-hospital mortality. However, this study only included a minimum number of critical patients (n=30; 12.4%). In turn, Higuera Iglesias et al.98 have analyzed the clinical course of 442 patients administered with oseltamivir during the pandemic in Mexico. The authors found that both the probability of developing pneumonia and the risk of suffering severe pneumonia increased with the length of delay in administering the antiviral drug. However, only a little over one half of the patients (54.4%) were hospitalized, and no mention was made of how many of these subjects required admission to the ICU. Lastly, Yu et al.,99 in over 1200 hospitalized but non-critical patients, found early treatment to be associated with a lesser development of radiological infiltrates and with a reduction in the viral elimination time.
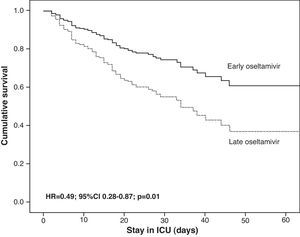
The GETGAG/SEMICYUC has published the only prospective study examining the impact of early treatment with oseltamivir during the pandemic in a large number of critical patients (n=657).82 This study found early treatment with oseltamivir to imply increased survival, but only in the more seriously ill patients, i.e., those with invasive mechanical ventilation who had received effective antiviral treatment (at least 4 doses). Furthermore, the impact was so notorious that one life could be saved for every 8 patients receiving early treatment with oseltamivir. These data derived from multivariate analysis were also confirmed by the corresponding propensity score, a statistical model that calculates the probability that a patient will receive early or late therapy, with adjustment for the possibility that the observed differences in clinical course are due to differences between groups and not to greater efficacy of early treatment (Table 4). However, the impact of antiviral treatment in less seriously ill patients (e.g., without mechanical ventilation) is difficult to establish, since the mortality rate recorded in these patients is low. Therefore, most studies center their primary objectives or endpoints on the time to resolution of the respiratory symptoms or fever, instead of on mortality. In the post-pandemic period during the winter of 2010–2011, and despite the observation of an increased delay in administration of the antiviral medication,37,74 early treatment with oseltamivir was also associated with an improved outcome and a 50% reduction in the risk of death (hazard ratio (HR)=0.49; 95%CI 0.28–0.87; p=0.01) (Fig. 3) (non-published data).
Mortality at discharge from the Intensive Care Unit according to the study population considered.
| Study population | Antiviral treatment | Mortality difference | OR | 95%CI | p-Value | |
| Early | Late | |||||
| Global | 15.9% | 23.1% | 7.2% | 1.45 | 0.96–2.21 | 0.06 |
| Non-ventilated | 1.6% | 2.6% | 1.0% | 1.65 | 0.19–13.9 | 0.62 |
| Invasive ventilation | 25.1% | 34.6% | 9.5% | 1.34 | 0.90–1.97 | 0.08 |
| Study groupa | 21.5% | 34.3% | 12.8% | 1.90 | 1.06–3.41 | 0.04 |
95%CI: 95% confidence interval; OR: odds ratio.
Patients with mechanical ventilation and effective antiviral therapy.
Adjusted survival analysis (Cox regression) in 397 critical patients admitted to the Intensive Care Unit in the post-pandemic period according to whether oseltamivir was administered early (≤48h) or late (>48h). The censored model after 60 days showed survival to be significantly greater in patients with early administration of the antiviral agent. ICU: Intensive Care Unit.
Lastly, we agree with the CDC (81) that patients with a high suspicion of influenza A/H1N1 should continue with treatment, independently of the negative results of the initial tests, unless an alternative diagnosis can be established or the clinical criteria suggest a low probability of influenza.
ConclusionAlthough there is a lack of randomized and controlled studies on the effect of antiviral treatment in critical patients with severe influenza A/H1N1 infection, the experience gained in these last two years allows us to recommend early administration of the antiviral drug (oseltamivir) (<48h from symptoms onset), at a dose of 75mg every 12h and with a duration of at least 7 days, or until clinical improvement becomes evident. The existing antivirals, particularly those formulated for intravenous administration, could prove to be the drugs of choice in future epidemics.
Antimicrobial treatment for bacterial coinfection. When, which treatment, and what is the impact upon mortality?Between 3 and 30% of all patients with viral pneumonia due to influenza A/H1N1 present bacterial coinfection at the time of admission.27,75,100,101 A number of studies have demonstrated a time relationship between the onset of influenza infection and bacterial pneumonia—this being attributed to possible synergy between the influenza virus and certain respiratory pathogens (particularly Streptococcus pneumoniae [S. pneumoniae]), which favors the presence of bacterial coinfections or overinfections.102–105 The present section examines the importance of bacterial coinfection in critical patients infected with influenza A/H1N1 and admitted due to respiratory failure, with the purpose of establishing: (1) the epidemiology of bacterial coinfection; (2) how to diagnose coinfection and what antibiotic treatment to use; and (3) the influence upon the patient prognosis.
EpidemiologyPrimary viral pneumonia is defined as the appearance of lung infiltrates (condensations) during the acute phase of influenza infection and affecting two or more lung lobes, with respiratory sample and blood cultures proving negative for bacterial growth. Confirmation of viral pneumonia due to influenza A is assumed when in addition to the above, identification of the influenza A/H1N1 virus is established by RT-PCR or virological culture.
Community-acquired or healthcare-related respiratory coinfection is defined as any bacterial respiratory infection diagnosed in a patient with influenza A/H1N1 within the first 48h of hospitalization. After this time period, bacterial coinfection is taken to be nosocomial.75
Bacterial coinfection has been associated to viral infection. The condition can manifest as simultaneous infection (coinfection) or as a complication secondary to influenza infection, manifesting when the viral infection is already in the resolution phase. Classically, Staphylococcus aureus (S. aureus) has been implicated in bacterial coinfection that tends to take a slow or torpid course, with lung infiltrations showing early cavitation (<72h), and which implies important mortality. In contrast, S. pneumoniae, Haemophilus influenzae (H. influenzae) and other streptococci tend to be responsible for later conditions manifesting in those patients who initially improve of their influenza infection and who after approximately one week experience clinical worsening (return of fever), with the appearance of new condensations on the chest X-rays. In these patients the prognosis tends to be more favorable.101
The incidence of detection of coinfection during the influenza A/H1N1 pandemic has varied among different countries. In Canada and Australia the detection rates were 32% and 20%, respectively, while in Spain the percentage reached 17.5%—this figure being considerably lower than the 28.6% recorded in a necropsy-based study in the United States. However, this study, which included 77 patients, had important design limitations, since the included cases did not correspond to a systematic analysis, and thus the series may not have been representative of patients with influenza A/H1N1 infection. Consequently, the observed frequency of coinfection cannot be taken as a measure of the prevalence of this complication, though it does confirm the participation of coinfection in the patients who have died.27,29,75,100
S. pneumoniae, H. influenzae and S. aureus were the most frequently isolated pathogens in patients with bacterial coinfection. Specifically, in Spain, S. pneumoniae was found to be responsible for coinfection in 54.8% of the cases, followed by methicillin-sensitive S. aureus (8%) and Streptococcus pyogenes (5.3%).
How to diagnose the condition and what antibiotic treatment to useDuring pandemic situations or in the period of seasonal influenza, patients with flu syndrome and acute respiratory failure presenting lung infiltrations and clinical manifestations of pulmonary infection, and who require hospitalization or admission to the ICU, should receive empirical antibiotic treatment (ATB) concomitant to antiviral therapy. In order for this strategy to minimize ATB overuse, it is necessary to either confirm or rule out the presence of coinfection by obtaining good quality respiratory samples (from deep locations if possible) immediately after orotracheal intubation, and whenever blood cultures and urinary antigen tests for S. pneumoniae and Legionella spp. are made.
There are no clinical or epidemiological data of help in distinguishing patients with bacterial coinfection. While the chest X-ray findings are nonspecific, they sometimes may provide an orientation as to the possible existence of bacterial coinfection. Specifically, if the interstitial lung condensations are accompanied by images of alveolar occupation (nodular or pseudonodular) or cavitations, the presence of bacterial coinfection should be suspected, particularly in the form of S. aureus. Although the presence of lobar or segmental infiltrates is suggestive of bacterial infection (despite their limited specificity), there have been descriptions of lobar condensations exclusively attributable to viral pneumonia due to influenza A/H1N1.101,106
Some biomarkers, especially procalcitonin, could be useful for deciding the start or early suspension of antibiotic treatment (ATB) in the case of negative bacterial cultures and antigenuria. Recently, a French multicenter study has suggested that procalcitonin <0.8μg/L offers a sensitivity of 91% and a specificity of 68%, with a negative predictive value of 91%, in reference to bacterial coinfection. Therefore, the absence of lobar condensations and a procalcitonin concentration of <0.8μg/L, together with the clinical course of the patient, could be used to decide the early discontinuation of ATB in the presence of negative microbiological findings.107
In adult patients, the empirical antibiotic treatment would be that recommended by the national guides of the SEIMC/SEMICYUC/SEPAR.108,109 In the case of patients with community-acquired pneumonia admitted to the ICU, the recommendation is a third generation cephalosporin (ceftriaxone or cefotaxime) plus a macrolide (some data suggest that the use of macrolides exerts an immune modulating effect, with an increase in the respiratory mucosal secretion of antiviral Ig A) or a fluoroquinolone. In the case of images suggestive of cavitation, or where gram staining shows a predominance of staphylococcus-type grampositive cocci, a switch to cloxacillin would be advisable. In Spain, empirical coverage for methicillin-resistant S. aureus (MRSA) would not be needed, except when the patient lives in a home for the elderly/sociosanitary center, or is known to have been previously colonized by MRSA. In such cases it would be advisable to add linezolid to the empirical treatment. In the case of patients with COPD, bronchiectasis, cystic fibrosis, or previous Pseudomonas aeruginosa colonization, we should administer an antipseudomonal betalactam (cefepime or piperacillin–tazobactam) instead of a third generation cephalosporin, associated to another ATB with action against P. aeruginosa, plus the macrolide.
Influence upon the prognosisThe mortality rate among patients with influenza A/H1N1 infection who require admission to hospital is in the order of 6–7% vs 18–41% in those requiring admission to the ICU. Specifically, in the series of patients admitted to the ICU in Spain, the mean mortality rate among those patients requiring ventilation support was 30%.11,21,27,72
There is some controversy regarding the impact of bacterial coinfection upon the prognosis of patients with influenza A/H1N1 infection. The postmortem studies made in patients affected by other pandemics, as well as the recently published information referred to patients with influenza A/H1N1 infection,100 have shown that an important number of deaths attributed to the viral infection corresponded to patients who simultaneously presented bacterial infections. However, in epidemiological studies such as the Spanish multicenter survey that analyzed the impact of coinfection upon mortality in the ICU during the recent pandemic, multivariate analysis failed to identify the presence of coinfection as an independent factor correlated to mortality—though it was associated to a significant increase in the duration of stay in the ICU, and to increased resource consumption (days on mechanical ventilation and use of vasoactive drugs).75 Palacios et al.110 have published similar results. The authors found coinfection with S. pneumoniae to be associated to increased severity. This apparent discrepancy could be related to the limitations of postmortem studies in assessing the impact of a disease, since they only express the frequency of presentation of a given disorder in samples that are usually not representative of the population under study.
ConclusionIn patients with pneumonia due to influenza A/H1N1, and given the possibility of bacterial coinfection, the prescription of empirical antibiotic coverage is advised (associating a betalactam with a macrolide), administered as soon as possible. The results of the cultures and the clinical or laboratory test findings will serve to decide suspension or continuation of the antibiotics. As a preventive measure, antipneumococcal vaccination in the population at risk is advised.
Coadjuvant corticosteroid treatment for the management of acute respiratory distress syndrome secondary to viral pneumonia due to influenza A/H1N1. When, why and what dose, and what is the impact upon mortality?Exogenous corticosteroids have potent antiinflammatory effects and have been used to modulate the host response for decades. Although these drugs classically have been accepted as treatment for refractory septic shock in the context of the measures designed to deal with severe sepsis,111 recent studies have questioned their benefit in terms of patient survival even at low doses.112 On the other hand, the efficacy of systemic corticosteroids has been extensively studied in ARDS. While these drugs play a clear role in situations where ARDS has been triggered by processes that respond to corticosteroids (e.g., acute eosinophilic pneumonia), in most cases the usefulness of corticosteroid therapy remains uncertain.113 In the 1970s and early 1980s, empirical corticosteroid therapy was widely used to treat ARDS, though this practice posteriorly became less frequent after several studies found that corticosteroids afforded no benefits and could even prove harmful.114,115 Since then, several metaanalyses and reviews have been published, offering conflicting perspectives in relation to corticosteroid therapy for ARDS.116–119
The most frequent pulmonary presentation in patients with influenza A/H1N1 infection is rapidly progressing viral pneumonia with bilateral alveolar infiltrates on the chest X-rays, and ARDS.120 The presentation of ARDS with severe refractory hypoxemia has been particularly common in patients with this disease process, and could be linked to an abnormal immune response.121 Several reports on the influenza A/H1N1 pandemic44 have described the use of empirical corticosteroid therapy in over one half of these patients, both as primary treatment and as rescue therapy in individuals with severe ARDS. The recent guidelines for the treatment of pandemic influenza A/H1N1 infection recommend that corticosteroids should not be used on a routine basis, although low doses can be considered in patients with septic shock who require vasopressors and are suspected to have adrenal gland insufficiency.10,89 Nevertheless, the supporting data remain scarce and controversial.122
A recent study123 has proposed the use of corticosteroids at moderate to low doses, in view of the resulting significant improvement of the lung damage. However, these results are very difficult to interpret, due to the important design limitations and small number of patients included in the study. Following the mentioned publication, different groups carried out multicenter analyses of the effects of the use of corticosteroids in patients with influenza A/H1N1 infection. The first of these studies124 was carried out in 220 patients admitted to Departments of Intensive Care Medicine all over the world by means of the registry of the European Society of Intensive Care Medicine, and showed that the use of corticosteroids not only affords no benefit in terms of patient outcome (HR=1.3; 95%CI 0.7–2.4; p=0.4), but moreover increases the rate of in-hospital pneumonia (OR=2.2; 95%CI 1.0–4.8) in those who receive such drug treatment. Likewise, no benefits were observed on performing the analysis in the subgroup of patients with ARDS. Two later studies yielded results pointing in this same direction. Brun-Buisson et al.125 analyzed 208 patients with ARDS without prior corticosteroid administration, and found the use of these drugs to be associated to an increase in mortality (HR=2.82; 95%CI 1.5–5.4; p=0.004) and in the risk of in-hospital pneumonia. Likewise, early corticosteroid treatment (<3 days from the start of mechanical ventilation) was associated to increased mortality. On the other hand, Kim et al.,126 on evaluating 245 critical patients, observed an increased prevalence of invasive bacterial and fungal infections, a longer stay in the ICU, and an increase in raw mortality among the patients who received corticosteroids (58% vs 27%; p<0.001). This impact upon mortality was confirmed by the multivariate statistical analysis, correcting the model for confounding factors (including immune suppression status), since the authors recorded a significant increase in mortality after 90 days (OR=2.63; 95%CI 1.43–4.82) in those who received corticosteroids.
The mentioned articles have some significant limitations that should be commented. Firstly, the populations studied were not comparable in terms of severity, since the patients treated with corticosteroids were more seriously ill, and the lack of benefits of corticosteroid therapy may have been conditioned by this fact. However, the multivariate analysis and propensity scores were adjusted for this and also for other confounding factors. Secondly, both the corticosteroid dosage and the timing of treatment were not standardized, thus further increasing the heterogeneity of the studies and the existing controversy. This aspect has been examined in a recent study by the GETGAG, analyzing only the impact of corticosteroid treatment upon viral pneumonia, and excluding patients who received corticosteroids as part of shock therapy, due to possible adrenal gland insufficiency in critical patients. This study likewise found no benefits of treatment with corticosteroids (data in press).
ConclusionOn the basis of the existing information, there is not enough scientific evidence to recommend the use of corticosteroids in patients with viral pneumonia due to influenza A/H1N1, and we must wait for conclusive data from randomized, double-blind trials that avoid the mentioned confounding factors. However, the only such study in course (Low Dose Corticosteroids as Adjuvant Therapy for the Treatment of Severe H1N1 Flu) has been suspended because of low patient recruitment rates.
Respiratory management of patients with acute respiratory failure due to influenza A/H1N1Acute lung injury (ALI) and ARDS are characterized in their early stages by an important local inflammatory response produced by intra- and extrapulmonary stimuli that give rise to considerable mortality. Such mortality has decreased in recent decades127 thanks to improved knowledge of the physiopathology of the disease and to global improvements in supportive treatment—though the percentage remains between 30 and 50%.128 In 90% of the cases, mortality in these patients is due to multiorgan dysfunction syndrome,127,129 and only 10% of the subjects die because of refractory hypoxemia.130 Thus, mortality in this syndrome is closely associated to the appearance of dysfunction affecting organs other than the lungs—fundamentally acute renal failure.127,130
Respiratory involvement during the pandemic had 5 main forms of presentation: (1) viral pneumonitis or primary viral pneumonia with severe ARDS; (2) asthmatic exacerbation episodes; (3) COPD exacerbation episodes; (4) bacterial coinfection associated to viral infection; and (5) bronchiolitis episodes in pediatric patients.
In Spain, viral pneumonitis with ARDS was present in 72.5% of the patients admitted to the ICU. The mortality rate was 33% in the subjects requiring intubation and ventilatory support.131 Moreover, in an important percentage of the cases, mortality could be directly attributed to refractory hypoxemia. A special characteristic of ARDS in this context was the large percentage of cases diagnosed in young patients, with a global median age of 35 years and no associated comorbidities (between 30 and 65% according to the different series). These patients were especially pregnant women and obese individuals, defined by a body mass index (BMI) of over 30kg/m2. In 40% of the cases the clinical manifestations of ARDS were associated to radiological findings in the form of opacities affecting all four lung quadrants (Fig. 4)—generally in association to early multiorgan failure, with septic shock and acute renal failure, and also other extrapulmonary manifestations of the disease (myocarditis, rhabdomyolysis and encephalitis).
Histologically, lung damage normally consists of diffuse alveolar alterations associated to necrotizing bronchiolitis and extensive hemorrhagic zones. Other findings are cytopathic damage and necrosis of the bronchial and alveolar epithelial cells, as well as necrosis, epithelial hyperplasia and squamous metaplasia of the large airways. Likewise, lung tissue studies have observed aberrant pulmonary immune responses with important expression of TLR-3 and IFN-gamma, and the invasion of CD8+ T and B cells.132
In view of the appearance of an important number of patients with severe ARDS and the need for prolonged ventilatory support in the ICU, different contingency plans were developed and different therapeutic options were analyzed for application in these patients. The contingency plan of the SEMICYUC (www.semicyuc.org/sites/default/files/plan_de_contingencia_gripe_a_semicyuc.pdf), published in 2009, proposed a series of norms for the management of these patients, to be updated in the light of the different studies published on the pandemic. As regards the respiratory management of these patients, mention can be made of the following aspects contemplated in the contingency plan.
Noninvasive ventilationThe role of noninvasive ventilation (NIV) in these patients has been the subject of controversy. On the basis of the evidence available at the time, the recommendation of the contingency plan of the SEMICYUC was to not use NIV in patients with acute respiratory failure secondary to ARDS due to influenza virus A/H1N1 infection, except in isolated cases. Approximately 30% of these patients were initially subjected to NIV, with a high failure rate (75%). The mortality rate among the patients in which NIV failed was 38%, and delays in intubation were associated to an increased mortality risk (OR=1.23; 95%CI 0.8–2.0).133 After carefully analyzing the results, it can be concluded that NIV possibly cannot be regarded as a technique of choice in these patients with ARDS, though it might be useful in centers that have great experience with the technique, and possibly using helmet-type interfaces134 which have been reported to offer good results, though in a very limited number of patients (n=5). In any case, we should follow the general recommendation to secure early intubation in these patients in response to the slightest evidence of NIV failure. This type of support would be viewed differently if the presentation of the disease were not in the form of ARDS.
Protective ventilatory strategiesThere is general agreement on the use of “lung-protecting ventilatory strategies” when mechanical ventilation is invasive. The general principle of these strategies is to optimize the ventilatory parameters so as to avoid the cyclic collapse and opening of the sealed alveolar units as far as possible, and thus also prevent excessive stretching or over-distension of the healthy lung parenchyma. The mechanisms causing damage are shown in Table 5, and the basic principles of protective ventilation destined to minimize all these mechanisms are described in Table 6. Such strategies should be used early in all patients at risk of suffering acute lung injury, in order to avoid lung damage secondary to progression. The mechanical ventilation strategy applied in patients with ARDS in the influenza A/H1N1 pandemic was to use a low circulating volume associated to high positive end-expiratory pressure (PEEP) values, thereby “keeping the lung open”.135 In studies published in Canada and the United States,11,41 between 68 and 80% of these patients were ventilated with controlled modes, involving a circulating volume of approximately 6ml/kg, and a pause pressure target of under 35cmH2O—though some authors report that in a number of cases the circulating volume had to be increased to 8ml/kg, especially in patients with severe hypoxemia. Use was also made of high PEEP values of up to 30cmH2O, which were maintained for a long time, since it was seen that even in the respiratory disconnection phase the patients were very sensitive (in terms of hypoxemia) to even small reductions in PEEP. A special characteristic of this population was the high ventilatory demand, with great difficulties in keeping the patients adequately adapted to the respirator—requiring abnormally high doses of sedative and neuromuscular blockers for prolonged periods of time.
General protective ventilation norms.
| Possible superiority of the pressure controlled ventilation methods |
| Circulating volume <10ml/kg of ideal weight |
| Pause pressure <30cmH2O |
| Respiratory frequency protocolized between 15 and 25rpm |
| FiO2<0.7 if O2 saturation >90% |
| PEEP over 10–12cmH2O (adjusted to lung mechanics and according to clinical response) |
| Use minimum sedation possible |
| Minimize the possibility of derecruitment in the disconnections and endotracheal aspirations |
| Use strategies to reduce the incidence of ventilator associated pneumonia |
PEEP: positive end-expiratory pressure.
Pulmonary recruitment and ventilation in prone decubitus (which combines lung recruitment with improved elimination of the inflammatory lung fluids, avoiding damage secondary to spread) form part of the usual treatment of patients with refractory hypoxemia. Lung recruitment maneuvering, in lungs amenable to recruitment, produces the re-expansion of previously collapsed pulmonary zones via a brief and controlled increase in transpulmonary pressure. The problem is that such maneuvering produces a variable response dependent upon a series of factors such as the type of lesion, the evolutive phase of the lung damage, the severity of the lesion, the prior lung volume history, and the recruitment maneuver applied.136 Recently, systematic reviews have been made137,138 of studies that sum 1170 patients with ARDS, showing significantly improved oxygenation with the use of recruitment maneuvers, but with no evidence of any decrease in mortality (RR=0.73; 95%CI 0.46–1.7), and there moreover are not enough data to analyze their effect upon the duration of mechanical ventilation or hospital stay. Therefore, it can be affirmed that lung recruitment maneuvers, if performed adequately and with sufficient transpulmonary pressure, can improve PaO2 in severely hypoxemic patients, though it is not possible to recommend their generalized use in patients with ARDS. In the case of ARDS due to influenza A/H1N1 infection, there have been individual case descriptions documenting the success of lung recruitment in improving oxygenation, but it is not possible to draw firm conclusions regarding its benefit in this particular patient population. In view of the characteristics of the disorder, it can be affirmed that ARDS due to influenza A/H1N1 infection is associated to important alveolar flooding, with the consequent increase in lung weight—giving rise to models of easily recruitable distress which therefore might benefit from recruitment maneuvers and the use of high PEEP values.
Regarding ventilation in prone decubitus, it can be concluded that there is enough evidence of its efficacy in patients with severe ARDS in terms of both improved oxygenation and a significant decrease in mortality, without increasing the risk of serious complications.139 During the influenza A/H1N1 pandemic, many cases and series were published involving this technique, with good results.140 At present, the use of high-frequency ventilation requires further information in order to improve the existing level of evidence with a view to drawing reliable conclusions. Although a recent metaanalysis published by Sud et al.,141 involving 6 studies and including approximately 190 patients per management arm, has described a significant reduction in mortality after 30 days (RR=0.77; 95%CI 0.61–0.98), more evidence is needed, since this metaanalysis was based on studies in reference centers, not blinded studies, and with a small number of patients. A clinical trial with a larger sample size is currently pending publication and may yield new data with which to establish recommendations.
Another therapeutic measure to be considered in refractory hypoxemia, when pulmonary arterial hypertension is moreover detected, may be inhalatory nitric oxide (NO), which is associated to transient improvements in oxygenation. However, no investigation group has clearly shown NO to improve the patient prognosis.142
ConclusionThe management of ARDS in patients with influenza A/H1N1 infection must be based on lung-protecting ventilation strategies (tidal volume <10ml/kg and plateau pressure <35mmHg) and on the use of high PEEP values adjusted to the lung mechanics of the patient, combined with ventilation in prone decubitus, muscle relaxation and recruitment maneuvers. NIV cannot be regarded as an option of choice in patients with ARDS, though it could prove useful in centers with great experience and in cases of respiratory failure associated to COPD exacerbation or heart failure. Thus, the recommendations of the 2009 contingency plan of the SEMICYUC remain applicable.
Management of the cardiovascular complications of influenza A/H1N1 infectionBetween 24.3% and 62.5% of the patients with influenza A/H1N1 infection admitted to the ICU suffer shock.11,21,27 Although the underlying etiology is fundamentally septic, concomitant cardiac involvement is observed in up to 12% of the patients, and in some of them these cardiac problems are the cause of admission to the ICU and even of patient death.143
EtiologyA number of causes of cardiac involvement have been described in these patients:
- (a)
Cardiac dysfunction secondary to ARDS: hypoxemia and pulmonary hypertension induce right ventricle dilatation, and in some cases dysfunction of both ventricles. Referred to by some authors as acute cor pulmonale,144,145 such dysfunction is observed in 25% of the patients and tends to be reversible as ARDS improves.
- (b)
Myopericarditis: this disorder is the result of direct viral action upon the myocardium. The prevalence of myocarditis in influenza infections varies between 0 and 11%, depending on the definition used.146 In infection due to influenza A/H1N1, many cases of fulminant myopericarditis have been reported in young patients without risk factors, and who required inotropic drugs and vasopressors, and even ventricular assist systems. The clinical picture is no different from that of myocarditis of other causes. Following the viral episode, the patients develop clinical signs of heart failure, together with hypotension and tachycardia. In the more serious cases the condition can evolve towards refractory cardiogenic shock and death.147 The myocardial involvement tends to be reversible provided cardiocirculatory support measures are adopted, together with early antiviral treatment in the form of oseltamivir.
- (c)
Exacerbation of pre-existing cardiovascular diseases: the influenza virus can cause alterations in endothelial function secondary to the systemic inflammatory process, inducing a procoagulant situation. The viral infection is also associated to atheroma plaque development and instability, which in turn can give rise to coronary ischemic episodes.148 A history of heart disease was present in 6–15% of the patients with influenza A/H1N1 infection during the pandemic of 2009.21 These patients may suffer destabilization as a result of the viral infection, with worsening of both systolic and diastolic ventricular function.
The diagnosis is based both on the clinical manifestations and on the complementary explorations and tests. The clinical condition is attributable to cardiac involvement, with a broad range of manifestations—though dyspnea is the most frequent symptom. Lower limb edemas or ascites are rare, and tend to manifest in patients with previous heart disease. The appearance or exacerbation of dyspnea, as well as the presence of signs of heart failure should cause us to suspect concomitant myocardial dysfunction.
Complementary testsElectrocardiogram: electrocardiographic alterations have been described in a large proportion of patients. The most common findings are T-wave inversion and ST-segment depression. These changes are usually transient and are not always correlated to elevations in cardiac enzymes and/or echocardiographic alterations. It is also possible to observe tachyarrhythmias (particularly supraventricular tachyarrhythmias), with a lesser frequency of bradyarrhythmias.149
Biochemical alterationsMyocardial enzymes: troponin elevation is seen in up to 46% of the patients admitted to the ICU. However, not in all cases does this have an impact upon contractile function as determined by echocardiography. Pro-type B natriuretic factor is increased in the presence of both right and left ventricular dysfunction of any cause. Its determination is useful for stratifying the prognosis, evaluating treatment efficacy, and for identifying subclinical cardiac decompensation or the need for invasive hemodynamic monitorization.
EchocardiographyEchocardiography is the technique of choice for the early diagnosis and management of patients with myocardial dysfunction. The most widely used parameters are the left ventricle ejection fraction (LVEF), the tricuspid annular plane systolic excursion (TAPSE) for assessing right ventricle function, and tissue Doppler ultrasound for the simultaneous evaluation of systolic and diastolic function. With tissue Doppler, 54% of the patients with troponin elevation or who require vasoactive drugs presented alterations in left ventricle function.150 Right-side dysfunction associated to ARDS is present in 8% of the cases.146 Pericardial effusion is not common, and when present it tends to be mild. Serial echocardiography in turn allows us to evaluate the persistence of alterations or the recovery of cardiac functions in a secure and noninvasive manner, and therefore to adjust drug treatment and circulatory and/or respiratory support, where considered appropriate.
Management and treatmentTaking into account that there are no recommendations based on adequate scientific evidence, the evaluation and introduction of therapeutic measures can be made following the indications given in Fig. 5. Although the direct action of antiviral drugs upon the myocardium is not well known, the early start of antiviral treatment with oseltamivir is associated to an increased survival rate. It is essential for all patients with suspected influenza A/H1N1 infection to be treated immediately.
Algorithm for the diagnosis and treatment of cardiovascular complications in patients with influenza A/H1N1 infection. IACB: intraaortic counterpulsation balloon; ECG: electrocardiogram; ECMO: extracorporeal membrane oxygenation; LVEF: left ventricle ejection fraction; Pro-BNP: type B natriuretic peptide; ACS: acute coronary syndrome; ARDS: acute respiratory distress syndrome; DICM: Department of Intensive Care Medicine; TAPSE: tricuspid annular plane systolic excursion; RV: right ventricle; LV: left ventricle.
It is advisable to initially evaluate cardiac function by echocardiography, and to repeat the exploration in the case of sudden or progressive clinical worsening. Left and right ventricle function should be assessed based on the LVEF and TAPSE, respectively, while systolic and diastolic function should be examined by tissue Doppler ultrasound. Depending on the clinical context of the patient, and after adequate hemodynamic monitorization, the most appropriate treatment should be started, based on inotropic and/or vasopressor drugs. Only in cases refractory to conventional treatment should the placement of an intraaortic counterpulsation balloon (IACB) be considered, and if this fails we should resort to a circulatory and/or respiratory assist device such as the extracorporeal membrane oxygenation (ECMO) technique. The use of IACB and of ECMO circulatory and/or respiratory assist devices complicates adequate hemodynamic monitorization, and it is not advisable to use monitoring systems based on analysis of the pulse wave profile, since the application of these circulatory assist devices would alter the measurements obtained. Most patients show recovery of cardiac and respiratory function with conventional treatment, without the need for assist devices.
ConclusionCardiovascular involvement in influenza A/H1N1 infection is frequent and secondary to the destabilization of pre-existing heart conditions including myocarditis, ischemic heart disease and right ventricle dysfunction. Early diagnosis and adequate monitorization allow us to start effective treatment and, in the more serious cases, assess the need for circulatory support systems.
Indications for extracorporeal membrane oxygenation in acute respiratory distress syndrome due to influenza A/H1N1One of the most notorious characteristics of the influenza virus A/H1N1 pandemic is the frequent appearance of ARDS, with important associated mortality. In many cases the seriousness of the patient condition, with hypoxemia refractory to conventional treatment, has favored the use of “rescue” therapies involving both drugs such as corticosteroids or prostaglandins, and non-pharmacological measures such as the different ventilatory support systems and strategies (alveolar recruitment maneuvers, ventilation in prone decubitus, nitric oxide, high-frequency ventilation). Extracorporeal membrane oxygenation (ECMO) is one of these ventilatory support options, and has often been used in patients with refractory hypoxemia during the influenza A/H1N1 pandemic,151,152 with satisfactory clinical results.
ECMO does not represent curative treatment in patients with refractory respiratory failure. The technique aims to provide respiratory and/or circulatory functional support, facilitating gas exchange and stabilizing the hemodynamic situation of the patient. This allows us to apply protective ventilation and reduce trauma due both to volume (volutrauma) and pressure (barotrauma), and also the pulmonary inflammatory response associated to invasive mechanical ventilation. With ECMO we gain time to continue with the diagnosis and the application of treatment measures, and are able to facilitate recovery of the initial or root cause underlying respiratory failure. However, these benefits are accompanied by a series of inconveniences inherent to the application of invasive techniques, with important material and personnel requirements, and a significant complications rate.
Scientific evidenceTo date, only three randomized studies have compared the use of ECMO vs conventional mechanical ventilation, and none of them included patients with influenza A/H1N1 infection (Table 7). The first two studies, published by Zapol et al.153 under the auspices of the United States National Institutes of Health (NIH) and by Morris et al.,154 revealed no reduction in hospital mortality with the use of ECMO. However, following the introduction of technological improvements in the pumps, oxygenators and cannulas of the ECMO circuit, the CESAR study,155 published in 2009, evidenced a decrease in mortality, with values at the limit of statistical significance. Nevertheless, on jointly assessing mortality and severe disability after 6 months, the difference was clearly favorable to ECMO (37% vs 53%; RR 0.69; 95%CI 0.05–0.97).
Studies on extracorporeal membrane oxygenation in patients with acute respiratory distress syndrome.
| Article/year | Type of study | Patients | Cases of influenza A | Hospital mortality |
| Zapol153/1979 | RandomizedMulticenter | ECMO, n=42Control, n=48 | No | ECMO 38/42 (90%)Control 44/48 (92%)RR 0.99 (0.87–1.12) |
| Morris154/1994 | Randomized | ECMO, n=21Control, n=19 | No | ECMO 14/21 (67%)Control 11/19 (58%)RR 1.15 (0.71–1.188) |
| Peek155/2009CESAR | Randomized | ECMO, n=45Control, n=45 | No | ECMO 33/90 (37%)Control 45/90 (50%)RR 0.73 (0.52–1.03) |
| ANZ-ECMO156/2009Australia–New Zealand | Case series | ECMO, n=68 | Yes all | ECMO 36/68 (53%) |
| Patroniti157/2011 Italian ECMO network | Case series | ECMO, n=49 | Yes all | ECMO 14/49 (29%) |
| Bonastre158/2011 SEMICYUC-CIBERES Spain | Case series | ECMO, n=9 | Yes all | ECMO 5/9 (55%) |
| Freed159/2010Canada | Case series | ECMO, n=6 | Yes all | ECMO 2/6 (33%) |
| Registry ELSO160/2011 adult patients | Case series | ECMO, n=238 | Yes all | ECMO 85/238 (36%) |
ECMO: extracorporeal membrane oxygenation.
Regarding patients with influenza A/H1N1 infection, only more or less extensive case series have been published to date, reporting hospital mortality rates in patients subjected to ECMO of between 29 and 55%. The level of evidence supporting the utilization of ECMO therefore corresponds to class III, and the degree of recommendation is weak.
Indications and contraindicationsWith the existing level of evidence, and considering the invasiveness of the technique and its potential complications, ECMO should be regarded as a rescue option in patients in which less invasive treatments have failed (e.g., optimization of mechanical ventilation and of alveolar recruitment, ventilation in prone decubitus, the use of nitric oxide, the administration of prostaglandins, or the use of high-frequency ventilation techniques where available).152 Its application must be restrictive, and ECMO therefore should only be indicated in the more seriously ill patients. The ECMO assessment and application guidelines are described in Table 8. Its use should be considered in those patients in which the estimated mortality is 50%, based on compliance with at least one of the criteria described in Table 8. In patients where the estimated mortality is 80%, immediate ECMO is indicated. Hemmila et al.,161 in a very large series, described greater mortality in those cases where before the application of ECMO the patients suffered severe acidosis with pH<7.10 (OR=8.4), or had required mechanical ventilation during >8 days (OR=5.5). In addition to the impossibility of securing systemic anticoagulation, the absolute contraindications of ECMO are those derived from terminal situations or previously greatly deteriorated patient conditions, as well as established multiorgan failure (MOF). In the case of venous–arterial ECMO, severe aortic insufficiency also should be regarded as an absolute contraindication. The relative contraindications of the technique in turn are derived from the poorer results obtained in elderly patients and obese individuals, and when ECMO use is delayed beyond 7 days of mechanical ventilation with high FiO2 and PEEP requirements.
Indications and contraindications of extracorporeal membrane oxygenation in patients with acute respiratory distress syndrome due to influenza A/H1N1 infection.
| Consider ECMO |
| If the estimated mortality is 50% as assessed by compliance with at least one of the following criteria: |
| PaO2/FiO2<150 with FiO2>90% |
| PaO2/FiO2<100 with PEEP≥10cmH2O |
| Murray score 2–3 |
| Oxygenation index >25 |
| Hypercapnia and respiratory acidosis with pH<7.25 |
| Indicate ECMO |
| If the estimated mortality is 50% as assessed by compliance with at least one of the following criteria: |
| PaO2/FiO2<80 with FiO2>90% |
| PaO2/FiO2<70 with PEEP≥15cmH2O |
| Murray score >3–4 |
| Oxygenation index >30 |
| Hypercapnia and respiratory acidosis with pH<7.25 during at least 6h |
| Con×traindicate ECMO |
| Absolute contraindications |
| Cerebral hemorrhage or other absolute contraindications to anticoagulation |
| Moribund patient |
| Decision to limit therapeutic effort |
| Prior functional dyspnea grade IV |
| Terminal chronic disease |
| Established multiorgan failure (≥2 organs without including respiratory apparatus, with ≥2 points on the SOFA scale) |
| Severe aortic insufficiency (in case of venous–arterial ECMO) |
| Relative contraindications |
| Mechanical ventilation during more than 7 days |
| Age >65 years |
| Body mass index >40kg/m2 |
| Aortic dissection (in case of venous–arterial ECMO) |
ECMO: extracorporeal membrane oxygenation.
In view of its complexity, with important instrumental, technical, training and qualified healthcare personnel requirements, ECMO preferentially should be available in third level hospital centers. Simultaneously, and in order to comply with the ethical norms referred to the equity of access to medical care, it is advisable to incorporate ECMO to reference hospitals, with a multidiscipline team composition in which an intensivist must always be present. The function of this team is to establish consensus-based protocols and to jointly evaluate with the physician caring for the patient the advisability of transferring the patient to the reference hospital for ECMO implantation. This type of centralized structure is effective in attending patients admitted to hospitals where ECMO is not available, and has been adopted with good healthcare, training and resource optimization results in countries such as Australia–New Zealand156 and Italy.157 It is also recommended by the Extracorporeal Life Support Organization160 in the United States. Based on these premises, Fig. 6 shows the management of patients with ARDS secondary to influenza A/H1N1 infection, according to the type of hospital in which they are admitted.
Algorithm for the management of patients with refractory acute respiratory distress syndrome secondary to influenza A/H1N1 infection, according to the level of hospital care. ECMO: extracorporeal membrane oxygenation; PEEP: positive end-expiratory pressure; ARDS: acute respiratory distress syndrome.
ECMO is a rescue technique in cases of influenza A/H1N1 infection with refractory ARDS. The supporting scientific evidence is weak, and ECMO is not a first choice technique. It is used when other less invasive options have failed. It should be applied in patients with a poor prognosis (estimated mortality >80%). The most effective and efficient model comprises centralization in reference hospitals and the creation of multidiscipline ECMO teams capable of implanting the technique in other hospital centers and of transferring patients. The clinical results obtained in large series reflect in-hospital survival rates of approximately 50–60%.
Prevention. Impact of vaccination—risk groupsIntroductionVaccination is one of the most effective measures for the prevention and control of certain diseases. In this sense, the best way to prevent influenza A/H1N1 infection is undoubtedly annual influenza vaccination comprising the updated prevalent circulating strains of the virus in each given season.
The pandemic spread of the new variant of influenza A/H1N1 during the year 2009, influenza A/California/7/2009 (H1N1), made it necessary to quickly prepare a monovalent vaccine including only the attenuated new strain, followed by inclusion of the latter in the formulation of the annual influenza vaccines (trivalent vaccines), starting with the season 2010–2011. In addition, the epidemiological data obtained since then have led to revision and updates of the vaccination recommendations on the part of most of the implicated healthcare organisms.
Types of vaccinesIn Europe, and in relation to seasonal influenza, use is only made of inactivated vaccines often formulated with adjuvants. Most are derived from embryonated chicken eggs via fractions of the virus obtained after treatment with detergents (split vaccines), or antigens or purified subunits. For patients allergic to egg proteins we also have vaccines obtained from viruses replicated in cell cultures. In the United States, the available inactivated vaccines do not use adjuvants. An attenuated live vaccine is also available, administered via the intranasal route (FluMist®). Recently, inactivated vaccines without adjuvants have been marketed, designed for intradermal administration in adults between 18 and 64 years of age, and which induce an equivalent immune response with a lesser amount of antigen and a smaller administered volume (0.1ml) than the conventional vaccines. In the United States, an inactivated vaccine has also been approved with a four-fold higher antigen concentration than the conventional vaccine (Fluzone—“High-Dose”®), for the immunization of individuals over 65 years of age. In the second half of the year 2009, the European Medicines Agency (EMA, previously EMEA) authorized three monovalent inactivated vaccines based on the pandemic influenza A/California/7/2009 (H1N1) strain: two with an oily adjuvant (scualene) based on recombinant virus (Pandemmrix® from GSK and Focetria® from Novartis), and a third vaccine (Celvapan® from Baxter) without adjuvant and which uses the complete virus. Posteriorly, and in the context of an emergency procedure, two other inactivated pandemic vaccines with oily adjuvants were authorized: Arepanrix®, produced by GSK, and Humenza®, from Sanofi-Pasteur. The pandemic vaccines were administered in a single dose during the 2009 season, together with the seasonal influenza vaccine. Starting in 2010 and 2011, the pandemic strain has been included in the trivalent seasonal influenza vaccines, together with antigens derived from the A/Perth/16/2009 (H3N2) and B/Brisbane/60/2008 strains in the northern hemisphere for the 2011–2012 season.162
The inactivated vaccine is administered via the deep intramuscular route, in the deltoid muscle. In nursing infants and small children, injection preferably should be made in the lateral zone of the thigh. In the northern hemisphere, the first cases of seasonal influenza can appear in November; as a result, the vaccination campaigns are generally activated in the month of October. The inactivated vaccine is safe in pregnancy, and its administration (preferably without adjuvant) is recommended in any stage of pregnancy in women with high-risk medical conditions, and from gestational week 14 in the rest of women. The attenuated live vaccine is administered via the nasal route and can cause mild respiratory symptoms. It can be used in healthy patients between 2 and 49 years of age. The vaccine is not recommended in patients with comorbidities, and is contraindicated both in severely immune depressed patients and in people living with them. In general, simultaneous administration of the seasonal influenza vaccine with other vaccines (whether live or inactivated) is not advised, with the exception of the antipneumococcal vaccine, which when indicated can be coadministered in the contralateral deltoid muscle.
Immunogenicity of the influenza vaccineThe response to vaccination is detected by measuring the serum titers of neutralizing antibodies and hemagglutination inhibiting antibodies in the vaccinated patient. Hemagglutination inhibiting antibody titers of over 1:32–1:40 are considered adequate. With the trivalent vaccines, protective antibodies are present from two weeks after vaccination, with reductions to below protective titers after 6–8 months—this in turn justifying annual revaccinations.163 The studies made with the pandemic A/H1N1 vaccine of 2009 showed children under 9 years of age to present lower response rates than older children and young adults. As a result, the United States Advisory Committee on Immunization Practices (ACIP) recommends that children between 6 months and 8 years of age vaccinated for the first time, and those who during the previous season had received a single dose, should be administered a second dose four weeks after the first. Immune depressed individuals show lower response rates than the general population, though immunization in these subjects does not improve with revaccination; as a result, neither the administration of two doses nor an increase in dosage is indicated in such cases.
IndicationsThe advisory committees of the United States and Canada (ACIP/NACI) currently recommend universal influenza vaccination from 6 months of age, except in the presence of contraindications. In most countries vaccination is indicated in risk groups, following the recommendations of the WHO,162 which prioritizes the vaccination of elderly people, institutionalized persons, those with chronic diseases, pregnant women, healthcare workers, children between 6 months and two years of age, and people with functions essential to society. In any case, annual revaccination is advised in order to ensure optimum protection, despite the fact that the vaccine may contain the same strains as in the previous season, since host immunity decreases in the months after vaccination or in natural infection with the influenza virus. This decrease in turn is faster in elderly people and in patients with comorbidities. The recommendations of the Interterritorial Council of the Spanish Ministry of Health164 referred to influenza vaccination are shown in Table 9.
Population groups of population in which influenza vaccination is recommended, according to the protocol for influenza vigilance approved by Interterritorial Council of the Spanish National Healthcare System.
| 1. People 65 years of age or older; especially among those living in closed institutions |
| 2. People under 65 years of age with a high risk of complications |
| Children (over 6 months of age) and adults with chronic cardiovascular or pulmonary diseases, including: bronchopulmonary dysplasia, cystic fibrosis and asthma. |
| Children/as (over 6 months of age) and adults with chronic metabolic diseases, including: diabetes mellitus, morbid obesity (body mass index ≥40kg/m2), renal failure, hemoglobin disorders and anemias, asplenia, chronic liver disease, severe neuromuscular diseases or immune suppression (including that caused by HIV infection, drugs or in transplant recipients), and diseases implying cognitive dysfunction (Down syndrome, dementias and others). |
| People living in homes for the elderly, institutions or centers that treat chronic patients of any age. |
| Children and adolescents between 6 months and 18 years of age, receiving prolonged treatment with aspirin, due to the possible development of Reye syndrome following influenza infection. |
| Pregnant women. |
| 3. People who can transmit influenza to individuals at high risk of suffering complications |
| Workers in both primary and specialized centers, and in public and private hospitals. |
| People working in geriatric institutions or in chronic patient care centers, especially those coming into continuous contact with vulnerable individuals. |
| Persons providing home care for high risk individuals or elderly people. |
| Persons (including children) living in the home with other individuals belonging to some high risk group, due to their special clinical condition (cited under point 2). |
| 4. Other groups in which vaccination is recommended |
| People working in essential public services (State security forces, firemen, civil protection personnel, people working in emergency healthcare services, people working in prisons and other internment centers). |
| International travelers: people at an increased risk of developing complications of influenza infection because of their age or special clinical conditions, who were not vaccinated during the influenza season and who are traveling to tropical regions at any time of year, or who are traveling to the southern hemisphere between the months of April and September. |
| People visiting areas with outbreaks of highly pathogenic avian influenza and who may come into close contact with poultry farms or experience intense exposure to avian species. |
| People who may come into contact with avian species suspected or known to be infected especially with highly pathogenic avian influenza. |
In the systematic review of the clinical trials with vaccines against seasonal influenza conducted in healthy adults and children, the efficacy in preventing influenza virus infection confirmed by laboratory testing was 70–90%, though the percentage may vary depending on the discrepancies between the vaccinal and circulating viral strains.165 In the general population, the clinical efficacy of the vaccine can be estimated by statistical case–control analyses of the incidence of influenza-like syndromes confirmed by laboratory techniques and which have required medical care. By means of the sentinel physician networks, the Influenza Monitoring Vaccine Effectiveness in Europe project has estimated the adjusted effectiveness of the trivalent inactivated vaccine for the 2010–2011 season. The recorded effectiveness was moderate: 42.3% (95%CI 7.3–69.0%), which is lower than the values obtained in the randomized clinical trials made in selected healthy populations.166 With due consideration of the limitations inherent to large observational studies, the cohorts studied in the United States in the 1990s showed influenza vaccination of the elderly population over 65 years of age to have an impact not only on respiratory disease—with a 29–32% reduction in hospital admissions due to pneumonia or flu167—but also on cardiac and cerebrovascular mortality,168 with a 48–50% reduction in global mortality of all causes.
Side effectsNo vaccine is absolutely safe. Moreover, paradoxically, when thanks to the efficacy of a vaccine concern about the disease it helps control decreases, fear of the risks associated to vaccine use is seen to increase. In this sense, continuous monitorization of vaccine safety is needed in order to ensure that the population continues to trust the vaccination programs. In the case of the influenza A/H1N1 vaccine, doubts about its efficacy and possible adverse effects have been one of the main causes limiting its use. Even among healthcare workers, which theoretically represent one of the best informed risk groups, low adherence to the vaccination campaigns has been documented.169 Furthermore, in centers with well informed and aware healthcare personnel, the influenza A/H1N1 vaccination rate was lower than for seasonal influenza (75.2% vs 93.8%).170 The vigilance systems that monitor the safety of the influenza A/H1N1 vaccines (both with and without adjuvants) generally report adverse events (AEs) rates similar to those recorded for seasonal influenza. The published AE rates vary greatly (0.2–41.7% of all vaccinated individuals), depending particularly on the study population and on the vigilance system involved.171,172 The incidence density varies between 9 and 31.8 AEs for every 100,000 administered doses. Problems in this sense are more frequent in the first 24h after vaccination in the younger population, and there are no gender differences.172,173 The most common AEs are considered mild (fever [15%], reddening-induration-local pain [38%], muscle pain [5–7%], rhinorrhea [10–15%], headache [12–27%], hysteria reactions), with incidence densities between 1.9 and 0.1 episodes/100,000 vaccinations,174 and which in some series represented over 90% of the total AEs.174 In addition, there have been reports of different types of allergic reactions, urticaria (10%), angioedema (0.5%), Schönlein-Henoch purpura (0.9%), anaphylaxis (0.6%), etc. On a point basis there have been reports of hematological AEs in the form of aplastic anemia or severe thrombocytopenia, and even episodes of sudden death.175 However, the AEs that have generated most concern are of a neurological nature, with isolated cases of encephalomyelitis,173,174 and particularly Guillain–Barré syndrome (GBS) and narcolepsy.
In the case of GBS, a number of studies have examined its possible association to the monovalent vaccine, though no consistent relationship has been demonstrated.173–178 Either no increase in the incidence of GBS after vaccination has been observed (31 expected cases; 7 observed cases), or the risk seems smaller with the influenza A/H1N1 vaccine than with the seasonal influenza vaccine (adjusted OR=0.9 vs 1.3). Other findings have been that both influenza infection and other respiratory infections and the seasonal vaccine constitute risk factors associated to the appearance of GBS, acting as confounding elements on analyzing the effects of the influenza A/H1N1 vaccine (non-adjusted OR=2.8; 95%CI 1.3–6; adjusted OR=1; 95%CI 0.3–2.7). Likewise, it has not been possible to identify a clear biological basis confirming the possible epidemiological link. On the other hand, following the 2009–2010 vaccination campaign, an abnormally large number of narcolepsy episodes were recorded in patients between 4 and 19 years of age in Finland and Sweden, and to a lesser degree in France.179 The estimated risk was 1 case/12,000 vaccinations, with a mean time to appearance of 52 days. This phenomenon appears to be associated with the monovalent vaccines containing adjuvant AS03. As a result, the EMEA (21 July 2011), while considering the overall risk-benefit ratio of this group of vaccines to be positive, recommended avoiding the Pandemmrix® vaccine in patients under 20 years of age, except when the trivalent seasonal influenza vaccine is not available.180 At present, studies are nearing completion that are hoped to be able to clarify this problem. Lastly, it must be remembered that pregnant women are at a special risk of developing complications associated to influenza, and although vaccination is advised in these cases, the observed vaccination rate is notoriously low (12–24%).181 However, although the available information is limited, the influenza A/H1N1 vaccine does not appear to increase the risk of maternal-fetal complications during pregnancy. The most common AE is spontaneous miscarriage (41.2%), followed by fetal death (6.5%).182 The most frequent AEs unrelated to pregnancy are non-anaphylactic allergic reactions, other local reactions, and the appearance of general symptoms. However, the most relevant finding is that there have been no unusual complication profiles in the pregnant women or in the fetuses. The rates corresponding to spontaneous miscarriage, fetal death, congenital anomalies and other complications appear to be no different from those described in the general population.183,184
ChemoprophylaxisChemoprophylaxis must always be regarded as a complement to vaccination. The use of adamantanes (amantadine and rimantadine) is not indicated, due to the resistance rates observed in the influenza A/H1N1 isolates. With the neuraminidase inhibitors (zanamivir or oseltamivir), the reported efficacy rates are 70–90%. Such therapy is indicated in patients at a high risk of developing complications due to influenza and who cannot receive the vaccine.185 In addition, it is particularly useful in high risk patients and in healthcare personnel who have suffered exposure during epidemic outbreaks (duration of 10 days in the case of home exposure, and 7 days in others). A minimum of two weeks of chemoprophylaxis is advised for the control of outbreaks, even in vaccinated individuals, maintaining treatment up to one week after identification of the last case. Chemoprophylaxis should not be administered during 48h before or two weeks after administration of the live-attenuated intranasal vaccine. In contrast, chemoprophylaxis has no effect upon the inactivated vaccine.
ConclusionInfluenza vaccination is advised of all patients at risk, though it may be necessary to extend this indication to all patients over 6 months of age, except in the presence of contraindications. Children should receive two doses spaced one month apart. Immune depressed individuals and the population at risk should receive one dose, with annual revaccination. The frequency of adverse events following administration of the influenza A/H1N1 vaccine is similar to that seen with seasonal influenza vaccination. Chemoprophylaxis must always be regarded as a complement to vaccination, and is indicated in individuals at high risk of developing complications, and in healthcare workers who have suffered exposure.
Andalusia: Pedro Cobo (Hospital Punta de Europa, Algeciras); Javier Martins (Hospital Santa Ana, Motril, Granada); Cecilia Carbayo (Hospital Torrecardenas, Almería); Emilio Robles-Musso, Antonio Cárdenas, Javier Fierro (Hospital del Poniente, Almería); Dolores Ocaña Fernández (Hospital Huercal—Overa, Almería); Rafael Sierra (Hospital Puerta del Mar, Cádiz); Ma Jesús Huertos (Hospital Puerto Real, Cádiz); Juan Carlos Pozo, R. Guerrero (Hospital Reina Sofía, Córdoba); Enrique Márquez (Hospital Infanta Elena, Huelva); Manuel Rodríguez-Carvajal (Hospital Juan Ramón Jiménez, Huelva); Ángel Estella (Hospital del SAS de Jerez, Jerez de la Frontera); José Pomares, José Luis Ballesteros (Hospital Universitario San Cecilio, Granada); Yolanda Fernández, Francisco Lobato, José F. Prieto, José Albofedo-Sánchez (Hospital Costa del Sol, Marbella); Pilar Martínez; María Victoria de la Torre; María Nieto (Hospital Vírgen de la Victoria, Málaga); Miguel Angel Díaz Castellanos (Hospital Santa Ana de Motril, Granada); Guillermo Sevilla (Clínica Sagrado Corazón, Sevilla); José Garnacho-Montero, Rafael Hinojosa, Esteban Fernández (Hospital Virgen del Rocío, Sevilla); Ana Loza, Cristóbal León (Hospital Universitario Nuestra Señora de Valme, Sevilla); Angel Arenzana (Hospital Virgen de la Macarena, Sevilla), Dolores Ocaña (Hospital de la Inmaculada, Sevilla), Inés Navarrete (Hospital Virgen de las Nieves, Granada), Medhi Zaheri Beryanaki (Hospital de Antequera); Ignacio Sánchez (Hospital NISA Sevilla Aljarafe, Sevilla).
Andorra: Antoli Ribas (Hospital Nuestra Señora de Meritxell, Andorra).
Aragón: Carlos Serón, Manuel Luis Avellanas, Arantxa Lander, S. Garrido Ramírez de Arellano, M.I. Marquina Lacueva (Hospital San Jorge, Huesca); Pilar Luque; Elena Plumed Serrano; Juan Francisco Martín Lázaro (Hospital Lozano Blesa, Zaragoza); Ignacio González (Hospital Miquel Servet, Zaragoza); Jose Ma Montón (Hospital Obispo Polanco, Teruel); Paloma Dorado Regil (Hospital Royo Villanova, Zaragoza).
Asturias: Lisardo Iglesias, Carmen Pascual González (Hospital Universitario Central de Asturias—HUCA, Oviedo); Quiroga (Hospital de Cabueñes, Gijon); Agueda García-Rodríguez (Hospital Valle del Nalón, Langreo).
Balearic Islands: Lorenzo Socias, Pedro Ibánez, Marcío Borges-Sa, A. Socias, Del Castillo A (Hospital Son Llatzer, Palma de Mallorca); Ricard Jordà Marcos (Clínica Rotger, Palma de Mallorca); José M. Bonell (USP. Clínica Palmaplanas, Palma de Mallorca); Ignacio Amestarán (Hospital Son Dureta, Palma de Mallorca).
Canary Islands: Sergio Ruiz-Santana, Juan José Díaz (Hospital Dr. Negrín, Las Palmas de Gran Canaria); Montserrat Sisón (Hospital Doctor José Molina, Lanzarote); David Hernández, Ana Trujillo, Luis Regalado (Hospital General la Palma, La Palma); Leonardo Lorente (Hospital Universitario de Canarias, Tenerife); Mar Martín (Hospital de la Candelaria, Tenerife); Sergio Martínez, J.J.Cáceres (Hospital Insular de Gran Canaria).
Cantabria: Borja Suberviola, P. Ugarte (Hospital Universitario Marqués de Valdecilla, Santander).
Castilla La Mancha: Fernando García-López (Hospital General, Albacete); Angel Álvaro Alonso, Antonio Pasilla (Hospital General La Mancha Centro, Alcázar de San Juan); Mª Luisa Gómez Grande (Hospital General de Ciudad Real, Ciudad Real); Antonio Albaya (Hospital Universitario de Guadalajara, Guadalajara); Alfonso Canabal, Luis Marina (Hospital Virgen de la Salud, Toledo); Almudena Simón (Hospital Nuestra Señora del Prado, Toledo); José María Añón (Hospital Virgen de la Luz, Cuenca).
Castilla y León: Juan B López Messa (Complejo Asistencial de Palencia, Palencia), Mª Jesús López Pueyo, Ortíz María del valle (Hospital General Yagüe, Burgos); Zulema Ferreras (Hospital Universitario de Salamanca, Salamanca); Santiago Macias (Hospital General de Segovia, Segovia); José Ángel Berezo, Jesús Blanco Varela (Hospital Universitario Río Hortega, Valladolid), A. Andaluz Ojeda (Hospital Universitario, Valladolid); Antonio Álvarez Terrero (Hospital Virgen de la Concha, Zamora), Fabiola Tena Ezpeleta (Hospital Santa Bárbara, Soria); Zulema Paez, Álvaro García (Hospital de la Virgen Vega, Salamanca).
Catalonia: Rosa Mª Catalán (Hospital General de Vic, Vic); Miquel Ferrer, Antoni Torres, Catia Cilloniz (Hospital Clínic, Barcelona); Sandra Barbadillo (Hospital General de Catalunya—CAPIO, Barcelona); Lluís Cabré, Igancio Baeza (Hospital de Barcelona, Barcelona); Assumpta Rovira (Hospital General de l’Hospitalet, L’Hospitalet); Francisco Álvarez-Lerma, Antonia Vázquez, Joan Nolla (Hospital del Mar, Barcelona); Francisco Fernández, Joaquim Ramón Cervelló, Raquel Iglesia (Centro Médico Delfos, Barcelona); Rafael Mañéz, J. Ballús, Rosa Mª Granada (Hospital de Bellvitge, Barcelona); Jordi Vallés, Marta Ortíz, C. Guía (Hospital de Sabadell, Sabadell); Joaquim Páez (Hospital Dos de Mayo, Barcelona); Jordi Almirall, Xavier Balanzo (Hospital de Mataró, Mataró); Jordi Rello, Elena Arnau, Marcos Pérez, César Laborda, Jesica Souto, Mercedes Palomar (Hospital Vall d’Hebron, Barcelona); Iñaki Catalán (Hospital Sant Joan de Déu, Manresa); Josep Mª Sirvent, Cristina Ferri, Nerea López de Arbina (Hospital Josep Trueta, Girona); Mariona Badía, Begonia Baseda-Garrido, Montserrat Valverdú-Vidal, Fernando Barcenilla (Hospital Arnau de Vilanova, Lleida); Mònica Magret (Hospital Sant Joan de Reus, Reus); M.F. Esteban, José Luna (Hospital Verge de la Cinta, Tortosa); Juan Mª Nava, J. González de Molina (Hospital Universitario Mutua de Terrassa, Terrassa); Zoran Josic (Hospital de Igualada, Igualada); Francisco Gurri, Paula Rodríguez (Hospital Quirón, Barcelona); Alejandro Rodríguez, Thiago Lisboa, Ángel Pobo, Sandra Trefler (Hospital Universitario Joan XXIII, Tarragona); Rosa María Díaz (Hospital San Camil, Sant Pere de Ribes, Barcelona); Eduard Mesalles, Fernando Arméstar (Hospital Germans Trias i Pujol, Badalona); Diego de Mendoza (Hospital M. Broggi, Sant Joan Despí).
Extremadura: Juliá-Narváez José (Hospital Infanta Cristina, Badajóz); Alberto Fernández-Zapata, Teresa Recio, Abilio Arrascaeta, Mª José García-Ramos, Elena Gallego (Hospital San Pedro de Alcántara, Cáceres); Fernándo Bueno (Hospital Virgen del Puerto, Plasencia); Mercedes Díaz (Hospital de Mérida, Mérida).
Galicia: Mª Lourdes Cordero, José A. Pastor, Luis Álvarez—Rocha (CHUAC, A Coruña); Dolores Vila (Hospital Do Meixoeiro, Vigo); Carmen Fernández González (Hospital Arquitecto Marcide, Ferrol); Javier Blanco Pérez, M Ortiz Piquer (Hospital Xeral—Calde, Lugo); Eleuterio Merayo, Victor Jose López-Ciudad, Juan Cortes Cañones, Eva Vilaboy, José Villar Chao (Complejo Hospitalario de Ourense, Ourense); Eva Maria Saborido (Hospital Montecelo, Pontevedra); Raul José González (Hospital Miguel Domínguez, Pontevedra); Santiago Freita, Enrique Alemparte, Ana Ortega (Complejo Hospitalario de Pontevedra, Pontevedra); Ana María López, Julio Canabal, Enrique Ferres (Clinica Universitaria Santiago de Compostela, Santiago).
La Rioja: José Luis Monzón, Félix Goñi (Hospital San Pedro, Logroño).
Madrid: Frutos del Nogal Sáez, Miguel Blasco Navalpotro (Hospital Severo Ochoa, Madrid); Mª Carmen García-Torrejón (Hospital Infanta Elena, Madrid); César Pérez-Calvo, Diego López (Fundación Jiménez Díaz, Madrid); Luis Arnaiz, S. Sánchez-Alonso, Carlos Velayos (Hospital Fuenlabrada, Madrid); Francisco del Río, Miguel Ángel González (Hospital Clínico San Carlos, Madrid); María Cruz Martín, José Mª Molina (Hospital Nuestra Señora de América, Madrid); Juan Carlos Montejo, Mercedes Catalán (Hospital Universitario 12 de Octubre, Madrid); Patricia Albert, Ana de Pablo (Hospital del Sureste, Arganda del Rey); José Eugenio Guerrero, María Zurita, Jaime Benitez Peyrat (Hospital Gregorio Marañón, Madrid); Enrique Cerdá, Manuel Álvarez, Carlos Pey (Hospital Infanta Cristina, Madrid); Montse Rodríguez, Eduardo Palencia (Hospital Infanta Leonor, Madrid); Rafael Caballero (Hospital de San Rafael, Madrid); Concepción Vaquero, Francisco Mariscal, S. García (Hospital Infanta Sofía, Madrid); Nieves Carrasco (Hospital Universitario La Princesa, Madrid); Isidro Prieto, A Liétor, R. Ramos (Hospital Ramón y Cajal, Madrid); Beatríz Galván, Juan C. Figueira, M. Cruz Soriano (Hospital La Paz, Madrid); Pedro Galdós, Bárbara Balandin Moreno (Hospital Puerta de Hierro, Madrid); Fernández del Cabo (Hospital Monte Príncipe, Madrid); Cecilia Hermosa, Federico Gordo (Hospital de Henares, Madrid); Alejandro Algora (Hospital Universitario Fundación Alcorcón, Madrid); Amparo Paredes (Hospital Sur de Alcorcón, Madrid); J.A. Cambronero (Hospital Universitario Príncipe de Asturias, Madrid); Sonia Gómez-Rosado (Hospital de Móstoles, Madrid); Luis Miguel Prado López (Hospital Sanitas La Zarzuela, Madrid); Andres Esteban, José Angel Lorente, Nicolas Nin (Hospital de Getafe, Madrid).
Murcia: Sofía Martínez (Hospital Santa María del Rosell, Murcia); F. Felices Abad (Hospital Universitario Reina Sofía, Murcia); Mariano Martínez (Hospital Universitario Virgen de la Arrixaca, Murcia); Sergio Manuel Butí, Bernardo Gil Rueda, Francisco García (Hospital Morales Messeguer, Murcia).
Navarre: Laura Macaya, Enrique Maraví-Poma, I. Jimenez Urra, L. Macaya Redin, A. Tellería (Hospital Virgen del Camino, Pamplona); Josu Insansti (Hospital de Navarra, Pamplona).
Basque Country: Nagore González, Pilar Marco, Loreto Vidaur (Hospital de Donostia, San Sebastián); Beatriz Santamaría, Tomás Rodríguez (Hospital de Basurto, Bilbao); Juan Carlos Vergara, Jose Ramon Iruretagoyena Amiano (Hospital de Cruces, Bilbao); Alberto Manzano (Hospital Santiago Apóstol, Vitoria);Carlos Castillo Arenal (Hospital Txagorritxu, Vitoria); Pedro María Olaechea, Higinio Martín (Hospital Galdakao-Usansolo, Vizcaya).
Valencia: José Blanquer (Hospital Clinic Universitari, Valencia); Roberto Reig Valero, A. Belenger, Susana Altaba (Hospital General de Castellón, Castellón); Bernabé Álvarez -Sánchez (Hospital General de Alicante, Alicante); Santiago Alberto Picos (Hospital Torrevieja Salud, Alicante); Ángel Sánchez-Miralles (Hospital San Juan, Alicante); Juan Bonastre, M. Palamo, Javier Cebrian, José Cuñat (Hospital La Fe, Valencia); Belén Romero (Hospital de Manises, Valencia); Rafael Zaragoza, Constantino Tormo (Hospital Dr. Peset, Valencia); Virgilio Paricio (Hospital de Requena, Valencia); Asunción Marques, S. Sánchez-Morcillo, S. Tormo (Hospital de la Ribera, Valencia); J. Latour (H. G. Universitario de Elche, Valencia); M. Ángel García (Hospital de Sagunto, Castellón).
Please cite this article as: Rodríguez A, et al. Recomendaciones del Grupo de Trabajo Enfermedades Infecciosas (GTEI) de la Sociedad Española de Medicina Intensiva, … Med Intensiva. 2012;36:103–37.