Mechanical ventilation in acute respiratory distress syndrome (ARDS) implies an increase in alveolar and transpulmonary pressure, giving rise to major alterations in pulmonary circulation and causing right ventricular functional overload that can lead to ventricular failure and thus to acute cor pulmonale. The condition is echocardiographically characterized by dilatation of the right ventricle and paradoxical movement of the interventricular septum, with the added alteration of left ventricular systolic function. It is important to take lung mechanical and hemodynamic monitoring into account when defining the ventilation strategy in such patients, optimizing lung recruitment without producing pulmonary over-distension phenomena that may lead to greater deterioration of right ventricle function. This approach is known as a right ventricle protective ventilation strategy.
La ventilación mecánica en el síndrome de distrés respiratorio agudo supone una elevación de la presión alveolar y transpulmonar que condiciona una alteración en la circulación pulmonar y supone una sobrecarga importante para la función del ventrículo derecho que puede fracasar dando lugar al cuadro clínico de cor pulmonale agudo. El cuadro se caracteriza ecográficamente por la dilatación del ventrículo derecho y por el movimiento paradójico del septo interventricular alterando también la función sistólica del ventrículo izquierdo. Es importante tener en cuenta la monitorización de la mecánica pulmonar y hemodinámica a la hora de plantear la estrategia ventilatoria de estos pacientes optimizando el reclutamiento pulmonar sin producir fenómenos de sobredistensión del pulmón que condicionen un mayor deterioro de la función del ventrículo derecho. Esta estrategia se ha denominado estrategia ventilatoria protectora del ventrículo derecho.
Acute respiratory distress syndrome (ARDS) is characterized by the appearance of inflammatory and necrotizing phenomena affecting the lung alveoli and spreading through the bloodstream to the entire body, giving rise to so-called biotrauma. However, ARDS is also characterized by alterations of the pulmonary circulation, and has always been associated to the development of pulmonary hypertension. There is a clear interaction between the pulmonary circulation and the heart, since both are connected in series, and this relationship is much more important and is clearly altered by the decrease in lung compliance and transpulmonary alveolar pressure elevation found in ARDS—particularly in patients subjected to mechanical ventilation (MV) with high intrathoracic pressure values.1,2
A classical paradigm of mechanical ventilation (MV) is that it induces systemic hypotension secondary to right ventricle (RV) failure - this in turn being associated to high pressures and fundamentally to the use of positive end-expiratory pressure (PEEP) ventilation. This paradigm arises from classical studies such as that published by Jardin et al.,3 showing progressive PEEP elevations to be associated to a gradual increase in central venous pressure and to a drop in arterial pressure. Thus, in routine clinical practice, when dealing with episodes of hypotension in a mechanically ventilated patient, it is common to initially resort to volume expansion, the introduction or dose escalation of inotropic drugs, the reduction of sedative agents, and also the lowering of PEEP levels in an attempt to reduce the intrathoracic pressure.
Purported right ventricle failure secondary to pulmonary hypertension, with a drop in arterial pressure, in relation to PEEP elevation, fundamentally occurs in situations in which mechanical ventilation is associated to other conditioning factors such as hypovolemia, the development of comorbidities giving rise to diminished lung compliance (increased intraabdominal pressure, restrictive lung disease, the presence of auto-PEEP or pneumothorax), and inadequate use of MV itself.3–9
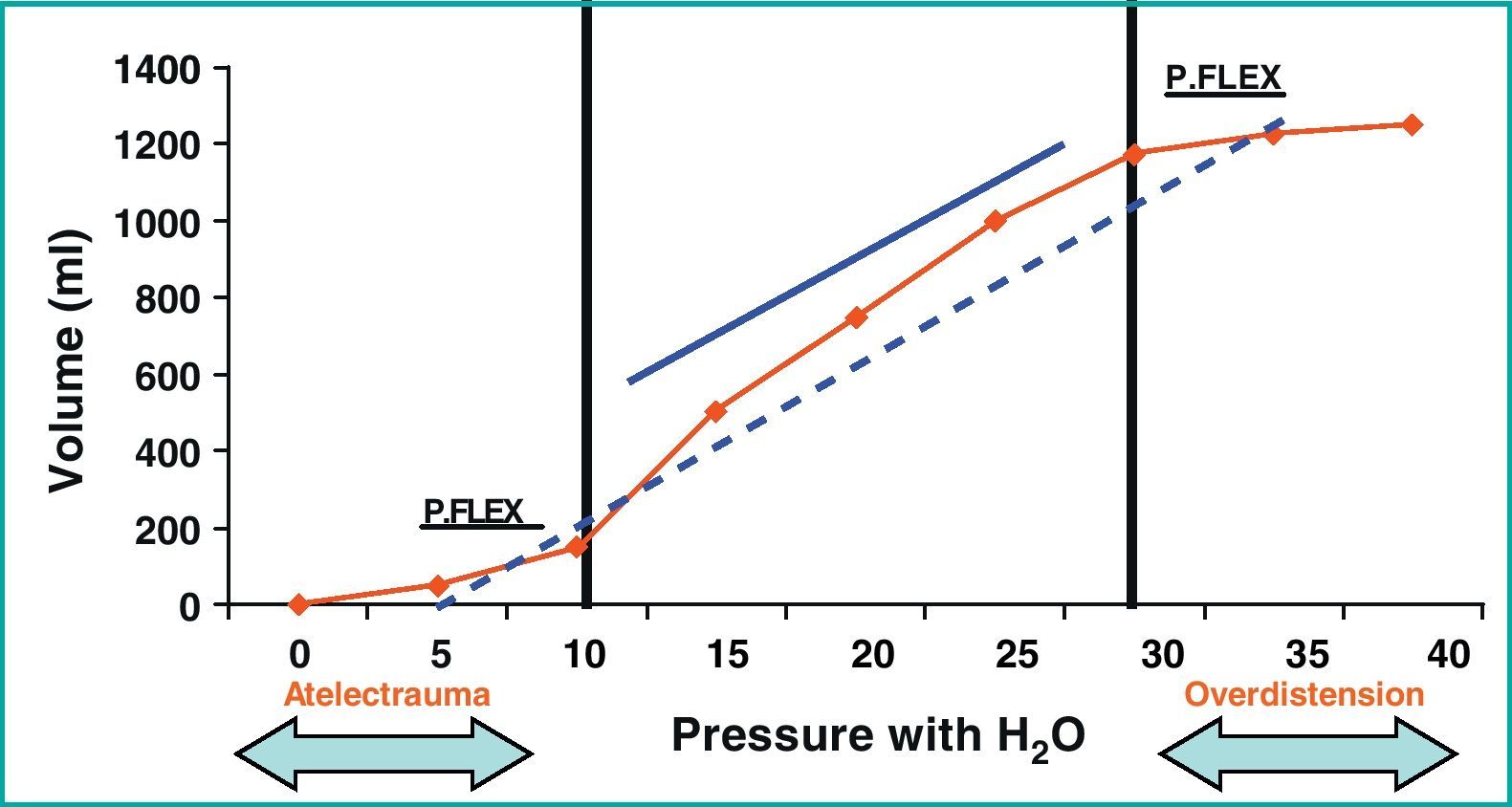
We now have a better understanding of lung and cardiac physiopathology in mechanical ventilation, and know that the application of excessive tension and the deformation of the lung tissues (fibrous skeleton, microvascularization, distal airways and juxta-alveolar tissues), which are conditioned by the stress (transpulmonary pressure) and strain (relationship between circulating volume and functional residual capacity) applied to 480million alveoli10 in the course of approximately 30,000cycles a day, give rise to lung parenchymal overdistension,10 with the consequent effect upon the pulmonary circulation and therefore on the right ventricle. The studies demonstrating the inverse relationship between high PEEP and right ventricle function were mostly carried out before the limitation of circulating volume in patients with ARDS, and such individuals were therefore ventilated with circulating volumes in excess of 10ml/kg ideal body weight—thereby resulting in important lung overdistension phenomena that conditioned these results (Fig. 1).
Characteristic pressure/volume (P/V) curve of a patient with acute respiratory distress syndrome, with a lower and upper inflexion point of 10 and 30cmH2O, respectively. The solid line shows the pressure–volume regimen of the lung-protecting strategies, while the broken line shows the traditional ventilation mode with lung overdistension phenomena. Cm, centimeter; Ml, milliliters; P. Flex, inflexion point; P/V, pressure/volume; ARDS, acute respiratory distress syndrome.
At present, and with the adoption of lung-protecting ventilatory strategies in patients with ARDS (Table 1),11–16 use is being made of greater mean pressures and probably greater pause pressures in these patients in relation to an increased PEEP level,17 yielding a decrease in mortality among these individuals as a result of the introduction of such strategies.18 This is fundamentally because we have learned that in order to adjust the circulating volume and PEEP, it is necessary to take hemodynamic and lung mechanical monitoring into account in these patients. Accordingly, in order to establish the ventilatory mode, it is important to know the lung recruitment capacity (to avoid atelectrauma phenomena) and balance it against adequate circulating volume (to avoid overdistension phenomena). Undoubtedly, an appropriate balance between lung recruitment and overdistension improves not only lung function but also heart function in patients with ARDS.
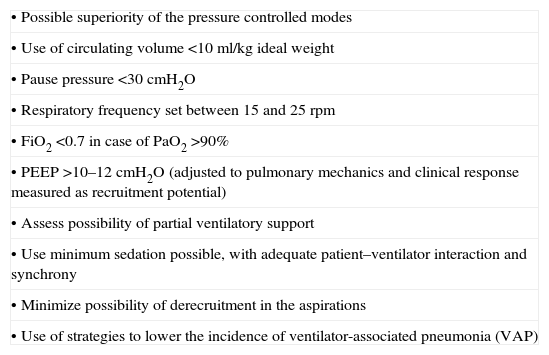
General principles of the protective ventilation strategies.
| • Possible superiority of the pressure controlled modes |
| • Use of circulating volume <10ml/kg ideal weight |
| • Pause pressure <30cmH2O |
| • Respiratory frequency set between 15 and 25rpm |
| • FiO2 <0.7 in case of PaO2 >90% |
| • PEEP >10–12cmH2O (adjusted to pulmonary mechanics and clinical response measured as recruitment potential) |
| • Assess possibility of partial ventilatory support |
| • Use minimum sedation possible, with adequate patient–ventilator interaction and synchrony |
| • Minimize possibility of derecruitment in the aspirations |
| • Use of strategies to lower the incidence of ventilator-associated pneumonia (VAP) |
The typical clinical picture in patients with ARDS subjected to MV is that of acute cor pulmonale (ACP), ultrasonographically characterized by RV dysfunction with dilatation, tricuspid regurgitation, paradoxical motion of the interventricular septum, and dilatation of the vena cava.19 In patients with ARDS in which a protective ventilation strategy is adopted and evaluation is carried out by ultrasound or with a pulmonary artery catheter, different studies have reported an incidence of between 22 and 27%.20–23 As an example, Mekontso Dessap et al.21 described a 20% prevalence of shunting due to a permeable foramen ovale in patients with ARDS subjected to MV with a mean PEEP of 10cmH2O and a pause pressure of 23cmH2O. The mentioned study moreover showed that the appearance of this shunt conditions PaO2 response to the PEEP increments.
Although it is logical to assume that the development of RV failure implies a poorer prognosis in these patients, the association is not entirely clear. In this sense, in the study of Osman et al.22 in which RF failure was defined from the data obtained with a pulmonary artery catheter, RV failure was not seen to be independently associated to mortality in the multivariate analysis.
Jardin et al.3,24 reported that the impact of RV failure upon the prognosis depends on the pause pressure. Accordingly, when the latter is under 27cmH2O, the incidence is 10% and its impact upon mortality zero. However, when the pause pressure exceeds 27cmH2O, the incidence is close to 35%, and a significant correlation to mortality is observed.
Vieillard-Baron et al.20 presented the data of a retrospective interventional study where on detecting RV failure the authors introduced changes in the respiratory settings—thereby reducing mortality among those patients in which such failure was corrected (conversion to the prone position and reduction of PEEP ventilation). In this study, the only factor found to be independently associated to the appearance of ACP in the multivariate analysis was PaCO2, with an odds ratio (OR) of 1.15 (95%CI 1.05–1.25).
All these data globally suggest that mortality in ARDS is conditioned by the severity of ARDS itself, the decrease in lung compliance, and lung collapse (reduction of functional residual capacity).
Causes of right ventricle failure in acute respiratory distress syndrome associated to mechanical ventilationARDS is able to intrinsically produce pulmonary hypertension due to its inflammatory and prothrombotic character; accordingly, patients with ARDS suffer alveolar damage combined with pulmonary vascular lesions, and with the appearance of microthrombi and pulmonary capillary obstruction. There have also been descriptions of remodeling of the pulmonary circulation (measured by hypoxia or hypercapnia), with muscle hyperplasia of the distal pulmonary arteries.19
Mechanical ventilation gives rise to an increase in intrathoracic pressure, which results in functional alterations of the pulmonary vascular circulation and thus conditions right ventricle function. This effect is dependent upon the transpulmonary pressure, which is the lung tissue distension pressure.25 We know that increased pause pressures give rise to an increased risk of RV overload—this being particularly relevant when using pause pressures of over 28cmH2O—and that an increased circulating volume implies an increased risk of RV overload (independently of the pressure reached) at values above 12ml/kg. In other words, the effect produced by mechanical ventilation is directly related to the degree of lung collapse and to the capacity of the lung to be recruited or not, and is associated to pulmonary compliance and the presence or not of overdistension phenomena.
Hager et al.26 reported a tendency towards gradually increasing mortality in relation to increases in pause pressure. Specifically, at any pause pressure level, patient mortality was greater with 12ml/kg of circulating volume – though the difference only proved significant with pause pressures above 35cmH2O. The effect upon lung compliance appears to be more related to circulating volume in relation to ideal body weight than to the actual pressure reached. In this context, although the first experimental models involved high pressures, we now know that there is a volume effect that is independent of the pressure reached. Dreyfuss et al.,27 in ventilated rats in which circulating volume input was limited by thoracic and abdominal cerclage (important overpressure in the airway with low volume), found no histological lesions in the lung parenchyma. However, lung damage was noted when overpressure was produced removing the cerclage, and thus with an increased circulating volume. Kolobow et al.28 found that sheep ventilated with high circulating volume can develop histologically manifest acute lung damage within 48h, even though the pressure reached was 30cmH2O.
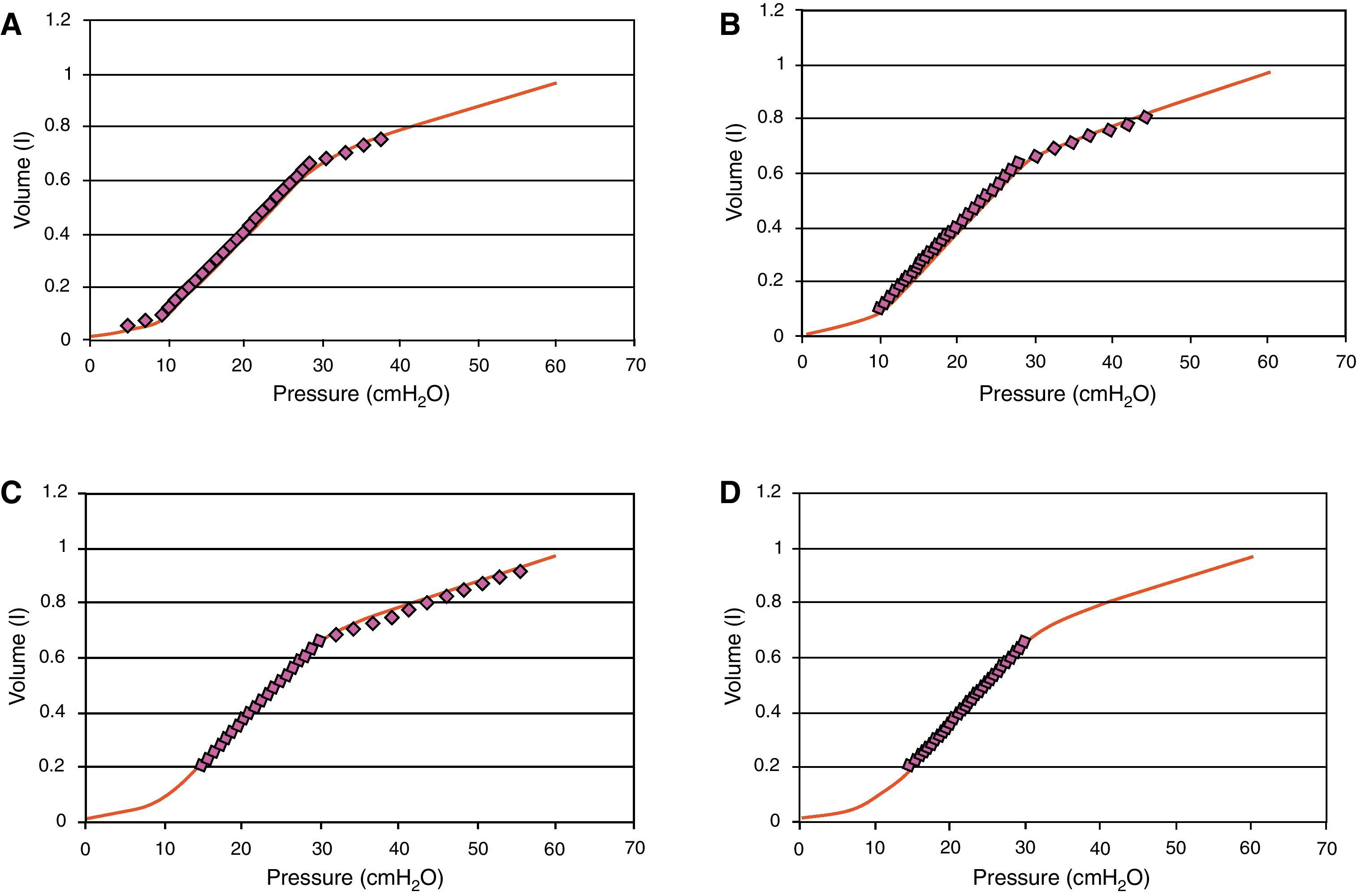
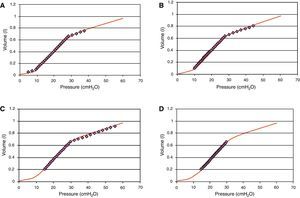
Regarding the effect of PEEP, in 1981 Jardin et al.3 reported that an increase in PEEP above 10cmH2O in patients with ARDS produces a drop in cardiac output related to RV overload, with displacement of the interventricular septum. However, this study and other similar publications were made at a time when we were still unaware of the clinical importance of reducing circulating volume and its effect upon lung compliance. In those days it was typical to ventilate these patients with a circulating volume of over 10ml/kg ideal weight, which undoubtedly in cases of severe ARDS induces overdistension phenomena that in turn worsen on gradually elevating PEEP without lowering the circulating volume. Fig. 2 shows the theoretical effect upon the pressure/volume curve of ventilation with a circulating volume of over 10ml/kg with different PEEP levels, and the reduction in overdistension that occurs on adopting a lung-protecting ventilatory strategy with a higher PEEP level.
Pressure/volume (P/V) curve of a patient with acute respiratory distress syndrome. The line formed by circles represents the real volume and pressure position. It is seen that on elevating PEEP from 5 (panel A) to 10 (panel B) and 15 (panel C) without lowering the circulating volume, an increased overdistension effect is obtained (most ventilation over the upper inflexion point). Panel (D) offers an example of protective ventilation, PEEP 15.
In other words, the effect of the PEEP increment upon RV function depends on its effect on lung recruitment; accordingly, if the PEEP increment induces lung recruitment, we observe a decrease in transpulmonary pressure secondary to an increase in lung compliance, with no effects upon the RV. In contrast, if the PEEP increment induces overdistension, we observe an increase in transpulmonary pressure and an increase in RV load.
Consequences for the ventilatory management of acute respiratory distress syndromeOn the basis of the considerations referred to the protection of pulmonary circulation and therefore of right ventricle function through mechanical ventilation in patients with ARDS, the ventilation mode should be adjusted in each individual patient according to the existing hemodynamic situation and the specific lung mechanics. Accordingly, we must distinguish which patients have truly recruitable lungs by using higher PEEP levels, and we should avoid unnecessary elevations in transpulmonary pressure, lung tissue collapse, and especially pulmonary overdistension phenomena.
All these principles are followed with the use of lung-protecting ventilation strategies, fundamentally reducing the circulating volume to under 10ml/kg ideal weight, with a PEEP level suited to the characteristics of the ventilated lung, and securing the maximum possible amount of recruitable lung tissue while limiting the pause pressure to 30cmH2O. In those cases of more severe ARDS with intense hypoxemia, it is advisable to consider the prone decubitus position,29–34 which in these cases allows an important reduction in transpulmonary pressure and PEEP level, and at the same time maintains recruitment capacity and therefore protects RV function.35
In future, it undoubtedly will be very important to improve individualization of the mechanical ventilation parameters through adequate clinical monitorization of the ventilated patient based on the hemodynamic and lung mechanics monitoring systems.36 In this context, mention should be made of the important role of thoracic and cardiac ultrasound in routine practice referred to the management of critical patients subjected to mechanical ventilation,37 and the introduction of new pulmonary monitorization systems allowing direct visualization of patient ventilation—not only lung morphology but also gas distribution within the lungs (impedance tomography techniques).38
It should be taken into account that the most important consideration is not the monitoring systems used but correct interpretation of the data obtained.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Gordo-Vidal F, Enciso-Calderón V. Síndrome de distrés respiratorio agudo, ventilación mecánica y función ventricular derecha. Med Intensiva. 2012;36:138–42.