Traumatic injuries represent a major health problem all over the world. In recent years we have witnessed profound changes in the paradigm of severe trauma patient resuscitation; new concepts regarding acute coagulopathy in trauma have been proposed; and there has been an expansion of specific commercial products related to hemostasis, among other aspects. New strategies in severe trauma management include the early identification of those injuries that are life threatening and require surgical hemostasis, tolerance of moderate hypotension, rational intravascular volume replacement, prevention of hypothermia, correction of acidosis, optimization of oxygen carriers, and identification of those factors required by the patient (fresh frozen plasma, platelets, tranexamic acid, fibrinogen, cryoprecipitates and prothrombin complex). However, despite such advances, further evidence is required to improve survival rates in severe trauma patients.
Los traumatismos son uno de los principales problemas de salud en todo el mundo. En los últimos años hemos presenciado profundas modificaciones en el paradigma de la resucitación del paciente traumatizado crítico, se han desarrollado nuevos conceptos en relación con la coagulopatía inducida por el trauma, así como hemos asistido a la expansión comercial de productos específicos relacionados con la hemostasia, entre otros. Las nuevas estrategias de resucitación en el trauma incluyen: identificar de manera precoz las lesiones que amenazan la vida del paciente, la detección de aquellas que precisan de un inmediato control quirúrgico o intervencionismo radiológico, tolerar una hipotensión moderada, reponer de manera racional el volumen intravascular, prevenir la hipotermia, evitar la acidosis, optimizar los transportadores de oxígeno, así como identificar aquellos factores necesarios para el paciente (plasma fresco congelado, plaquetas, ácido tranexámico, fibrinógeno, crioprecipitados y complejo protrombínico). Sin embargo, a pesar de estos avances, se necesitan más evidencias para reducir las tasas de mortalidad de los pacientes traumatizados graves.
Traumatic injuries remain a major healthcare, social and economic problem throughout the world. In recent years there have been profound changes in the paradigm of severe trauma patient resuscitation; new concepts regarding acute coagulopathy induced by trauma (CIT) have been proposed; and there has been an expansion of specific commercial products related to hemostasis, among other aspects. To a large extent, all these changes have been promoted by the experience gained from armed conflicts over the last decade, in which strategies are evaluated and subsequently transferred to the civil setting.1–6
General considerations on the new strategies of resuscitation in traumaDamage control resuscitation (DCR) is defined as the global series of structured interventions that take place during the management of serious trauma characterized by a high risk of mortality due to hemorrhagic shock. These interventions must begin at the injury site, and must be maintained both in the operating room and in the Intensive Care Unit (ICU).1,2 Thus, in damage control resuscitation, effort should focus on the early identification of potentially fatal injuries, with simultaneous rational intravascular volume replacement tolerating mild hypotension, the vigorous prevention of hypothermia, control of acidosis (or prevention of acidosis progression), oxygen transporter optimization, and emphasis on the correction of CIT.3,4 The approach to this type of coagulopathy includes the early transfusion of fresh frozen plasma (FFP) and platelets, with recognition from the physiopathological perspective of those factors which may be needed by the patient (fibrinogen, cryoprecipitates and prothrombin complex) and modulation of the hyperfibrinolysis state making use of tranexamic acid (TXA).2,5,6 All these interventions are time dependent and must be carried out parallel to damage control surgery (DCS) – the aim of which is to afford emergent management of those surgical lesions that prove life-threatening, leaving their definitive repair for a latter stage.
Failure to control any of the abovementioned aspects gives rise to a vicious circle known as the “lethal triad” (hypoperfusion/acidosis, hypothermia and coagulopathy).2 Some authors postulate that these three factors, classically associated to mortality in trauma, should in fact be complemented by two additional factors: hypoxia and hyperglycemia. This “lethal pentad” results in a decrease in oxygen available at tissue level, secondary to hypoperfusion, with the utilization of aerobic pathways that generate increased lactic acid levels and lower pH. These pathways are less efficient in energy terms and limit endogenous heat production–thereby worsening the existing exposure hypothermia. This circumstance, and the replacement of fluids and blood products without prior warming, gives rise to an unsustainable situation resulting in patient death secondary to refractory hemorrhagic shock.1
Guaranteeing adequate tissue perfusion with rational fluid administrationDuring the initial phase of trauma resuscitation, the blood pressure targets should be moderate, allowing systolic blood pressure (SBP) values of between 80 and 90mmHg.4–6 This situation of permissive hypotension is achieved by delaying the start of fluid therapy or by limiting the infused volume. The theoretical benefits of such a strategy are the maintenance of optimum/suboptimum tissue perfusion with the aim of avoiding clot disruption and minimizing hypothermia, the dilution of coagulation factors and blood losses through uncontrolled foci.5–8 It must be taken into account that blood pressure is a surrogate marker of tissue oxygenation; consequently, such blood pressure control should be viewed as a dynamic concept in which the thresholds are adjusted on an individualized basis in each case, considering the patient history (arterial hypertension, pregnancy, etc.), the expected delay until in-hospital care can be provided, and the type of injuries evidenced after trauma. As an example, in patients with severe traumatic brain injury (TBI) or acute spinal cord damage, hypotension could have deleterious effects upon secondary injuries.9 In patients of this kind, the recommended mean blood pressure value would be ≥80mmHg.4
For decades, fluid administration has been the standard of practice in circulatory resuscitation of trauma patients in shock, attempting to preserve organ perfusion by replacing the blood losses. Oliguria is often present during the early stages of resuscitation. Early-stage efforts to restore urine output would inevitably generate volume overload and an increased third space. It is known that a liberal and aggressive fluid strategy has deleterious consequences for CIT and is related to the appearance of abdominal compartment syndrome or lung injury.6,7 Different in vitro studies have shown that the concentrations of coagulation factors and fibrinogen are directly proportional to the volume administered to the patient after trauma.10,11 The study conducted by Bickell et al.12 in a group of penetrating trauma patients revealed benefits in terms of survival when a lesser fluid volume was used during resuscitation. However, later studies have obtained variable results.13,14 The latest review of the Cochrane Database, assessing ideal timing of the start of resuscitation with fluids as well as the amount of volume to be administered in patients with hemorrhagic shock, does not allow firm conclusions to be drawn.15
The use of crystalloids in initial resuscitation is the standard of practice and the recommendation established for both advanced trauma life support (ATLS) and the different clinical practice guides.4 Nevertheless, the use of high-dose isotonic saline solutions may give rise to a situation of hyperchloremic acidosis.7 Immunological studies of the different types of fluids show that the l-isomer of Ringer lactate solution could induce less inflammatory and immune dysfunction, as well as fewer electrolyte disorders. However, it must be emphasized that Ringer lactate in patients with brain damage would not be a choice, because of its hypotonic characteristics.16
The use of colloids has been unable to demonstrate benefits with respect to crystalloids in the critical patient. Although all fluids are able to cause dilutional coagulopathy, colloids produce anomalies in fibrinogen polymerization and lesser clot stability–the effect being dose dependent.17 One of the latest clinical trials, the multicenter CRISTAL study involving 2857 patients, has revealed no differences in mortality after one month between the use of crystalloids and colloids.18 Recently, the European Pharmacovigilance Risk Assessment Committee has recommended suspension of the marketing of colloids containing hydroxyethyl-starch, due to their association to the appearance of renal failure and increased mortality among critical patients.19 Accordingly, the European guides of 2013 place special emphasis on the need for a more adjusted use of colloids in general, referred both to time and to the volume administered, and insist on the importance of adhering to the doses specified in the Summary of Product Characteristics.4
Resuscitation with small doses of hypertonic saline solution (7.5%) in hemorrhagic shock acts as an effective plasma expander and plays a positive immune modulating role. Given its lesser volume and weight in comparison with other fluids, hypertonic saline solution allows for easier transport and storage, especially in the military and pre-hospital settings. To our knowledge, however, hypertonic saline solution has not been shown to be superior to isotonic saline in the resuscitation of critical patients.20
Vasopressors in severe trauma, specifically noradrenalin, are used in life-threatening situations of arterial hypotension refractory to resuscitation with fluids. Although their use in the experimental setting has revealed a decrease in fluid requirements, with lesser blood losses and improved survival, studies in humans have produced contradictory results, and the early administration of such drugs in trauma has even been associated to increased mortality.21
Since correction of the macrocirculatory hemodynamic parameters does not guarantee the resolution of tissue hypoperfusion, the initial measurements of lactate levels or base defects, and their subsequent clearance, can allow us to identify patients with incomplete resuscitation or with unresolved problems such as underdiagnosed serious lesions.22,23 Recently, a prospective study has shown early lactate clearance (0–2h) to be an important prognostic factor. The follow-up of lactate levels therefore could be very useful in the context of trauma resuscitation.22
Avoiding coagulopathy induced by traumaPatient age, personal history and genetic characteristics, and the type of injury or its seriousness, among others, are non-modifiable factors following trauma.24 We therefore must act upon those potentially reversible factors that contribute to worsen CIT. In this regard, bleeding in trauma patients can often be controlled provided early and appropriate measures are taken. However, hemorrhagic shock remains the cause of death in over 40% of all serious trauma patients. Between 33% and 56% of these deaths occur in the pre-hospital period, with the documentation of up to 81% within the first 6h of admission to hospital. Following the first 24h, the main cause of death in trauma patients tends to be multiorgan failure or intracranial hypertension, in cases of serious TBI.25
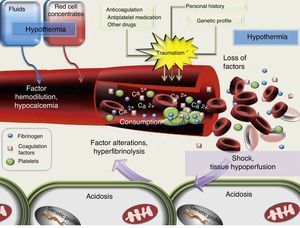
It must be taken into account that up to 25–43% of all trauma patients that reach the hospital have coagulation disorders.26–28 The origin of CIT is multifactorial, and is illustrated in Fig. 1. Of the different causes, mention should be made of the loss of coagulation factors through the bleeding sites, the dilution of coagulation factors (as a consequence of fluid infusion or the administration of red cell concentrates [RC] without associated FFP), the activation of fibrinolysis, and alterations in protease function secondary to acidemia, hypothermia and shock.29
At present, dilutional coagulopathy is recognized as one of the elements that favor CIT.10,11,17 However, it has been shown that such alterations can be independent of the volume of fluids used during pre-hospital care.17,27,28 Many studies have related the severity or extent of trauma to coagulation disorders.27,28 In this regard, CIT worsens in the presence of shock at hospital admission, since hypoperfusion promotes hyperfibrinolysis (through the activation of thrombomodulin and protein C) independently of the presence of hypothermia, acidosis or the dilution of factors, which manifest posteriorly in cases of inadequate resuscitation.17,29,30 Recent studies define CIT as disseminated intravascular coagulopathy with a fibrinolytic profile.31 Hypothermia has been described as an independent mortality risk factor in trauma.27,29 The correction of hypothermia improves the coagulation times and platelet activity.2,32 A drop in pH to 7.2 is associated to a 50% decrease in the generation of thrombin, a reduction in the activity of coagulation factors Va and Xa, and an increase in fibrinolysis. This decrease may reach 90% when the pH drops to 7. However, at experimental level, the correction of acidosis following the administration of bicarbonate does not improve CIT.32 This indicates that despite normalization of the pH values, if hypoperfusion secondary to hemorrhagic shock is not controlled, CIT will persist.32,33 It should be underscored that the concentrations of calcium–a crucial ion in blood coagulation–are influenced by the acid–base equilibrium and by the citrate administered with blood products. Hence, a lack of control of their levels is related to both patient mortality and the transfusion needs.3,34
The management of CIT based on conventional coagulation studies is not advised, since the information they offer always comes late and does not reflect the situation of the patient on a real-time basis.3,35,36 For this reason, the implementation of viscoelastic tests in the trauma emergency ward is becoming increasingly widespread, in the same way as in major surgery.36,37 It is considered that CIT could have a series of specific characteristics with the use of these techniques.38 Analysis using the ROTEM (ROtation ThrombElastometry Method) shows that there is an alteration in clot formation time (CFT), clot amplitude after 5min (CA5), maximum clot firmness (MCF), and alpha-angle. In brief, clot formation is said to be slow, with a 40% reduction in its firmness.38 This information would allow the real-time and immediate adoption of specific actions for controlling coagulopathy at the patient bedside.37
Coagulopathy induced by trauma is associated to increased transfusion needs, a greater incidence of multiorgan dysfunction, a longer ICU stay and higher mortality rates.24,28,30 Consequently, in recent years the trauma management strategies have focused on ways to avoid the progression of CIT.2,3,29
Proportional transfusion of blood productsThe replacement of blood losses after trauma through the transfusion of blood products has changed substantially in recent decades. The use of these products is known to increase morbidity-mortality after trauma.39 Although whole blood transfusions are still used in certain military scenarios, the protocols were reoriented years ago toward the administration of specific blood components (RC, FFP or platelets).40 The aim of this strategy is to avoid transmissible diseases and supply only those elements which the patient needs. However, while this concept is valid in controlled situations and elective surgery, it proves ineffective during the management of traumatic hemorrhagic shock.41,42
The proportions in which these components should be infused have also been the subject of study and debate. Computational models have shown the need to increase the amount of FFP and platelets administered to patients after trauma.42 Therefore, the definition of massive bleeding which we use, and which was described in the 1990s as the need for over 10 red cell concentrate units in under 24h, does not precisely reflect the way in which transfusions currently should be carried out in severe trauma patients.35,41,43 The recently published results of the PROMMPT study, which included 1245 patients in 10 trauma centers, indicate a decrease in mortality when the ratios of FFP and platelets versus RC approach 1:1.26 These data are consistent with those of previous studies which found FFP:RC ratios of under 1:2 to be associated with decreased mortality after trauma.1,41,44,45 Likewise, the use of cryoprecipitates or fibrinogen administered in relation to the number of infused RC also improves survival.3,46 On the basis of the described clinical trials, a lack of homogeneity is seen in the administration of the specified ratios, particularly during the first few hours.26,36 In brief, it can be concluded that the current recommendations comprise the transfusion of high FFP:RC and platelet:RC ratios (between 1:1 and 1:2), with transfusion performed in a constant manner during the resuscitation of trauma patients.1,26,41,44,45
Of all trauma patients receiving blood products, 36% will require activation of the massive transfusion protocol (MTP). In broad terms, these cases represent only 2.6% of all serious traumatisms, but the associated mortality rate is so high that careful care and coordination with the blood banks is required.3 Since blood product availability upon patient arrival in hospital must be considered, it is advisable to implement an MTP in those centers that attend severe trauma cases, in order to avoid unnecessary delays.1 As an example, in military scenarios, routine plasma thawing is carried out on a daily basis, or methods are adopted to ensure prompt availability of FFP (universal donor plasma, rapid thawing units, specific plasma formulations), in order to optimize the management of these patients and try to reach appropriate transfusion ratios.2,3,47 In this respect, different scores have attempted to predict patients with massive bleeding upon arrival in hospital, based on the use of clinical variables and basic explorations. A recent study comparing some of these scales has shown the Trauma Associated Severe Hemorrhage Score (TASH) to offer the best correlation, making it possible to identify those patients who do not require activation of the MTP.43Table 1 describes the TASH, as well as the probability of massive bleeding according to the score reached.48
Probability of massive bleeding according to the Associated Severe Hemorrhage Score (TASH).
| Variable | Value | Score |
| Hemoglobin (g/dl) | <7 | 8 |
| <9 | 6 | |
| <10 | 4 | |
| <11 | 3 | |
| <12 | 2 | |
| Base excess (nM) | <−10 | 4 |
| <−6 | 3 | |
| <−2 | 1 | |
| SBP (mmHg) | <100 | 4 |
| <120 | 1 | |
| HR (bpm) | >120 | 2 |
| Free intraabdominal fluid/positive ECO-FAST | Yes | 3 |
| Extremities | ||
| Clinically unstable pelvic fracture | 6 | |
| Open femoral fracture/luxation | 3 | |
| Sex | Male | 1 |
| TASH score | ||
| TASH score | Probability of massive transfusion |
| 1–8 | <5% |
| 9 | 6% |
| 10 | 8% |
| 11 | 11% |
| 12 | 14% |
| 13 | 18% |
| 14 | 23% |
| 15 | 29% |
| 16 | 35% |
| 17 | 43% |
| 18 | 50% |
| 19 | 57% |
| 20 | 65% |
| 21 | 71% |
| 22 | 77% |
| 23 | 82% |
| 24 | >85% |
ECO-FAST: focused assessment sonography in trauma; HR: heart rate; SBP: systolic blood pressure.
Reproduced from Maegele et al.48
Other parameters may also be predictive of the need to activate the MTP, including the presence of hypocalcemia or the results of the ROTEM.34,38 In this latter case, the clot amplitude after 5min (CA5) with a cutoff point of 35mm allows us to identify those patients at risk of developing massive bleeding after trauma.38
Prohemostatic drugsTo date, the most promising results referred to decreased trauma mortality are those published by the CRASH-2. This study evaluated the use of tranexamic acid (TXA) within the first 8h after trauma, and documented a significant decrease in overall mortality after one month and in mortality due to massive bleeding versus placebo.49 The antifibrinolytic profile of the drug based on inhibition of the conversion of plasminogen to plasmin is the hypothesis that could best explain this decrease in mortality, taking into account as commented above the hyperfibrinolysis state that characterizes severe trauma.30 However, it should be noted that the use of TXA did not reduce the transfusion requirements of the patients. An analysis of the timing of TXA showed that administration in the first 3h optimized the results obtained.49 Hence the early use of TXA is recommended by the trauma patient guides–the suggestion being to start administration during pre-hospital patient management.4 A cost-effectiveness analysis conducted by the National Health Service on the use of TXA showed that utilization of the drug resulted in a cost increment of only 64 USD for each life saved.
It should be noted that there was no comparative increase in the incidence of ischemic adverse effects after the administration of TXA in over 20,000 patients.49 However, retrospective series posterior to the CRASH-2 have described an increased risk of pulmonary and venous thrombotic events, despite a documented decrease in mortality rate among polytraumatized patients.50 Recently, a series of recommendations have been published that referred to future lines of research and their priority in relation to TXA. The purpose of these recommendations (explained in Table 2) is to improve knowledge of the specific mechanism of action of the drug, and its safety profile, among other aspects.51 Some of these lines of research have already been started in specific sub-populations of patients with TBI associated to intracranial bleeding injuries, based on the CRASH-3 study.52
Recommended lines of investigation according to priority and gaps in knowledge regarding the use of tranexamic acid (TXA).
| Category | Doubts and future lines of investigation |
| Priority 1 | |
| Safety | Specifically investigate the increase in mortality risk due to bleeding in the group of patients administered TXA beyond 3h after trauma. Determine whether the studies made showed an increase in the risk of deep venous thrombosis and pulmonary thromboembolism. |
| Investigate thromboembolic risk during DCS. | |
| Study the potential complications in patients in which TXA could be contraindicated, including patients with brain trauma, and the impact upon the development of seizure crises after surgery. | |
| Evaluation of the patients in the clinical trials who died, to establish the presence of microthrombi. | |
| Animal models | Development of animal models for studying the efficacy, safety and mechanisms of action of TXA. |
| Additional tests of efficacy and definition of patients that may benefit from TXA | Designing of clinical trials to define the efficacy of the drug in the military and civil settings following the latest management recommendations. |
| Mechanism of action | Information on the mechanism of action in traumatic hemorrhage. Different mechanisms have been proposed but have not been tested. |
| Efficacy and safety in head injuries without associated lesions | Is the information on the potential efficacy and safety in patients with traumatic/hemorrhagic brain injuries accurate? |
| Pre-hospital use (military scenario) | Potential use in the pre-hospital setting, cases of deferred evacuation or limitation of the supportive measures (no blood products or only plasma) and modification of the dose/time window if immediate administration following injury is possible. |
| Interactions with other fluids or drugs, impact upon storage on the battlefield. | |
| Compatibility with the concept of remote guided damage control resuscitation. | |
| Priority 2 | |
| Optimization of knowledge on the time window for administration | Efficacy and safety of TXA with administration at different timepoints after trauma. What is the best time for administration? When has the time window been exceeded? |
| Use of TXA in combination with other blood products | How would its usefulness be improved if administered with other blood products? |
| Alternative administration routes | Potential use of other administration routes (intravenous, oral, intraosseous, etc.). |
| Priority 3 | |
| Interaction with the inflammatory and coagulation cascades | Identify inflammation and coagulation time patterns. Investigate how they are influenced by the administration of TXA in different moments. |
| Dosing | All the available data are based on a single regimen. Could other dosing schemes increase efficacy and safety? How are the pharmacokinetics affected in trauma? |
| Use with common resuscitation fluids | Potential use of TXA in combination with new generation fluids. |
| Microcirculation | Effects of TXA upon microcirculatory blood flow. |
| Interaction with other drugs | Interaction with antithrombotic prophylaxis, prohemostatic drugs (such as recombinant activated factor vii), antiseizure drugs and other substances. |
TXA: tranexamic acid; DCS: damage control surgery.
Reproduced from Pusateri et al.51
Coagulopathy induced by trauma (CIT) is associated to a decrease in factors I, II, V, VII, VIII, IX and X, mediated by the activation of protein C.30 The most notorious decrease corresponds to fibrinogen, and its levels are not seen to improve until damage control has been achieved.10.11,17,46 Retrospective studies have shown an increase in the fibrinogen:RC administration ratio to be correlated to patient survival.3,46 Administration can be made individually or associated to factors VIII and XIII in the form of a cryoprecipitate. Prescription is therefore advised according to the results of the ROTEM or when the detected plasma levels are below 1.5g/l.37,40
The prothrombin complex allows us to administer large doses of coagulation factors (II, VII, IX and X) in a small volume, compared with FFP. It is clearly indicated in patients under the effect of certain anticoagulant drugs.35 The rest of the reported experiences, guided by the ROTEM, have not yet been validated.37
Lastly, the clinical trials that have evaluated the use of recombinant activated factor VII have evidenced a lesser need for blood products, as well as improvement of bleeding in closed trauma.53 However, since there is no clear decrease in mortality, recombinant activated factor VII is not regarded as a first line treatment option–its administration being restricted to compassionate use when other measures prove ineffective.4
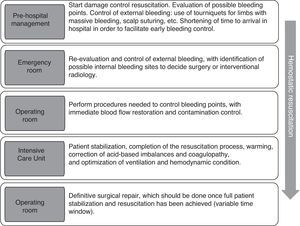
Control of bleeding: damage control surgeryAs has been commented above, the aim of damage control surgery (DCS) is to minimize the surgical times, since definitive management of the injuries from the start would worsen the situation of the patient and could even prove fatal, due to accelerated body heat loss caused by exposure during surgery, the difficulty of providing adequate volume replacement, and the increased presence of acidosis and coagulopathy.2,5,6,54 The concept of DCS, initially referred to major abdominal injuries characterized by uncontrollable bleeding and unstable pelvic fractures, has been expanded to cover all types of injuries: renal, retroperitoneal, vascular (of the extremities), thoracic, cerebral, etc. Hemostasis is usually achieved by arterial ligation, balloon tamponade catheters, vascular shunts or packing techniques.55 The resuscitation strategies are to be maintained during DCS and should be considered as important as anatomical repair itself.5 There is no concrete time window for definitive treatment of the injuries after DCS. As can be seen in Fig. 2, management should be decided according to the clinical condition of the patient, the type of lesion and the planned DCS. As a result, it is often implemented beyond the classically proposed first 48h.54,55
It is recommended that the time elapsed between trauma and surgery should be minimized in patients who require surgical control of bleeding.4 It is therefore crucial to establish an early diagnosis of such injuries. Apart from the traditional diagnostic methods, other complementary techniques such as focused assessment sonography in trauma (ECO-FAST), spiral CAT, digital angiography, etc., not only help to locate the injuries but moreover are able to assess their extent and impact with a view to afford a better surgical approach.56
Patients requiring DCS are those who are at an increased risk of death due to uncontrolled bleeding and in whom the parameters and situations cited in Table 3 are observed.2,5
Situations amenable to damage control surgery.
| 1. | High-energy trauma with penetrating or blast injuries |
| 2. | Coagulopathy present with impossibility of hemostasis |
| 3. | Major vascular damage |
| 4. | Multiple organ damage |
| 5. | Estimated surgical time for definitive repair > 90min |
| 6. | Severity indexes such as ISS > 25 |
| 7. | Vital signs: SBP <70mmHg, pH <7.1, Temp. <34°C or lactate >6mmol/l |
| 8. | Hemodynamic instability |
| 9. | Need for massive transfusion |
ISS: injury severity score; SBP: systolic blood pressure; Temp.: temperature.
(a) Abdominal packing: This early laparotomy technique allows us to identify serious injuries and sources of bleeding. When the surgical measures prove ineffective, packing may represent the first step within the concept of damage control. It can be used to compress liver rupture or directly apply pressure to bleeding points. The technique allows future attempts to secure complete hemostasis through arteriography and/or application until CIT has been corrected. In some cases aortic clamping and bypass may prove necessary to reduce bleeding and redistribute the blood flow to the heart and brain.4 Contamination can be adequately controlled by temporary ligation or exteriorization to the skin, in the case of ruptured ureters or damaged hollow organs, or by sealing intestinal damage without terminal anastomoses. If abdominal wall closure is not possible, the organs are covered with sterile dressings that are affixed to the wall margins, or using opened infusion solution bags or Opsite (“Vacpac”).5 In these circumstances, laparotomy should be revised in under 48h, once the bleeding risk has decreased.4 On occasion of the revision, the packing is removed and a thorough exploration of the zone is carried out.
The main complication of the procedure is the development of abdominal compartment syndrome, with an incidence of up to 15%, and which causes an important increase in mortality. The syndrome is a consequence of massive fluid support during resuscitation on one hand, and of organ damage caused by the traumatism on the other. Both mechanisms produce intestinal edema that gives rise to abdominal hypertension.7
(b) Arteriography and embolization: This is one of the most effective methods and allows minimally invasive control of bleeding in areas that are sometimes inaccessible or incoercible from the surgical perspective, and it can be used as a therapeutic complement to packing.57 Embolization should be as distal as possible in the culprit artery, in order to lessen the risk of tissue ischemia and lactic acidosis. This technique is indicated: (1) in the presence of hemodynamic instability or signs of active bleeding, once bleeding on non-abdominal origins has been discarded; and (2) when the CAT scan evidences contrast extravasation.
At present, the possibility of equipping the trauma emergency operating room with an interventional radiology team trained to act in resuscitation scenarios has been suggested. This project, known as Resuscitation with angiography, percutaneous techniques and operative repair (RAPTOR), could increase the management options targeted to the bleeding site, minimizing delays and in-hospital patient transfer.58 However, while interesting, this initiative is currently difficult to apply in many hospitals.
Pelvic damage control surgeryThe pelvic volume increases significantly following unstable pelvic fracture. This increase in volume favors bleeding and the massive hemorrhage caused by such fractures–thereby contributing to hemorrhagic shock. In hemodynamically stable patients with pelvic fracture, an abdominopelvic CAT scan is advised, due to the high incidence of associated intraabdominal injuries.4,59
- a.
External fixation: The use of external fixation reduces displacement of the fracture and thus lessens pelvic volume, but is unable to limit blood loss in patients with active pelvic hemorrhage. An increased need for blood product transfusions in the first 24–48h has been observed with external fixation in comparison with pelvic circumferential compression, probably as a result of the promptness with which such compression is applied.59
- b.
Pelvic circumferential compression: Although the theoretical models indicate that compression can help control bleeding, no subsequent studies have quantified its true effect in terms of the hemodynamic stabilization of unstable patients.59 It should be mentioned that when using pelvic circumferential compression, excessive fracture reduction should be avoided, the points of support must be taken into account, and it must be remembered that the nerve roots may be compressed in the case of sacral fractures.
- c.
Pelvic packing: In addition to closure and stabilization of the pelvic fracture, the hematoma tamponade effect afforded by pre-, extra- or retroperitoneal packing may reduce or arrest venous bleeding. Pelvic packing could help control intrapelvic bleeding on an early basis and offer a better moment for more selective management of the bleeding site. The technique can be combined with posterior laparotomy if necessary. This can help lessen the high mortality rate observed in patients with major pelvic injuries that require laparotomy as primary intervention.4
- d.
Arteriography and embolization: This technique is currently accepted as an effective option for the control of arterial bleeding when control has not been possible through stabilization of the fracture. It is indicated: (1) in the presence of hemodynamic instability or signs of active bleeding, once bleeding on non-pelvic origins has been discarded; (2) when the CAT scan evidences contrast extravasation; and (3) in patients over 60 years of age with major pelvic fractures (vertical, “open book” or “butterfly” fractures), independently of the hemodynamic condition.59 Several authors indicate that survival can be improved by permissive hypotension until stabilization of the fracture and/or arteriography becomes possible.5,6
The European guides recommend that patients with pelvic ring disruption under conditions of hemorrhagic shock should undergo immediate stabilization. Furthermore, in the presence of hemodynamic instability despite adequate stabilization of the pelvic ring, preperitoneal packing is advised, with arteriography and embolization if needed, and/or surgical control of the bleeding.4
Damage control surgery of the extremitiesThe use of a tourniquet is restricted to life-threatening bleeding in patients with open wounds of the extremities, until surgical resolution becomes possible. The use of this technique has traditionally been described in the context of catastrophes or military actions. The tourniquet should be applied for as short a time as possible, with a suggested maximum of 2h.4 Important fractures are mainly stabilized with external fixators instead of definitive primary osteosynthesis. The latter can be indicated at a later stage (4–14 days), once the patient has been stabilized.5
DiscussionMost of the objectives of resuscitation in trauma have been addressed in the course of this study and are reflected in Table 4.
Objectives of resuscitation in the severe trauma patient.
| Variable | Objective |
| Heart rate | <100bpm |
| Arterial pressure | |
| During initial resuscitationPatients with TBI or ASI | Systolic: 80–90mmHgMean: ≥80mmHg |
| After bleeding control | >100mmHg |
| Urinary output | 0.5–1ml/kg/h |
| Control of hypothermia | Temperature >35°C |
| Restore blood volume | Fluid therapyConsider use of inotropic vasopressor drugs |
| Carbon dioxide | Normoventilation (in absence of endocranial hypertension) |
| Correction of coagulopathy | Transfusion of blood products and antifibrinolytic agents |
| Fibrinogen | >1.5g/l |
| Ionic calcium | >0.9mmol/l |
| Correction of acidosis (lactic, hyperchloremic, others) | Treat underlying causeIf pH <7: sodium bicarbonate+hyperventilation+calcium |
| Lactate | ≤2mmol/l |
| Base excess | >−6mEq/l |
ASI: acute spinal cord injury; TBI: traumatic brain injury.
Although we have offered an in-depth exploration of the physiopathological knowledge referred to trauma, the review of the current literature cannot be considered complete. The phenomena triggered after injury, and their consequences, are still not fully known. We are therefore unable to design resuscitation strategies targeted to the individual and to the concrete events occurring in each patient.1 At present, attention centers on ensuring perfusion and restoring hemostasis, though consistent and fully supporting evidence is lacking in this regard. As an example, the study published by Khan et al.33 shortly before the final draft of the present article found that hemostatic resuscitation failed to achieve CIT control or restore tissue perfusion until 24h after trauma, i.e., until the time when damage and bleeding control had been achieved. On the other hand, it should be commented that much of the available evidence comes from military scenarios, with their specific particularities, and such data cannot always be extended to the civil setting.
In sum, despite the profound conceptual changes in pre-hospital care and management in the first hours after trauma, further work is needed to promote knowledge related to trauma patients, in order to evaluate the current strategies adopted in our Units.60 In this respect we need more quality trials allowing adequate consolidation of changes in the management of severe traumatisms, as has recently occurred with the use of tranexamic acid.49 Lastly, we must underscore the need to implement critical trauma resuscitation protocols in our own Units and hospitals with a view to ensure the early identification of bleeding foci, their early management through surgical or interventional techniques, and the implementation of a massive transfusion protocol to shorten the management times in the control of massive bleeding.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Egea-Guerrero JJ, Freire-Aragón MD, Serrano-Lázaro A, Quintana-Díaz M, Grupo de Trabajo de Trauma y Neurointensivismo de SEMICYUC. Objetivos y nuevas estrategias de resucitación en el paciente traumatizado grave. Med Intensiva. 2014;38:502–512.