Due to the increase in isolation of Candida spp. in critically ill patients, and the high mortality and economic costs which this infection entails, a study was made of the risk factors associated to candidemia in critically ill patients from 7 intensive care units in Colombia.
Materials and methodsA multicenter matched case–control study was conducted in 7 intensive care units of 3 university hospitals. Data on overall length of hospital stay (including both general wards and the intensive care unit) were recorded.
ResultsA total of 243 subjects (81 cases and 162 controls) between January 2008 and December 2012 were included. In order of frequency, Candida albicans, Candida tropicalis and Candida parapsilosis were isolated. The main identified risk factors were: overall length of hospital stay >25 days (OR 5.33, 95%CI 2.6–10.9), use of meropenem (OR 3.75, 95%CI 1.86–7.5), abdominal surgery (OR 2.9, 95%CI 1.39–6.06) and hemodialysis (OR 3.35, 95%CI 1.5–7.7). No differences in mortality between patients with candidemia and controls were found (39.5 versus 36.5%, respectively; p=0.66).
ConclusionsIn Colombia, a long hospital stay, abdominal surgery, the use of meropenem and hemodialysis were identified as risk factors for candidemia.
Determinar los factores de riesgo asociados a candidemia en pacientes críticos de 7 unidades de cuidados intensivos de Colombia.
Materiales y métodosEstudio de casos y controles pareado, multicéntrico, retrospectivo, en 7 unidades de cuidados intensivos de 3 hospitales universitarios. Se tomaron datos de duración de la estancia hospitalaria global (incluyendo salas generales) y en la unidad de cuidados intensivos.
ResultadosSe incluyeron 243 participantes (81 casos y 162 controles) entre enero de 2008 y diciembre de 2012. Se aislaron en orden de frecuencia C. albicans, C. tropicalis y C. parapsilosis. Los principales factores de riesgo identificados fueron: tiempo de estancia hospitalaria global > 25 días (OR 5,33; IC 95% 2,6-10,9), uso de meropenem (OR 3,75; IC 95% 1,86-7,5), cirugía abdominal (OR 2,9; IC 95% 1,39-6,06) y hemodiálisis (OR 3,35; IC 95% 1,5-7,7). No se encontraron diferencias en mortalidad entre los grupos de pacientes con candidemia y el grupo control (39,5 frente a 36,5%; p = 0,66).
ConclusionesSe identificaron como factores de riesgo para candidemia en Colombia la larga estancia hospitalaria, la cirugía abdominal, el uso de meropenem y la hemodiálisis.
Among the infections acquired in the Intensive Care unit (ICU), the isolation of Candida spp. in blood cultures is characterized by high morbidity–mortality.1,2 A retrospective analysis of the study on the prevalence of infections in intensive care in Europe (EPIC II) found Candida spp. infection in blood to be associated to high mortality rates and an important use of intensive care resources.3,4 In Colombia, the genus Candida accounts for 99% of all fungal species and 5.2% of the total microorganisms isolated in blood.5 Gudlaugsson et al.6 reported an attributable 30-day mortality rate of 38% and an overall mortality rate of 49% in patients with nosocomial candidemia.
Candidemia has increased in recent years in critically ill patients, due to expansion of the risk population and increased patient survival, exposure to a greater number of invasive procedures, immunosuppression, the use of broad-spectrum antibiotics, alteration of the host innate or acquired immune mechanisms, or mucosal barrier alterations associated to the use of central catheters, renal failure subjected to hemodialysis, abdominal surgery, or neutropenia, among other factors.7–12
Different clinical predictive algorithms have been developed and validated,4,13 involving both colonization indices and risk factors, and which in combination with clinical judgment facilitate the diagnosis and opportune and adequate treatment of disorders of this kind.14–17
We consider that in Colombia, the risk factors for candidemia in critical patients may be different from those found in regions of Europe and North America, since epidemiological differences are found in the Colombian population, in the same way as in other countries in Latin America: non-Candida albicans species cause over 50% of the episodes of candidemia – the most prevalent being Candida parapsilosis and Candida tropicalis.3,6,15 In view of these possible circumstances, we must establish our own approach to the diagnosis and management of such disorders.
The aim of the present study was to determine the risk factors associated to the isolation of Candida spp. in the blood cultures of patients admitted to 7 ICUs of three university hospitals in Colombia; establish the frequency of the different species of Candida that cause candidemia in the ICU; and describe the epidemiological and clinical characteristics of the patients who suffer candidemia, as well as the mortality rate in the studied population.
Material and methodsAn analytical, paired case–control multicenter observational study was carried out.
We collected the microbiological and clinical data related to the isolation of Candida spp. in the blood cultures of patients admitted to hospital between the years 2008 and 2012 in a high-complexity private center with two ICUs–one polyvalent and the other cardiovascular (23 ICU beds)–in the city of Bogota, and in a hospital of similar characteristics in the city of Neiva, with two polyvalent ICUs and a total of 21 ICU beds. All three centers shared common characteristics as regard the treated populations, with an important representation of patients with complex medical, cardiovascular and traumatic diseases. The blood cultures were processed in the Microbiology Laboratory of each of the institutions using the automated systems MicroScan® (Siemens) or Vitek® (Biomerieux).
Cases were defined as patients with the isolation of Candida spp. in the blood cultures performed in the ICU after 48h of stay in the Unit, between the years 2008 and 2012. For each case we selected two controls over 18 years of age, admitted for over 48h to the same ICU, with no isolation of Candida spp. in any culture, and no use of antifungal drugs. The controls were paired as having been admitted to the same ICU as the cases, in the same period (±3 days), and presenting a similar age (±5 years). Pregnant women were excluded from both groups, since the study institutions usually do not assist this population, as well as patients with neutropenia (defined as a neutrophil count of <500cells/mm3), in order to avoid selection bias in the critical population. The cases and controls were analyzed from the time of admission to each institution, covering the stay in hospital wards before admission to intensive care, and stay in the ICU.
The study variables included: patient age, gender, the APACHE II score upon admission to the ICU, the day of blood culture sampling for the cases, and diabetes mellitus defined as a history or diagnosis of the disease reflected in the case history upon admission. The diagnosis leading to admission was defined as the principal diagnosis in the case history corresponding to hospital admission. The total stay in hospital was determined as the time from hospital admission to discharge; the stay up until development of the invasive fungal infection (for the cases) was regarded as the time from hospital admission to the day of blood culture positivity for Candida. The duration of stay in the ICU was likewise recorded in both groups.
Immunocompromised patients were defined as those subjects with a history of any of the following: chemotherapy, HIV infection/AIDS, bone marrow transplantation or corticosteroid use for more than 7 days. Broad-spectrum antibiotic treatment was defined as the prescription of any of the following drugs: carbapenems, quinolones, third and fourth generation cephalosporins, and piperacillin/tazobactam. We identified those patients with abdominal surgery and cardiovascular surgery, since the study included two cardiothoracic ICUs. Other surgeries were defined as any surgical procedure not involving access to the abdominal cavity during hospitalization. Dialysis was taken to include continuous and intermittent renal replacement therapy in chronic or acute patients. We also documented the need for parenteral nutrition and the presence of severe sepsis or septic shock.
The study was approved by the Ethics Committee and the Infections and Epidemiological Vigilance Committee of each of the participating centers. Patient data confidentiality was observed at all times.
Statistical analysisThe sample size was calculated using StatCalc, part of the Epi Info™ package, with a significance level of 95%, a statistical power of 80%, two controls per case, a percentage control exposure rate of 40% and a percentage case exposure rate of 69%. Using an odds ratio (OR) of 3.3 based on the study published by Barberino et al.15 in Brazil for the variable use of antibiotics and candidemia, the resulting sample size was found to be 105 (35 cases and 70 controls).
The variables were described per categories, with calculation of central tendency and dispersion measures for continuous variables, and frequencies for categorical variables.
The SPSS® version 17 statistical package was used. The Student t-test was applied for the comparison of variables exhibiting a normal distribution, with the Mann–Whitney–Wilcoxon test as applicable, and the chi-squared test for comparing categorical variables. The Kolmogorov–Smirnov normality test was used to evaluate the distribution of the populations. The odds ratio (OR) was calculated as association measure, with the corresponding confidence intervals, and statistical significance was considered for p<0.05. A multivariate analysis was carried out with those variables that reached statistical significance in the univariate analysis.
ResultsThe 7 ICUs of the three participating institutions included a total of 81 cases and 162 controls. The institution with the largest number of ICU beds contributed 51% of the cases and controls, the hospital with 23 ICU beds contributed 31%, and the hospital with 21 beds contributed 18%. We included patients requiring intensive care and admitted either directly to the Units or from the general Hospital wards.
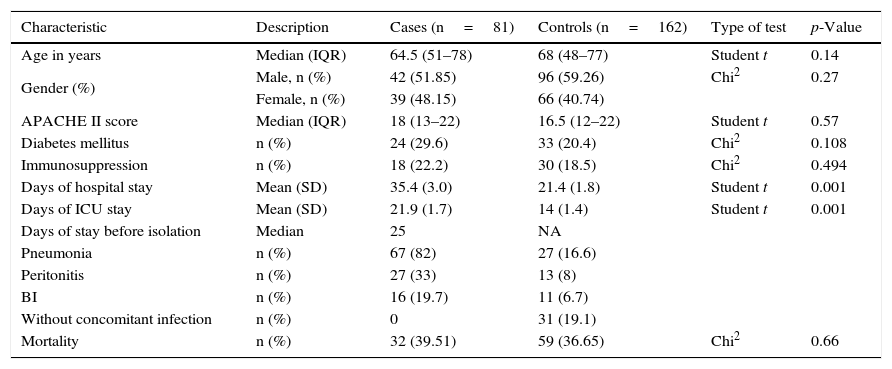
The participants in both groups did not differ in terms of the severity of disease, age, gender or comorbidities such as diabetes mellitus (Table 1). The median age of the cases was 64.5 years (interquartile range [IQR] 51–78), versus 68 years (48–77) for the controls. The median APACHE II score was 18 (13–22) for the cases and 16.5 (12–22) for the controls. The mortality rate was similar in both groups, with 32 fatalities among the cases (39.51%) and 59 in the control group (36.65%), p=0.66.
Characteristics of the study population.
| Characteristic | Description | Cases (n=81) | Controls (n=162) | Type of test | p-Value |
|---|---|---|---|---|---|
| Age in years | Median (IQR) | 64.5 (51–78) | 68 (48–77) | Student t | 0.14 |
| Gender (%) | Male, n (%) | 42 (51.85) | 96 (59.26) | Chi2 | 0.27 |
| Female, n (%) | 39 (48.15) | 66 (40.74) | |||
| APACHE II score | Median (IQR) | 18 (13–22) | 16.5 (12–22) | Student t | 0.57 |
| Diabetes mellitus | n (%) | 24 (29.6) | 33 (20.4) | Chi2 | 0.108 |
| Immunosuppression | n (%) | 18 (22.2) | 30 (18.5) | Chi2 | 0.494 |
| Days of hospital stay | Mean (SD) | 35.4 (3.0) | 21.4 (1.8) | Student t | 0.001 |
| Days of ICU stay | Mean (SD) | 21.9 (1.7) | 14 (1.4) | Student t | 0.001 |
| Days of stay before isolation | Median | 25 | NA | ||
| Pneumonia | n (%) | 67 (82) | 27 (16.6) | ||
| Peritonitis | n (%) | 27 (33) | 13 (8) | ||
| BI | n (%) | 16 (19.7) | 11 (6.7) | ||
| Without concomitant infection | n (%) | 0 | 31 (19.1) | ||
| Mortality | n (%) | 32 (39.51) | 59 (36.65) | Chi2 | 0.66 |
SD: standard deviation; BI: bloodstream infection; NA: not applicable; ICU: Intensive Care Unit.
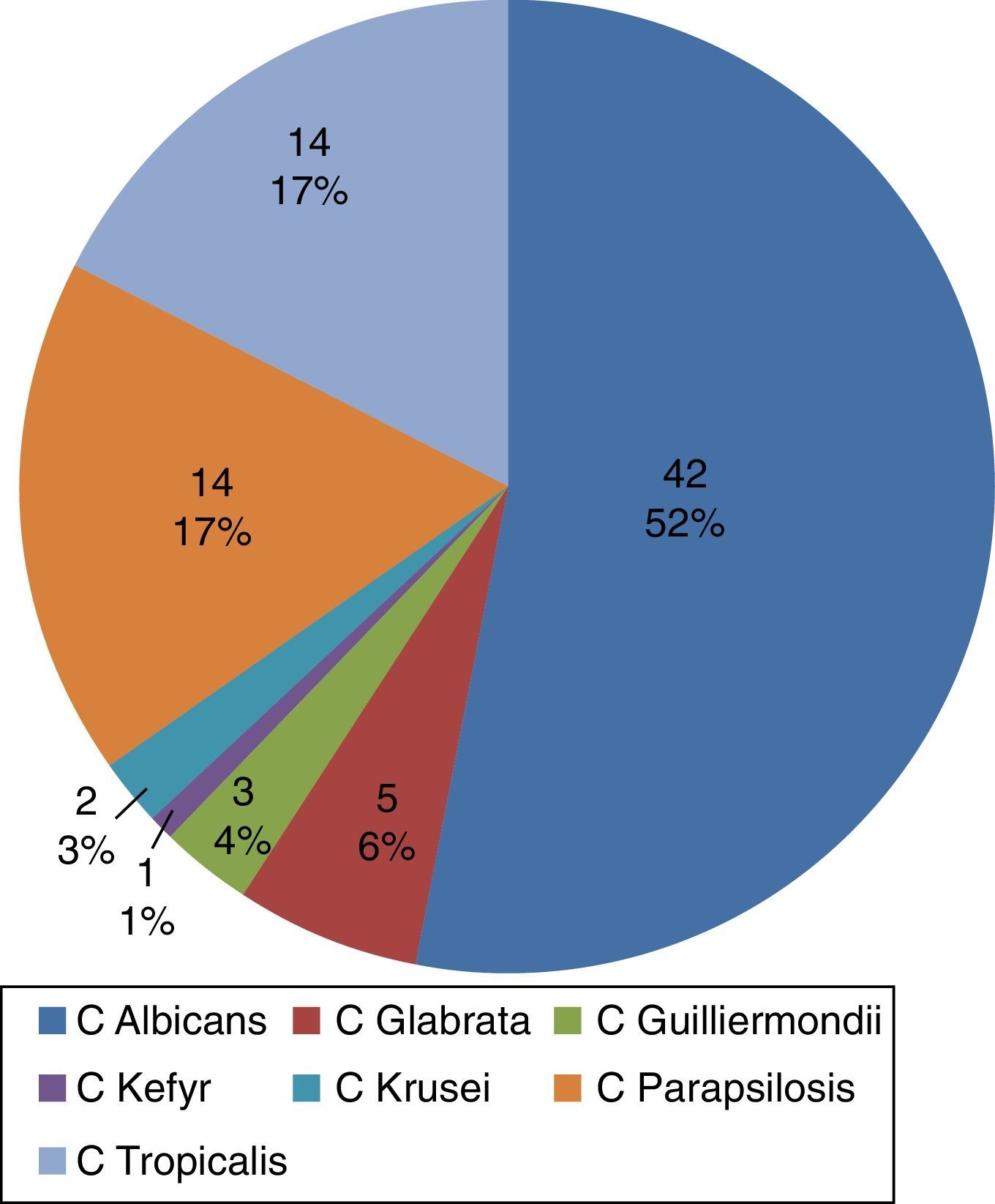
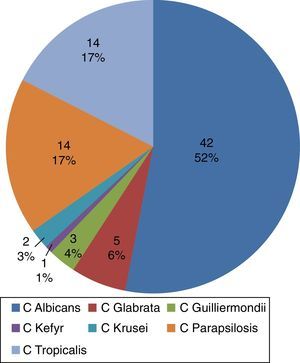
The most frequently isolated Candida species in blood were C. albicans, C. tropicalis and C. parapsilosis (Fig. 1). The diagnoses upon admission were grouped according to the affected organ system: cardiovascular disease in 34.5%, gastrointestinal disorders in 24.6%, and lung disease in 25.3%. The main infectious processes concomitant to candidemia were pneumonia (nosocomial or ventilator-associated pneumonia) in 33% of the cases, and catheter-related bloodstream infection (septicemia) (20%). The main infectious diagnoses among the controls were peritonitis and pneumonia.
The distribution of broad-spectrum antibiotic use among the cases was as follows: meropenem (64%), piperacillin/tazobactam (41.9%), and fourth generation cephalosporins (27.1%). The main antibiotics used in the controls were: piperacillin/tazobactam (28.4%) and meropenem (27.6%).
The mean stay in hospital for the controls was 21.4 days (standard deviation [SD] 1.4), versus 35.4 days (SD 3.0) for the cases. In turn, the mean stay in the ICU for the controls was 14 days (1.4), versus 21.9 days (SD 1.7) for the cases. The median stay before the isolation of Candida spp. in blood cultures was 25 days for the cases. These data (over 14 days in the ICU or more than 25 days of general hospital stay) were considered in establishing the cutoff point for comparing the impact of the variable as a candidemia risk factor between groups.
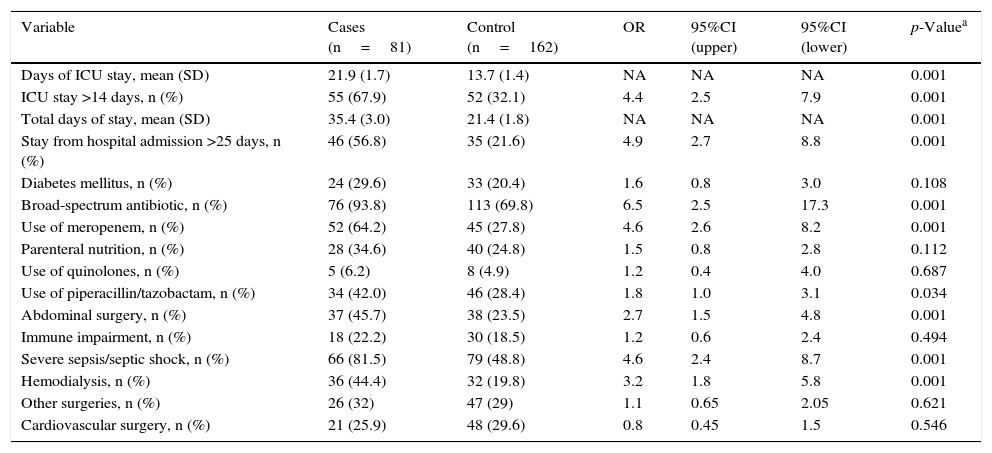
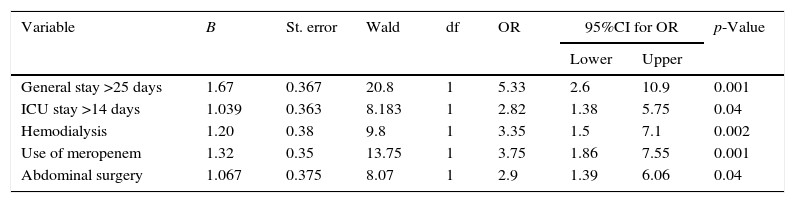
A first univariate analysis was carried out (Table 2). The factors showing statistical significance in this analysis in turn were entered in the multivariate model (Table 3). The identified risk factors for candidemia were global hospital stay >25 days (OR 5.33; 95%CI 2.6–10.9)–the mean stay in the ICU for the cases being 21.9 days (SD 1.7) versus 14 days (SD 1.4) among the controls–the use of meropenem (OR 3.75; 95%CI 1.86–7.5), previous abdominal surgery (OR 2.9; 95%CI 1.39–6.06), and the application of hemodialysis (OR 3.35; 95%CI 1.5–7.7).
Univariate analysis.
| Variable | Cases (n=81) | Control (n=162) | OR | 95%CI (upper) | 95%CI (lower) | p-Valuea |
|---|---|---|---|---|---|---|
| Days of ICU stay, mean (SD) | 21.9 (1.7) | 13.7 (1.4) | NA | NA | NA | 0.001 |
| ICU stay >14 days, n (%) | 55 (67.9) | 52 (32.1) | 4.4 | 2.5 | 7.9 | 0.001 |
| Total days of stay, mean (SD) | 35.4 (3.0) | 21.4 (1.8) | NA | NA | NA | 0.001 |
| Stay from hospital admission >25 days, n (%) | 46 (56.8) | 35 (21.6) | 4.9 | 2.7 | 8.8 | 0.001 |
| Diabetes mellitus, n (%) | 24 (29.6) | 33 (20.4) | 1.6 | 0.8 | 3.0 | 0.108 |
| Broad-spectrum antibiotic, n (%) | 76 (93.8) | 113 (69.8) | 6.5 | 2.5 | 17.3 | 0.001 |
| Use of meropenem, n (%) | 52 (64.2) | 45 (27.8) | 4.6 | 2.6 | 8.2 | 0.001 |
| Parenteral nutrition, n (%) | 28 (34.6) | 40 (24.8) | 1.5 | 0.8 | 2.8 | 0.112 |
| Use of quinolones, n (%) | 5 (6.2) | 8 (4.9) | 1.2 | 0.4 | 4.0 | 0.687 |
| Use of piperacillin/tazobactam, n (%) | 34 (42.0) | 46 (28.4) | 1.8 | 1.0 | 3.1 | 0.034 |
| Abdominal surgery, n (%) | 37 (45.7) | 38 (23.5) | 2.7 | 1.5 | 4.8 | 0.001 |
| Immune impairment, n (%) | 18 (22.2) | 30 (18.5) | 1.2 | 0.6 | 2.4 | 0.494 |
| Severe sepsis/septic shock, n (%) | 66 (81.5) | 79 (48.8) | 4.6 | 2.4 | 8.7 | 0.001 |
| Hemodialysis, n (%) | 36 (44.4) | 32 (19.8) | 3.2 | 1.8 | 5.8 | 0.001 |
| Other surgeries, n (%) | 26 (32) | 47 (29) | 1.1 | 0.65 | 2.05 | 0.621 |
| Cardiovascular surgery, n (%) | 21 (25.9) | 48 (29.6) | 0.8 | 0.45 | 1.5 | 0.546 |
SD: standard deviation; CI: confidence interval; NA: not applicable; OR: odds ratio; ICU: Intensive Care Unit.
Multivariate analysis. Risk factors for the development of candidemia.
| Variable | B | St. error | Wald | df | OR | 95%CI for OR | p-Value | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| General stay >25 days | 1.67 | 0.367 | 20.8 | 1 | 5.33 | 2.6 | 10.9 | 0.001 |
| ICU stay >14 days | 1.039 | 0.363 | 8.183 | 1 | 2.82 | 1.38 | 5.75 | 0.04 |
| Hemodialysis | 1.20 | 0.38 | 9.8 | 1 | 3.35 | 1.5 | 7.1 | 0.002 |
| Use of meropenem | 1.32 | 0.35 | 13.75 | 1 | 3.75 | 1.86 | 7.55 | 0.001 |
| Abdominal surgery | 1.067 | 0.375 | 8.07 | 1 | 2.9 | 1.39 | 6.06 | 0.04 |
CI: confidence interval; OR: odds ratio; ICU: Intensive Care Unit.
Abdominal surgery and hemodialysis were identified in our study as risk factors for the development of candidemia, in concordance with the observations of other authors.1 In contrast to other publications,12,13 parenteral nutrition was not identified as a significant risk factor, possibly because of its low frequency of use in both study groups. On the other hand, on comparing the controls versus the cases, the median days of stay prior to isolation of the fungus was also identified as a risk factor. In this regard, the greater probability of exposure to antibiotics and to diagnostic and therapeutic interventions in the course of longer admissions may have played a contributing role, since prolonged stay–whether in the general hospital ward or in the ICU–is unlikely as an isolated factor to increase patient vulnerability to infection.
A case–control study carried out in Nebraska (USA) with the purpose of developing a predictive tool for invasive candidiasis identified the following risk factors: broad-spectrum antibiotic use (OR 2.21; 95%CI 1.29–3.79; p<0.005); central venous catheter use (OR 4.42; 95%CI 1.95–10.02; p<0.001); abdominal surgery (OR 3.40; 95%CI 2.02–5.70; p<0.001); parenteral nutrition (OR 3.80; 95%CI 2.18–6.63; p<0.001) and immunosuppressor therapy (OR 3.80; 95%CI 2.18–6.63; p<0.001). The results of the study showed the tool to offer high negative predictive value, and suggest the need to develop a similar instrument at local level, allowing identification of the true population at risk and thereby contributing to reduce inappropriate antifungal use.16
With regard to previous antibiotic use, the prescription of meropenem was found to exert a significant effect. This may be explained by the widespread use of this drug in all the Units, since meropenem was prescribed in 64% of the cases and in 24% of the controls–reflecting that the individuals with candidemia had important prior exposure to antibiotic medication.13,18
Pasero et al.19 using logistic regression analysis, evaluated the main predictors of candidemia in a cardiovascular ICU among patients admitted for over 48h. They identified parenteral nutrition, severe sepsis, the APACHE II score, and an ICU stay of over 20 days as risk factors. Only one of these factors (ICU stay) was identified as a risk factor in our study, which included two cardiovascular ICUs.
Our findings are also consistent with those published by Ostrosky-Zeichner et al.13,17 in a large retrospective cohort study involving patients admitted to intensive care for at least four days in 9 hospitals in the United States and Brazil. The observed incidence of fungal infection was 3%, and the main candidemia risk factors were seen to be systemic antibiotic use, central venous catheterization, parenteral nutrition, and hemodialysis.15 In coincidence without own observations, these authors associated broad-spectrum antibiotic use to a risk of developing candidemia.
Other risk factors for invasive candidiasis described in the literature, such as diabetes mellitus, failed to reach statistical significance in our study. With respect to parenteral nutrition, the absence of a significant association can probably be attributed to its low frequency of use in both of our groups, due to the early enteral nutrition protocols that are used in our ICUs. Likewise, the absence of a relationship between candidemia and immunosuppression could be explained by the few patients with hematological malignancies or other conditions characterized by immune impairment treated in our ICUs during the study period.
On the other hand, significant differences were observed in terms of stay in the ICU–the duration of stay being longer in the patients who developed candidemia in our series compared with other publications in which candidemia occurred in the first 10 days of admission to intensive care. In our study, fungal septicemia was of late onset.
In concordance with the findings of other authors, the mortality rate was high,20,21 though without significant differences between the two groups. We feel that this may have been because the controls were selected from the same postoperative and polyvalent ICUs, with patients presenting similar APACHE II scores upon admission.
In coincidence with the international literature1,19 and with the data published in 2010 by Cortés et al.4 in an epidemiological study of systemic fungal infections in high-complexity hospitals in Colombia and in the Latin American registry,2,15 we found the Candida species most frequently isolated in blood cultures to be C. albicans (52%), with a tendency to decrease in proportion with respect to non-Candida albicans species (C. parapsilosis, C. tropicalis, C. glabrata), which were found in 40% of the cases.
The strengths of our study are the control of bias, with an adequate sample size and the inclusion of populations comparable in terms of variables other than the presence of candidemia (i.e., age, gender, APACHE II score, diabetes, immunosuppression, etc.).
As to the limitations of the study, it must be mentioned that the analyzed broad-spectrum antibiotics did not include those drugs exclusively destined to cover resistant grampositive bacteria, such as vancomycin. However, this aspect has been more frequently cited in the pediatric population. Likewise, it was not possible to analyze the days of exposure to central intravascular devices, since the required information was not available. Nevertheless, we were able to identify the main risk factors for the development of candidemia in critical patients at local level.
ConclusionsThe factors associated to the appearance of candidemia in critical patients in Colombia were found to be a global hospital stay of over 25 days and an ICU stay of over 14 days; the use of broad-spectrum antibiotics (mainly meropenem); a history of major abdominal surgery; and hemodialysis. Of note is the presence of two risk factors not contemplated by the most widely used predictive tools in intensive care, such as the Candida Score: the use of antibiotics and prolonged hospital stay. No association to parenteral nutrition was recorded. Despite the high mortality rate associated to candidemia, no differences in this regard were observed between the two study groups.
Conflicts of interestThe authors of the present study declare that they have no conflicts of interest referred to the presentation, evaluation and possible publication of the manuscript.
Please cite this article as: Ortíz Ruiz G, Osorio J, Valderrama S, Álvarez D, Elías Díaz R, Calderón J, et al. Factores de riesgo asociados a candidemia en pacientes críticos no neutropénicos en Colombia. Med Intensiva. 2016;40:139–144.