Lung transplant recipients are at high risk of suffering many complications during the immediate postoperative period, such as primary graft dysfunction, acute graft rejection or infection. The most common symptom is the presence of acute respiratory failure, and the use of biomarkers could be useful for establishing an early diagnosis of these conditions.
Different biomarkers have been studied, but none have proven to be the gold standard in the differential diagnosis of acute respiratory failure.
This paper offers a review of the different biomarkers that have been studied in this field.
Los receptores de un trasplante pulmonar tienen un alto riesgo de presentar numerosas complicaciones durante el postoperatorio inmediato, como la disfunción primaria del injerto, el rechazo agudo del injerto o las infecciones. El síntoma más común será la presencia de insuficiencia respiratoria aguda, y el uso de biomarcadores podría ser de gran utilidad para establecer un diagnóstico precoz de estas entidades.
Hasta la fecha, se han estudiado diferentes biomarcadores, pero ninguno ha demostrado ser el gold estándar en el diagnóstico diferencial de la insuficiencia respiratoria aguda.
En este artículo se expone una revisión de los diversos biomarcadores que han sido estudiados en este campo.
In the last 20 years, lung transplantation has become an established practise for prolonging survival among patients with advanced-stage lung disease. According to the registry of the International Society for Heart and Lung Transplantation,1 a total of 3272 transplants were carried out in the year 2009, and during the first month mortality was fundamentally attributable to primary graft dysfunction (PGD) (27.1%), followed by infections (20.1%). In turn, almost 4% suffered acute rejection.
Thus, lung transplant recipients are at a high risk of developing many complications during the immediate postoperative period, including PGD, acute graft rejection of the development of infections, as commented above. The most frequent manifestation in all these clinical conditions is acute respiratory failure (ARF). For this reason, the differential diagnosis of these conditions can be very difficult to establish, and may have important consequences, since the treatment required in each case differs in certain aspects. Thus, in the presence of acute rejection, we need to increase the level of immunosuppression; in the case of PGD, immunosuppression must be lowered; and in patients with infections we must prescribe antibiotic treatment. In this context, although the diagnosis of PGD is fundamentally clinical, distinction between rejection and infection often requires histological evaluation of the samples obtained by fibrobronchoscopy with transbronchial biopsy. The use of this technique is limited, however, since it is invasive and has potential complications that can prove serious – particularly in patients with severe ARF.
Despite the existence of preventive measures against PGD,2 such as the optimization of lung preservation, the minimization of ischemia time, and the avoidance of barotrauma during lung donor maintenance, once the damage has been established, the treatment is similar to that applied in patients with respiratory distress syndrome. In any case, a survival rate of 80% in the first year after transplantation, and of 50% after 5 years of follow-up, is considered acceptable.
The fact that the lungs are in direct contact with the exterior, among other factors, contribute to the need for high levels of immunosuppression; despite such immunosuppression, however, the acute rejection rates remain high.3
Different biomarkers have been investigated with the aim of improving and anticipating the diagnosis of these disorders. A biomarker is defined as a parameter or characteristic that can be objectively measured and evaluated as an indicator of normal biological processes, pathological conditions, or responses to drug treatment.4 An ideal biomarker is a parameter that can be recorded quickly from a sample obtained in a minimally invasive manner, and which is simple to preserve and handle. In addition, an ideal biomarker should be sensitive, reproducible, predictive and cost-effective. The lack of biological markers capable of predicting the early onset, progression and severity of disease has had a negative impact upon the identification and development of effective drug treatments for improving morbidity and mortality among critical patients.
Many biomarkers potentially useful for the differential diagnosis of post-transplantation ARF have been studied. However, their use in daily clinical practise is very limited, since the available supporting evidence is scarce. The present study offers a clinical review of the available evidence referred to the usefulness of the different biomarkers in application to the differential diagnosis of ARF in the immediate postoperative period of lung transplantation.
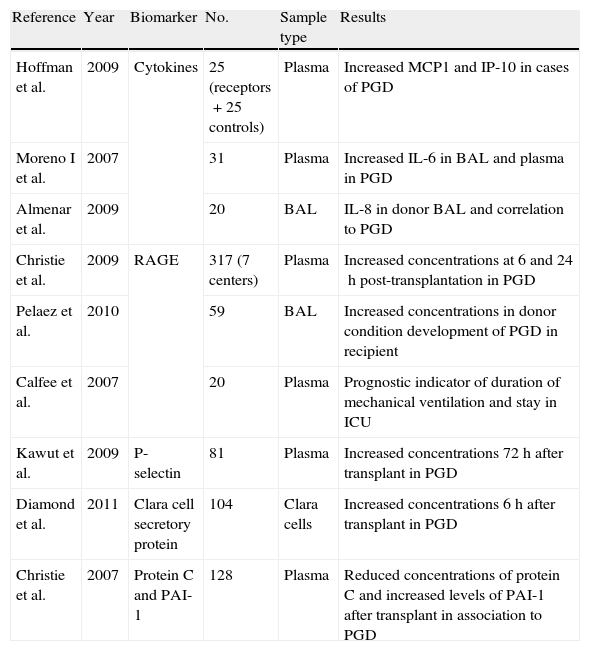
Primary graft dysfunctionPrimary graft dysfunction (PGD) is a form of acute lung injury occurring in the immediate post-transplantation period, and which has been defined by the consensus document of the International Society for Heart and Lung Transplantation as hypoxemia manifesting in the first 72h after lung transplantation, with pulmonary infiltrates evidenced on the chest X-rays.5 The prevalence of PGD ranges widely from 10–40%,6,7 and its appearance has prognostic implications, since it is associated with increased morbidity-mortality in the Intensive Care Unit (ICU).6,8,9 At clinical level, PGD has been almost exclusively associated with ischemic damage occurring during lung preservation and posterior reperfusion–though factors related to donor maintenance may also play an important role.10 The physiopathology of PGD is characterized by an increase in the concentration of inflammatory and endothelial and epithelial dysfunction biomarkers.11,12 For this reason, an analysis has been made of the usefulness of different biomarkers in the diagnosis of primary graft dysfunction, taking into account the degree of PGD (Table 1).
Principal biomarkers studied in relation to primary graft dysfunction.
| Reference | Year | Biomarker | No. | Sample type | Results |
| Hoffman et al. | 2009 | Cytokines | 25 (receptors+25 controls) | Plasma | Increased MCP1 and IP-10 in cases of PGD |
| Moreno I et al. | 2007 | 31 | Plasma | Increased IL-6 in BAL and plasma in PGD | |
| Almenar et al. | 2009 | 20 | BAL | IL-8 in donor BAL and correlation to PGD | |
| Christie et al. | 2009 | RAGE | 317 (7 centers) | Plasma | Increased concentrations at 6 and 24h post-transplantation in PGD |
| Pelaez et al. | 2010 | 59 | BAL | Increased concentrations in donor condition development of PGD in recipient | |
| Calfee et al. | 2007 | 20 | Plasma | Prognostic indicator of duration of mechanical ventilation and stay in ICU | |
| Kawut et al. | 2009 | P-selectin | 81 | Plasma | Increased concentrations 72h after transplant in PGD |
| Diamond et al. | 2011 | Clara cell secretory protein | 104 | Clara cells | Increased concentrations 6h after transplant in PGD |
| Christie et al. | 2007 | Protein C and PAI-1 | 128 | Plasma | Reduced concentrations of protein C and increased levels of PAI-1 after transplant in association to PGD |
PGD: primary graft dysfunction; IL-6: interleukin 6; IL-8: interleukin 8; IP-10: interferon induced protein; BAL: bronchoalveolar lavage; MCP1: monocyte chemotactic protein-1; PAI-1: plasminogen activator inhibitor; RAGE: receptor for advanced glycation end products; ICU: Intensive Care Unit.
Cytokines are low molecular weight proteins secreted by different immune cells. They play a key role in inflammation and in regulation of the immune response. Different studies have examined the usefulness of cytokine determination in the diagnosis of PGD. In this sense, it has been shown that elevated interleukin 8 (IL-8) levels in the immediate post-transplantation period are significantly correlated to the subsequent development of PGD.13 On the other hand, studies of changes in the expression of different cytokines and chemokines during the immediate post-transplantation period14 have revealed an increase in the plasma levels of monocyte chemotactic protein-1 (MCP-1) and IP-10, a protein induced by gamma-interferon (IFN-γ), implicated in the recruitment of monocytes and lymphocytes, in those patients that develop PGD. These results suggest that macrophage activation induced by IFN-γ, and the attraction of monocytes and effector T cells, could play an important role in the pathogenesis of PGD. In fact, there are data indicating that IP-10 could be an important factor in cardiac and renal post-transplantation injury.15–19 On the other hand, the concentration of interleukin 6 (IL-6), in both bronchoalveolar lavage (BAL) and in plasma, measured in the first hours after transplantation, is directly related to the development of PGD.20 Likewise, elevated IL-8 concentrations in donor BAL favor the development of PGD and imply a prolongation of mechanical ventilation in the transplant recipient.13
Receptor for advanced glycation end productsStudies have also been made of the usefulness of receptor for advanced glycation end products (RAGE) in the diagnosis of PGD after lung transplantation. RAGE is a marker of type I alveolar cell damage,21 and is a receptor of the immunoglobulin family.22 RAGE is present in different tissues, and is expressed at low concentrations under normal conditions. Over-regulation of this marker has been associated to different disorders ranging from atherosclerosis to Alzheimer's disease.23 Although its function at lung level has not been clarified, RAGE is regarded as a marker of the severity of acute lung injury.24–26 In lung transplant patients, a positive correlation has been reported between RAGE in donor BAL and the subsequent development of PGD in the recipient.27 Likewise, an association has been observed between increased plasma concentrations of the marker 6 and 24h after lung transplantation and the development of PGD.28 On the other hand, it has been reported that the plasma concentration of RAGE four hours after graft perfusion may be of prognostic significance–high plasma RAGE being correlated to a prolongation of mechanical ventilation and stay in the ICU after transplantation.29
P-selectinAnother of the proposed biomarkers for predicting PGD is P-selectin, a platelet activation marker.30–33 Previous studies have shown neutrophil adhesion to the pulmonary vascular endothelium, diapedesis, and infiltration of the vessel wall to be a key event in the development of PGD.34,35 In this sense, the platelets are involved in neutrophil sequestration, activation and mobilization toward the interstitial and alveolar space. In lung transplant patients with PGD, the plasma P-selectin levels have been correlated to the appearance of grade III PGD.9
Clara cell secretory proteinAnother proposed biomarker is Clara cell secretory protein, which appears to participate in the repair and protection of the respiratory epithelium, in toxin detoxification, and in the production of surfactant.36 A positive correlation has been observed between plasma Clara cell secretory protein levels in the immediate post-transplantation period and the development of PGD.37 These results support the importance of epithelial damage in the origin of PGD.
Protein C and plasminogen activator inhibitorProtein C and plasminogen activator inhibitor (PAI-1) have also been proposed as biomarkers of PGD, and have been the subject of a multicenter study comprising 6 centers and 128 patients.38 The results showed a decrease in protein C and an increase in PAI-1 before transplantation and 6, 24, 48 and 72h after transplantation to be associated with the development of grade III PGD–thus evidencing the importance of coagulation markers in the development of PGD.
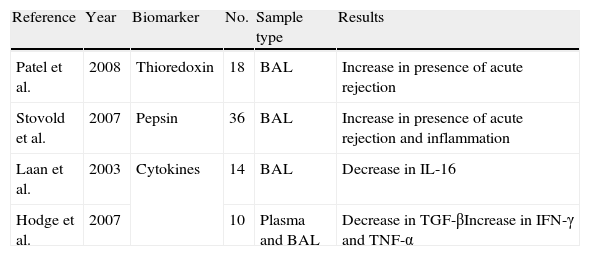
Acute rejectionAcute rejection is also a frequent complication in the immediate postoperative period of lung transplantation. Its estimated incidence is 36% in the first year after transplantation, and compared with other organs, the lungs appear to be at an increased risk of rejection.39 On the other hand, the appearance of acute rejection can have important consequences for patient prognosis, since immunosuppressive treatment of acute rejection episodes increases the risk of infections.39 As has been commented above, the clinical characteristics of the disease are very similar to those of other complications that manifest during the same period, and this complicates the diagnosis. To date, bronchoscopy with the obtainment of a transbronchial biopsy has been the technique of choice for diagnosing acute rejection. Very few biomarkers have been investigated in application to the diagnosis of acute graft rejection (Table 2).
Principal biomarkers studied in relation to acute graft rejection.
| Reference | Year | Biomarker | No. | Sample type | Results |
| Patel et al. | 2008 | Thioredoxin | 18 | BAL | Increase in presence of acute rejection |
| Stovold et al. | 2007 | Pepsin | 36 | BAL | Increase in presence of acute rejection and inflammation |
| Laan et al. | 2003 | Cytokines | 14 | BAL | Decrease in IL-16 |
| Hodge et al. | 2007 | 10 | Plasma and BAL | Decrease in TGF-βIncrease in IFN-γ and TNF-α |
IFN-γ: gamma-interferon; IL-16: interleukin 16; TGF-β: transforming growth factor-beta; TNF- α: tumor necrosis factor-alpha; BAL: bronchoalveolar lavage.
Some studies have analyzed the usefulness of thioredoxin, a protein that regulates oxidative metabolism40 and which exerts antiinflammatory effects in different tissues.41 Results of initial studies in experimental models revealed an association between the appearance of acute graft rejection and high levels of this protein.42 More recently, studies have been made of the levels of thioredoxin in BAL and in transbronchial biopsy samples from transplant patients.43 The results show an increase in the concentration of thioredoxin in BAL of patients with histological criteria of acute graft rejection.
PepsinPepsin is an enzyme that hydrolyzes proteins in the stomach. Consequently, when found in respiratory samples, pepsin indicates the aspiration of gastric contents. Studies of the concentration of pepsin in BAL samples of lung transplant patients have revealed higher pepsin concentrations in patients presenting acute rejection.44 These results suggest that acute rejection is conditioned not only by immunological factors but also by direct aggression upon the grafted organ, such as that resulting from the microaspiration of gastric contents.
CytokinesInterleukin 16 (IL-16) is a CD4 receptor ligand that participates in antigen presentation. It is known that CD4+ lymphocytes are implicated in the development of acute rejection, and that IL-16 can inhibit the activity of this complex.45 Its concentration has been shown to decrease in episodes of acute rejection,45 in the same way as the levels of TGF-β in CD4+ and CD8+ blood cells. In contrast, the levels of gamma-interferon and tumor necrosis factor-alpha (TNF-α) have been seen to increase in these same cells in BAL samples,46 thus suggesting synergic action of both molecules, with activation of the development of acute rejection through the activation of epithelial cells, and demonstrating that acute rejection episodes are associated to a decrease in the levels of Th3 type cytokines (TGF-β) and an increase in the levels of type Th1 cytokines (IFN-γ and TNF-α).
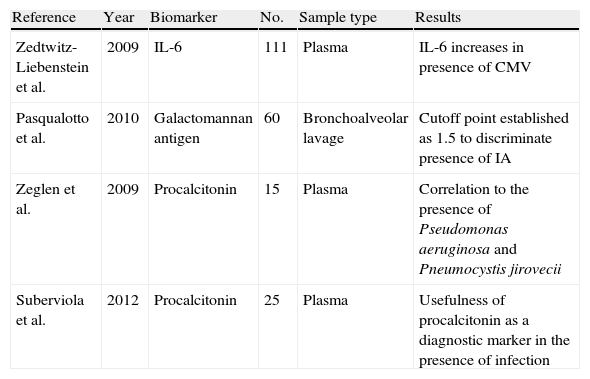
InfectionDespite the experience gained in the field of lung transplantation, supported by advances in the form of new surgical techniques and the introduction of new immunosuppressor drugs, the infections which transplant recipients may develop in the postoperative period are an important cause of morbidity and mortality. This problem is particularly manifest in the immediate postoperative period, since it is in this period when the risk of acute rejection is greater and higher levels of immune suppression are therefore needed.47 Moreover, the patient is in the hospital setting, with an increased risk of nosocomial infections. Table 3 shows the main biomarkers related to the development of the different infectious processes.
Principal biomarkers studied in relation to infection.
| Reference | Year | Biomarker | No. | Sample type | Results |
| Zedtwitz-Liebenstein et al. | 2009 | IL-6 | 111 | Plasma | IL-6 increases in presence of CMV |
| Pasqualotto et al. | 2010 | Galactomannan antigen | 60 | Bronchoalveolar lavage | Cutoff point established as 1.5 to discriminate presence of IA |
| Zeglen et al. | 2009 | Procalcitonin | 15 | Plasma | Correlation to the presence of Pseudomonas aeruginosa and Pneumocystis jirovecii |
| Suberviola et al. | 2012 | Procalcitonin | 25 | Plasma | Usefulness of procalcitonin as a diagnostic marker in the presence of infection |
IA: invasive aspergillosis; CMV: cytomegalovirus; IL-6: interleukin 6.
Studies have been made of the usefulness of the determination of IL-6 and interleukin 10 (IL-10) concentrations in the diagnosis of cytomegalovirus (CMV) infection.48 The results revealed an increase in the concentration of IL-6 in plasma and in BAL samples from patients colonized by CMV. However, the great variability observed in the concentration of IL-6 between different patients made it difficult to establish its usefulness in predicting the appearance of CMV infection. In contrast, no correlation to the levels of IL-10 was observed.
Galactomannan antigenAnother pathogen that can colonize the graft in this same time period is Aspergillus spp. Approximately 6–16% of all patients may be colonized by Aspergillus, and some studies have shown 9% of all post-transplantation deaths to be attributable to invasive aspergillosis.49 In recent years, the detection of galactomannan antigen in hematological patients has been found to be reliable in establishing an early diagnosis of Aspergillus infection.50 Such detection can be made in BAL or serum samples, though serum assaying yields a larger number of false-positive results.50 In a study involving 60 post-transplantation BAL samples, 8 of which were diagnosed with invasive aspergillosis according to the criteria of the European Organization for Research and Treatment of Cancer/Mycoses study group in the diagnosis of invasive fungal diseases and radiological criteria, an optical density of 1.5 in the results was established as the best cutoff point for the diagnosis of this condition, with a sensitivity of 100% and a specificity of 90%.50 However, the authors pointed out that the lack of a standard for the bronchoscopic technique poses an important limitation that must be taken into account when interpreting these results.
ProcalcitoninProcalcitonin (PCT) is a 116-amino acid peptide produced and secreted under normal conditions by the thyroid gland as a precursor of calcitonin. The basic inducer of OCT is bacterial wall lipopolysaccharide. In contrast, the secretion of PCT is not stimulated, or is very weakly stimulated, by viral infections and autoimmune processes. Moreover, PCT concentration appears to be closely related to the severity of respiratory infection of bacterial origin.51
Studies have been made of the plasma concentrations of PCT in the context of infections caused by Pseudomonas aeruginosa and Pneumocystis jirovecii in lung transplant patients. In this regard, the concentration of PCT was seen to increase with the presence of both infections,52 thus suggesting that determination of the concentration of the peptide might be useful for differentiating between acute rejection and infection.53
In addition, it has recently been demonstrated54 that serial PCT determinations are correlated to the presence of infectious complications.
Limitations of the studiesThe studies carried out to date have a series of limitations that complicate interpretation of the results obtained. A first problem is the limited number of patients included in the different studies. Secondly, the included patients are very heterogeneous–a fact that makes it difficult to extrapolate the results obtained. Lastly, there are also differences in sample collection and processing, thus indicating the need to standardize such techniques. It is therefore important to emphasize that no ideal biomarker of help in the differential diagnosis of post-transplantation ARF has been defined to date.
Future lines of researchNew faster and more sensitive diagnostic techniques would allow us to establish a more effective diagnosis, based on the precise quantification of a concrete biomarker. In this sense, metabolomics allows the total assessment of metabolites in an organism, and represents a global evaluation of the biochemical and physiological condition of the patient. The detection of these markers can be made in different samples (fluids, cell types and tissues). Critically ill patients might benefit from these techniques, since they frequently suffer metabolic deregulations.55
Another aspect that may be of interest is research based on microRNA (miRNA), which has been shown to have many applications, such as for example in the early detection of lung cancer or rheumatoid arthritis.56–58 MicroRNA consists of small, non-coding molecules which nevertheless are important for the regulation of genic expression. These molecules are implicated in regulation of the development of the immune system and cell proliferation. For these reasons, research has recently focused on the relationship between the appearance of kidney and liver graft rejection and different types of microRNA. The results have revealed an association between certain types of microRNA and acute rejection.59 However, to date the studies in the field of transplantation include very few patients and have fundamentally focused on these kinds of transplantations.60,61
ConclusionsThe success of lung transplantation is largely dependent upon its management in the immediate postoperative phase. Close monitoring of the evolution of the graft is therefore necessary from the immediate post-transplantation phase, in order to anticipate any problems capable of affecting the outcome. In this context, the analysis of the different biomarkers capable of contributing to the differential diagnosis of post-transplantation ARF is very important. Although some studies offer encouraging results, no ideal biomarker has yet been described capable of substantially improving the management and prognosis of these patients. The use of new techniques, such as the analysis of microRNA, could lead to further advances in this respect.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Ruano L, et al. Papel de los biomarcadores en el diagnóstico diferencial de la insuficiencia respiratoria aguda en el postoperatorio inmediato del trasplante pulmonar. Med Intensiva. 2013;37:416–422.