Spain has become one of the most active countries in donation after controlled cardiac death, using normothermic abdominal perfusion with ECMO in more than 50% of all donors – a situation contributed to by the creation of mobile teams to support hospitals lacking this technology. The donation process must be respectful of the wishes and values of the patients and their relatives, especially if there is pre mortem manipulation, and the absence of cerebral perfusion should be guaranteed. The liver is the most benefited organ by reducing biliary complications as well as the loss of grafts. In renal transplantation, the technique could contribute to reduce the incidence of delayed graft function. In addition, the procedure is compatible with surgical rapid recovery in hypothermia when there is also lung donation. The future lies in the consolidation of cardiac donation by extending normothermic perfusion to the thoracic cavity.
España se ha convertido en uno de los países más activos en donación en asistolia controlada incorporando la perfusión abdominal normotérmica con ECMO en más del 50% de los donantes, a lo que ha contribuido la creación de equipos móviles para apoyo a hospitales carentes de esta tecnología. El proceso de donación debe ser respetuoso con los deseos y valores del paciente y sus familiares, especialmente si hay manipulación pre mortem, y debe asimismo garantizar la ausencia de flujo cerebral. El hígado es el órgano más beneficiado al reducirse las complicaciones biliares, así como la pérdida de injertos. En el trasplante renal podría contribuir a reducir la incidencia de retraso en la función inicial del injerto; además, el procedimiento es compatible con la cirugía súper rápida en hipotermia cuando también hay donación pulmonar. El futuro pasa por la consolidación de la donación cardíaca al extender la perfusión normotérmica a la cavidad torácica.
Controlled asystole donation (CAD) started its path in Spain approximately 10 years ago.1 The growing need of transplant organs added to a progressive decrease of the donation potential under conditions of brain death (BD). Also, we were witnessing changes in end-of-life patient care based on the recommendations made by the Spanish Society of Critical Intensive Medicine and Coronary Units on life-support treatment adequacy (LSTA) that acknowledged the inappropriateness of prolonging the treatment beyond a situation of futility.2,3 In this context a national program was implemented to develop organ donation after the prior, independent decision of LSTA. Following a pilot CAD project, the development of a new regulatory framework bak in 20124 and the publication of the National Consensus Document on Donation in Asystole,5 the foundations for its clinical practice were laid.
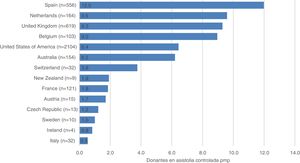
The protocolization of this activity, as well as the training of the professionals involved and the institutional support granted to the program have placed Spain as one of the most active countries regarding CAD (Fig. 1).6 Back in 2018 over 100 hospitals in our country had already developed, at least, one CAD process, and 28% of the donors registered became donors in asystole donation (Fig. 2).7
Donors in CAD per million inhabitants worldwide in 2018.
Donors in CAD per million inhabitants (pmi) reported to the Global Observatory on Donation and Transplantation. The absolute number of donors in CAD donors is seen in between brackets.
Source: Global Observatory on Donation and Transplantation (http://www.transplant-observatory.org/).
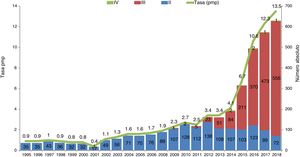
Evolution of CADs in Spain.
Evolution of CADs in Spain in absolute number and rate per million inhabitants (pmi).
Maastricht type ii: death after unsuccessful resuscitated cardiorespiratory arrest; Maastricht type iii: death after withdrawal of life-sustaining therapies; Maastricht type iv: cardiorespiratory arrest after brain death.
Source: Spanish National Transplant Organization (ONT).
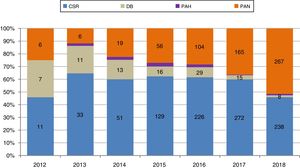
The increased activity of CAD progressively added oabdominal normothermic regional perfusion with extracorporeal membrane oxygenation (ANRP-ECMO) as a technique used to preserve organs in situ. Its theoretical benefits should become evident in a larger number of organs available, as well as in the better results reported after the transplant. Throughout these years, ANRP-ECMO has coexisted with other organ harvesting procedures: cold abdominal perfusion with triple-lumen, double-balloon catheter (which has progressively fallen in disuse after gaining momentum at the beginning), and superfast surgery (SFS). The use of ANRP-ECMO has been gaining relevance to the point that, to this date, it is applied to over 50% of all CAD procedures performed (Fig. 3). The boom of this technique in Spain has been followed by use in other neighboring countries such as France, Italy, and the United Kingdom.8
Evolution of the different in situ preservation and harvesting techniques in controlled asystole donation in Spain.
in situ preservation and harvesting techniques in CAD in Spain, in percentages and absolute numbers over the years.
ANRP, abdominal normothermic regional perfusion; DB, double balloon; HAP, hypothermic abdominal perfusion; SFS, super-fast surgery.
Source: Spanish National Transplant Organization (ONT).
The objective of this article is to describe the ANRP-ECMO technique in CAD and the complications associated with its use, to analyze the evidence available on its impact on the effectiveness of the process and the results reported after the transplant, approach its logistical issues and ethical challenges, and anticipate its future role regarding heart transplants from these donors.
The abdominal normothermic regional perfusion technique in controlled asystole donationThe potential CAD donors are patients with catastrophic brain damage or end-stage cardiac, respiratory or neurodegenerative disease in whom the LSTA decision has been made after being considered futile and in anticipation of an early death. Once the family has made such decision, the organ donation option can be considered, and if it consistent with the values and principles of their loved one, the donor can be prepared for donation.
Unlike donors under conditions of BD, organs in CAD undergo ischemic insult due to progressive hypotension and hypoxia from the LSTA until cardiac arrest (CA) plus the 5 min of observation required by the law before death is certified.4
To minimize ischemic insult, it is imperative to quickly initiate organ protection. SFS has been the method of choice for many years, and it is the standard of use in most countries.9 It consists of laparotomy and/or sternotomy immediately after death, depending on whether there will be abdominal organ donation, lung donation or both followed by cannulation of the abdominal aorta and/or pulmonary artery, perfusion of preservation cold fluid and local cooling, and harvesting of the corresponding organs. On the other hand, ANRP-ECMO has positioned itself in Spain as an alternative that is being used more and more frequently. The procedure, which is more complex than SFS, includes several stages.
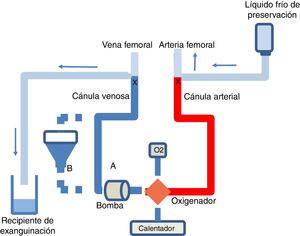
CannulationIt can be performed both pre- and post-mortem. The Spanish legislation does not prohibit performing pre-mortem procedures for better organ preservation as long as a specific authorization for that purpose has been obtained (often a consent by proxy decision making).5 It is necessary to heparinize and cannulate the potential donor’s femoral arteries and veins for their eventually connection to the ECMO circuit under the corresponding sedoanalgesia (Fig. 4). The cannulas often used (18-Fr to 21-Fr venous cannulas and 15-Fr to 19-Fr arterial cannulas) are smaller in caliber compared to those used in critically ill patients since only the abdominal cavity will be perfused.
Diagram of the ECMO circuit and exsanguination procedure in CAD.
ECMO circuit without (A) or with (B) reservoir, perfusion pump, oxygenator, oxygen source, and heater. At the end of the ANRP-ECMO technique the arterial and venous cannulas are clamped, the cold perfusion fluid is perfused via the arterial cannula, and the venous cannula is eventually used for exsanguination.
Cannulation is a critical stage in the entire ANRP-ECMO process since it can determine its success or failure. Hemorrhage, impossibility to cannulate, and occlusion balloon migration are among the problems that have been described, which have resulted in the ANRP-ECMO suspension, organ harvesting through SFS or, in some cases, the death of the donor.10
Although cannulation in therapeutic ECMO can be central or peripheral depending on the cases,11 in pre-mortem CAD preparation access is peripheral, whether through local surgery, percutaneously or mixed, just in case any of these procedures has complications.12 Although it cannot be claimed that one technique is better than the other, the development of percutaneous techniques using Seldinger technique has made it easier for intensivists to participate in the procedure.13
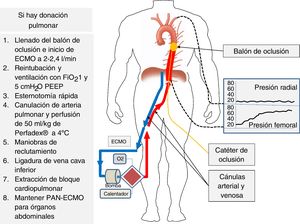
An occlusion balloon catheter should be placed in the contralateral femoral artery, and then advanced empty until reaching the thoracic aorta and, once the patient has died and before ECMO is initiated, it is filled up with fluid to occlude the thoracic aorta and prevent ascending flow and the reperfusion of heart and brain, which is one of the major concerns when using ANRP-ECMO.14 The position of the balloon should be controlled radiologically before the LSTA and the left radial artery monitored during the entire procedure to ensure the absence of flow (Fig. 5).15
Donor’s cannulation and monitoring for ANRP-ECMO.
The left radial artery is catheterized followed by arterial and femoral vein cannulation while the occlusion catheter is placed at thoracic aorta level. After death, the occlusion balloon becomes filled, and when perfusion with ECMO is initiated, the progressive increase of nonpulsatile pressure at femoral level and the absence of pressure at radial level become evident, which confirms the correct occlusion of the aorta. If lung harvesting should be performed, it will follow the steps shown on the figure right side.
In some countries like the United Kingdom, where pre-mortem manipulation is not allowed, and in some Spanish hospitals that have decided to prepare donors after they have died, post-mortem cannulation and surgical clamping of the thoracic aorta are used. In these cases, warm ischemia time prolongs depending on the experience of the surgical team. Former studies have described average times of 7 min between the skin incision and cannulation,16 and more prolonged warm ischemia times compared to pre-mortem cannulation.17
Life support withdrawalWhen the potential donor’s preparation has come to an end, the team proceeds to LSTA in the operating room or in the intensive care unit (ICU), depending on the protocol in place in each hospital, and then the procedure is initiated.
Abdominal normothermic regional perfusionOnce death is certified, in donors cannulated before the LSTA, the aortic occlusion balloon is filled, and perfusion progressively initiated with ECMO until reaching, in just a few seconds, flows between 2 L/min and 2.4 L/min. When cannulation is performed post-mortem ECMO is initiated after the aorta has been clamped. It is necessary to maintain an average blood pressure of 60–65 mmHg, a temperature between 96.8 °F and 98.6 °F, and oxygen flow on a 1/1 initial ratio with the ECMO flow. The patient will be transfused and administered bicarbonate if necessary to ensure hematocrit levels >25% and 7.35–7.45 pH. Also, the oxygen source flow will be modified to ensure normal arterial gases. Frequent analytical control should be performed, at least every 30 min, including arterial gases, lactate, hematocrit and hemoglobin, ionogram, and liver and kidney profiles.
In liver validation, early AST/ALT values <3 times the upper limit of normal, and final AST/ALT values <4 times the upper limit of normal are suggested to declare a feasible liver.18 Before retrieving the abdominal organs, ECMO is interrupted, the arterial cannula used to perfuse the cold preservation fluid, and the venous cannula used for exsanguination (Fig. 4).
Among the organ selection criteria, the warm ischemia time, and the macroscopic aspect of the organ together with the analytical values are assessed. The total warm ischemia time (from LSTA to ANRP-ECMO) should be <30–45 min, and functional warm ischemia (from systolic blood pressure ≤60 mmHg to ANRP-ECMO) <20–30 min for the liver. The lung, pancreas, and kidney admit a maximum total warm ischemia time of 60 min, although this criterion has been modified lately based on experience.5
The maximum duration of ANRP-ECMO has not been established definitively, but most series use times that go from one hour and a half until 2 h for the lack of definitive studies on this regard.10,19
Contribution of abdominal normothermic regional perfusion to abdominal organ donationDamage to transplant organs is often due to ischemia, from the interruption of organ circulation to the perfusion of the preservation hypothermic solution and the release of toxic metabolites was a result of reperfusion.20 CAD adds to the ischemic stress that occurs while dying. Therefore a greater incidence of delayed graft function (DGF) has been described in the case of kidney transplant, though with similar long-term progression compared to kidney transplant from donation after brain death (DBD).21 In the liver, which is especially sensitive to ischemia, the risk of early graft dysfunction, bile duct lesions, and retransplant, and less graft survival is greater compared to organ transplants from DBD.22 Ischemic cholangiopathy, one of the most serious complications of liver transplant, is more frequent in CAD graft recipients with a 16%–29% rate compared to DBD (3%–17%).23 There seems to be a direct correlation with ischemia time, especially with the time from asystole to clamping prior to the perfusion of cold preservation fluid.24
To minimize these risks, several strategies have been considered to reduce warm ischemia time, select donors with probable early death, shorten cold ischemia times or improve the selection of transplant recipients.25 The strategy that has provided the best results has been the use of ANRP-ECMO since reverts metabolic deviations, re-establishes cellular physiology after energy depletion, and clears the metabolites resulting from ischemia. This way preconditioning is provided first as a back-up of static cold preservation followed by warm ischemia in the recipient, thus attenuating the ischemia-reperfusion lesion.18,26
ANRP-ECMO is a remarkable improvement regarding harvesting viable abdominal organs for transplant compared to SFS.27,28 Also, unlike the latter, it turns organ obtention into a «quiet» procedure that is similar to extractions under BD conditions, with enough time to analyze biochemical parameters, assess, and monitor the functional recovery of liver metabolism, and the attenuation of ischemic insult prior to liver retieval.19
The ANRP-ECMO superiority in liver transplants in CAD has been confirmed in 2 recent retrospective multicenter studies. The largest series published so far by Hessheimer et al.17 includes all livers transplanted in CAD in Spain from June 2012 through December 2016. The comparison between 95 liver transplants in CAD managed with ANRP-ECMO and 117 harvested with SFS showed fewer biliary complications (8% vs 31%), a lower rate of ischemic cholangiopathy (2% vs 13%), and a lower risk of graft loss (12% vs 24%) with the ANRP-ECMO technique. The British series of Watson et al.,29 that compared 43 liver transplants in CAD to ANRP-ECMO vs 187 liver transplants retrieved through SFS found fewer early dysfunctional grafts (12% vs 32%), fewer graft losses at 30 days (2% vs 12%), less stenosis of biliary anastomosis (7% vs 27%), and lack of ischemic cholangiopathy (0% vs 27%). This lack of ischemic cholangiopathy has been reported even with donors >65.30 In view of these studies, it seems justified to consider that the ANRP-ECMO technique to perform liver transplants in CAD contributes to a successful transplant.28
Regarding kidney transplants in CAD, the rates of primary nonfunctional graft, DGF, and 1-year survival are 4%, 58%, and 93%, respectively.1 Despite the high rate of DGF, both the mid- and long-term survival rate of these kidneys and function are similar to the ones reported in donation after brain death (DBD). Also similar are the results obtained with kidney transplants in CAD with expanded criteria compared to the same type of graft in DBD.21
In view of this information, is there a reason that justifies the use of the ANRP-ECMO technique in CAD where there is only kidney harvesting? Although it is difficult to answer this question, this technique can help minimize potential organ damage or loss that may result from surgical accidents derived from SFS.31 Also, the ANRP-ECMO technique seems to reduce the rate of DGF and, therefore, hospital stay.32 More studies are necessary to confirm these findings. If a reduction of DGF is confirmed, a comparison of the costs resulting from the use of ECMO and those associated with a greater need for dialysis after the transplant should be made.
The pancreas is a hard-to-get organ; therefore, CAD represents another source added to BD, both in isolation33 and in the combined pancreas-kidney transplant.34 Although favorable results have been published regarding pancreatic transplant using the ANRP-ECMO technique, experience is still limited.16,19
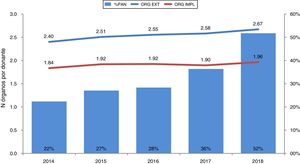
Effectiveness in the donation process is another favorable fact of ANRP-ECMO since the average number of harvested and implanted organs per donor goes up year after year, which is consistent with its growing use (Fig. 6). Also, more organs are harvested and implanted when the ANRP-ECMO technique is used compared to SFS (3.0 and 2.4 vs 2.2 and 1.8, respectively according to data from the Spanish National Transplant Organization-ONT).
Effectiveness of the use of the ANRP-ECMO technique in CAD processes in Spain.
Effectiveness of the use of the ANRP-ECMO technique. Its use has been going up every year until it has been used in 52% of all donors in CAD back in 2018. Consistent with this, the number of organs harvested and implanted has been going up too.
% ANRP, percentage of use of abdominal normothermic regional perfusion with respect to the overall number of donors in CAD; ORG. HARV., number of organs harvested per donor; ORG. IMPL., number of organs implanted per donor.
Lung transplant in CAD provides similar and even better results compared to those performed in DBD.35,36 However, in our country only 10% of CAD donors are lung donors.37 In CAD the lungs are retrieved with SFS, decreasing lung temperature rapidly with topical cooling. Although chest hypothermia is harmful to the liver and abdominal normothermia damages the lung, if the ANRP-ECMO technique is used in a multiorgan donor, the British groups have described a combined lung harvesting technique with SFS plus ANRP-ECMO using post-mortem cannulation and lung topical cooling with 4 L of a cold saline solution (39.2 °F) in each hemothorax.38
The Marqués de Valdecilla Hospital published a variation of this technique, adding pre-mortem interventions to minimize functional warm ischemia and the complications associated with recovery; this way it was not necessary to use 4 L of a cold saline solution, which minimized the risk of transdiaphragmatic cooling of the liver.19 Recently, an experience with 19 cases of CAD with this type of combined harvesting was published (the largest series published to this date), with excellent results regarding effectiveness (16 of the 19 livers harvested and 37 of the 38 lung grafts were transplanted successfully), with identical survival rates compared to those of the control group of organs from BD.39
Unlike the lungs from uncontrolled asystole donation where use of ex situ devices is advised for lung validation and reconditioning,40,41 in the experiences with lungs in CAD, the use of ex situ devices is not something common being only used in cases with very long functional warm ischemia times or edematous lungs.36
Given the excellent results of lung transplants in CAD, the use of this combined technique should be encouraged in multiorgan donors. The description of this combined procedure is shown on Fig. 5. Once a patient has been pronounced dead and after the ANRP-ECMO technique is initiated, the thoracic surgeon should perform a fast sternotomy. At the same time, the donor is re-intubated and ventilated with oxygen at 100% and a PEEP of 5 cm H2O. Afterwards, the pulmonary artery is cannulated and a preservation solution (Perfadex®, 50 mL/kg) is perfused through the cannula. Only the lungs are cooled topically with 1 L of a saline solution at 39.2 °F. Once the lungs have been preserved, alveolar recruitment maneuvers are performed, if necessary, in case of atelectasis. Then, the heart-lung block can be extracted. Prior to the extraction of the cardiopulmonary block, the inferior vena cava should be ligated immediately below the right atrium to separate the thoracic and abdominal compartments and the cardiopulmonary block should be retrieved simultaneously leaving the ANRP-ECMO technique working for as long as it is required as if we were dealing with an abdominal extraction only.
The most important complication of the procedure is the loss of flow in the ECMO circuit pump due to bleeding in the thoracic cavity after the cardiopulmonary block has been extracted or due to loss of venous return when the vena cava has been ligated and the two compartments (chest and abdomen) have been isolated. To prevent this, the azygos vein should be ligated followed by careful hemostasis of the thoracic cavity. In addition, the donor should be administered 1–1.5 L of a saline solution before the vena cava is ligated to compensate for any possible loss of venous return. A variation of the procedure has been described with satisfactory results avoiding the ligation of the vena cava initially, improving the volume of venous return, and eventually preventing the complication described above.42
We should mention that, after clamping the vena cava, all the vascular, jugular, subclavian and upper limb accesses are lost, which means that the administration of fluids and/or blood derivatives can only be performed via a femoral catheter or through the ECMO reservoir (Fig. 4).
Role of mobile extracorporeal membrane oxygenation teams in the promotion and management of controlled asystole donationAccording to the recommendations made by the Extracorporeal Life Support Organization,43 ECMO is a complex technique that should take place in centers experienced in heart surgery, heart and/or lung transplant, and cardiothoracic ICUs. On the other hand, there are currently over 100 hospitals in our country that have CAD programs underway, many of which lack the necessary means to implement ANRP-ECMO processes. The experience published on the benefits of this procedure, especially regarding liver donation, confirms how importance it is to look for the necessary means so that this preservation technique can become available in all centers with CAD programs. In the section with recommendations on the use of ECMO regarding organ donation in the document of the Spanish Society of Critical Intensive Medicine and Coronary Units (SEMICYUC), it is recommended that «if this technology is not available, cooperation agreements should be established with hospitals that actually have it and can take to other places».44
Mobile ECMO teams are already a reality in several autonomous communities throughout our country. They basically go to CAD-capable hospitals to support the preservation and harvesting of abdominal organs.45–47 To this date, 13 centers can already use these machines, and according to data from the Spanish National Transplant Organization (ONT), over the last 2 years, these centers have participated in 26% of controlled asystole donations managed with the ANRP-ECMO technique.
The makeup of these teams is variable. However, they should all include a surgeon, a nurse or a perfusionist plus an intensivist who should work together with the donor hospital transplant coordination and with the harvesting surgeons.
Ethical aspects of abdominal normothermic regional perfusionThe use of the ANRP-ECMO technique has led to ethical discussions regarding the patient’s manipulation before death, information on the procedure in the informed consent forms or the re-establishment of circulation after the diagnosis of death.14Pre-mortem cannulation or the administration of heparin have been considered an aggression to the patient’s integrity for the benefit of a third party. However, in organ donation from living donors, for example, citizens accept healthcare acts on their own body that are not aimed at improving their health, but the health of others. This right to help other human beings should not be violated through a paternalistic approach to it. The debates on pre-mortem cannulation should observe the principle of patient’s self-sufficiency, that is, give the patient the chance to donate their organs and achieve the best possible result from this donation, as a sign of respect to everyone’s freedom to manage their own biography.
The principle of nonmaleficence is not violated with pre-mortem cannulation. There is no harm intended and the patient’s welfare is guaranteed using the proper sedoanalgesia. The principle of nonmaleficence compels us to avoid obstructing the right to decide freely, the right to the principle of patient’s representation, the right of donation as a comprehensive part of end-of-life care or the principle of respect for the patient’s life project, values, and ideals.
When the patient cannot make his own decisions, these should be based on a proxy decision making process using both a subjective criterion (implementing the patient’s instructions on the type of healthcare he prefers if he becomes incapacitated), and the substituted judgment criterion (the proxy makes the same decision the patient would if he could make it himself).48
To make decisions regarding end-of-life care it is essential that the free decision should be made after receiving appropriate information. In the case of pre-mortem cannulation or heparin administration, it is mandatory to have an informed consent document that registers this process of informing to the family seeking their comprehension and approval.5
It has been suggested that re-establishing circulation with the use of ECMO would invalidate death pronouncement if brain perfusion has not been properly excluded, since death is determined by the permanent cessation of blood flow towards the brain.14 However, this risk associated with the ANRP-ECMO technique is more of a technical issue rather than an ethical problem. The use of an aortic block protocol with further verification of intra-aortic occlusion and invasive monitoring of left radial artery pressure during the entire entire procedure of preservation and extraction guarantees the absence of brain reperfusion.15,18
The future of normothermic perfusion: heart donationThe heart had not been considered a transplantable organ in this type of donors until the early 21st century because it was considered that ischemic insult after CA caused irreversible myocardial damage. However, in 2008 the Denver team (Colorado, United States) published the first 3 cases of pediatric heart transplant in CAD.49 The protocol included pre-mortem heparinization and cannulation. The donors’ mean age was 3.7 days, the mean time from the LSTA until the death was 18.3 min, and the time since CA until death occurred ranged between 75 s and 3 min, which was controversial at that time. To minimize ischemia time, both donors and recipients were placed in adjacent operating rooms. The recipients survival rate at the 6-month follow-up was 100%.
A year later, the Papworth Hospital group (Cambridge, United Kingdom) harvested the heart of a 57-year female CAD donor. Following the donor’s death and once the supra-aortic trunks had been clamped to prevent brain reperfusion, a thoracoabdominal normothermic regional perfusion system was established with ECMO (TANRP-ECMO). The heart was harvested despite 23 min of warm ischemia, and the authors considered the possibility that these hearts could be used for transplants.50
Back in 2015, St. Vincent Hospital (Sidney, Australia) published the first 3 adult patients with heart transplants in CAD. The donors were <40, and the maximum warm ischemia time was 30 min. The hearts were harvested through SFS, protected with chemical cardioplegia, and transferred to an ex situ portable system (Organ Care System® Transmedics) primed with the donors own blood for preservation, resuscitation, and if necessary, transfer purposes.51 This same group recently published their own experience with 23 heart transplants in CAD with a 96% 1-year survival rate. They claim that their results were consistent with those obtained with DBD.52
The same Australian protocol was used almost simultaneously in the United Kingdom at Papworth53 and Harefield54 hospitals, and more recently in Manchester55 with heart extraction using SFS followed by transfer to the OCS for organ recovery and assessment before the transplant. In turn, the Papworth group used a different protocol (based on their previous experience50) consisting of in situ heart extraction through TANRP-ECMO followed by ex situ preservation using the OCS. In this case, after the patient was pronounced dead, the donor underwent a median sternotomy incision followed by clamping of the supra-aortic trunks to exclude brain circulation. After cannulating the ascending aorta and the right atrium were, TANRP-ECMO was initiated in the potentially transplantable organs (heart, lung, liver, pancreas, and kidneys). The absence of brain flow was confirmed through carotid Doppler ultrasound and myocardial function was assessed using the Swan-Ganz catheter, transesophageal echocardiography, and pressure-volume curves. With this procedure Messer et al. published the cases of 9 patients transplanted with a 100% survival rate and no episodes of rejection back in 2016.56 The hearts were place in OCS for preservation and transportation purposes except for one case in which the graft was directly transplanted prior cold preservation into a recipient who was waiting in the operating room next door.57
Until 2019, the number of hearts transplanted in CAD between Australia and the United Kingdom was 105. The Papworth group has performed 40 transplants through direct ex situ removal and harvesting, and 20 with TANRP-ECMO and ex situ harvesting with results consistent with those of recipients of donation after brain death (DBD).58
In 2019, Tchana-Soto et al. published the first 2 non-British European cases with a modified protocol.59 These authors performed pre-mortem peripheral cannulations with clamping of supra-aortic trunks after death and before initiating the TANRP-ECMO. Afterwards, the heart is weaned from ECMO and assessed in situ before being transplanted into a recipient of the same hospital. The authors say that, added to the good clinical course of both recipients, this procedure reduces costs associated with ex situ heart evaluation and preservation significantly.
As a matter of fact there are fewer hearts available for transplant as a result of a change in the donor’s profile, which has triggered the use of expanded criteria regarding the acceptance of these organs. Considering heart transplants in CAD, as it has been the case in other countries, could increase the number of hearts available when this procedure becomes implemented in our country.
ConclusionsCAD started in Spain nearly 10 years ago, favored by the intensivists’ gradual awareness of the importance of end-of-life care and LSTA in critically ill patients. Its increased use has positioned Spain as one of the most active countries that perform this form of donation today. Also, ANRP-ECMO is being used more often to the extent that it is used in over 50% of donors, thus becoming a leading procedure.
The technique requires the donor’s careful cannulation, before or after his death, and based on the different protocols used. Also, solid knowledge of ECMO perfusion techniques.
Guaranteeing the correct occlusion of the thoracic aorta to avoid brain reperfusion has been cause for concern, as well as both an ethical and a technical requirement.
ANRP-ECMO partially reverts the damage caused by the ischemic insult of the dying stage until the moment death is pronounced, which is a significant improvement in abdominal organ harvesting, especially liver extraction, with a lower rate of ischemic cholangiopathy. This technique is not incompatible with the simultaneous extraction of lungs even though SFS with hypothermia is used to extract these.
Although still unavailable in many hospitals, the creation of mobile ECMO teams has triggered the use of this system in hospitals in which this technology is still unavailable.
Finally, although the heart was not initially considered a transplantable organ due to the ischemic insult suffered after the CA, the recent Australian and British experiences have proven that heart transplants in CAD are feasible, which opens an important field of improvement increasing the number of hearts that are available for transplant.
Conflicts of interestsNone reported.
We wish to thank Dr. Elisabeth Coll, Head of the Spanish National Transplant Organization (ONT) for her critical review of the manuscript and for the data provided.
To the National Transplant Organization for both the information provided on this occasion, as well as for its invaluable work over the years.
Please cite this article as: Rubio Muñoz JJ, Dominguez-Gil González B, Miñambres García E, del Río Gallegos F, Pérez-Villares JM. Papel de la perfusión normotérmica con oxigenación de membrana extracorpórea en la donación en asistolia controlada en España. Med Intensiva. 2021;46:31–41.