Frailty is a relatively new concept for intensivists, and is defined as a status of increased vulnerability to stressors associated with reduced reserve and function of different physiological systems. Supporting the hypothesis that frailty may be an important predictor of poor prognosis among older patients admitted to Intensive Care Unit (ICU), this study seeks to evaluate the association between frailty at ICU admission and short and long-term mortality.
DesignAn unmatched case-control study was carried out.
SettingIntensive Care Unit.
Patients or participantsPatients≥80 years of age admitted to the ICU for medical reasons.
InterventionsNone.
Main variables of interestThe primary outcome was 30-day mortality, while secondary outcomes were ICU mortality and mortality at one year.
ResultsMost of the patients were classified as frail at ICU admission (55.3%). The prevalence of frailty was higher among those who died than in those who were alive within 30 days from ICU admission (62.3% vs 48.3%, p=0.01). One-year mortality was higher in frail (84.4%) than in non-frail patients (65.2%, p<0.001). In the logistic regression analysis, after adjusting for potential confounders such as chronic diseases, clinical complexity, cause of ICU admission and use of advanced procedures, frailty was seen to be significantly associated to one-year mortality, but not with ICU mortality or 30-day mortality.
DiscussionThe admission of geriatric patients to the ICU is increasing. Frailty assessment may play an important role in the clinical evaluation of such individuals for triage, but should not be considered a priori as an exclusion criterion for admission.
«Fragilidad» es un concepto relativamente nuevo para los intensivistas, y se define como un estado de mayor vulnerabilidad frente a los estresores asociados con una reducción de las reservas y del funcionamiento de distintos sistemas fisiológicos. Basándose en la hipótesis de que la fragilidad podría ser un importante factor predictivo de un mal pronóstico en pacientes ancianos ingresados en la unidad de cuidados intensivos (UCI), este estudio tenía por objeto evaluar la asociación entre la fragilidad en el momento del ingreso en la UCI y la mortalidad a corto y largo plazo.
DiseñoEstudio de casos y controles sin emparejamiento.
ÁmbitoUnidad de cuidados intensivos.
Pacientes o participantesPacientes ≥80 años ingresados en la UCI por motivos médicos.
IntervencionesNinguna.
Variables de interés principalesLa variable principal fue la mortalidad a 30 días, mientras que las variables secundarias fueron la mortalidad en la UCI y al cabo de un año.
ResultadosLa mayoría de los pacientes se clasificaron como frágiles en el momento de su ingreso en la UCI (55,3%). La prevalencia de la fragilidad fue más alta entre quienes fallecieron que en el caso de los que seguían con vida a los 30 días de su ingreso en la UCI (62,3% frente al 48,3%; p=0,01). La mortalidad a un año fue más elevada en los pacientes frágiles (84,4%) que en los no frágiles (65,2%; p<0,001). En la regresión logística, tras el ajuste de los posibles factores de confusión, como las enfermedades crónicas, la complejidad clínica, el motivo del ingreso en la UCI y el uso de procedimientos avanzados, la fragilidad resultó estar significativamente asociada con la mortalidad a un año, pero no con la mortalidad en la UCI ni al cabo de 30 días.
DiscusiónEl ingreso de pacientes geriátricos en la UCI está aumentando con el paso del tiempo. La evaluación de la fragilidad podría desempeñar un papel importante en su evaluación clínica para el triaje, pero no debería considerarse a priori como un criterio de exclusión para el ingreso.
According to the World Health Organization, older people are a rapidly growing proportion of the world's entire population. In fact, in 2015, this population rose by 55 millions and the proportion of the older people reached 8.5% of the total population.1 This trend unavoidably leads to an increasing demand for health care resources, including those provided by intensive care units (ICU). Actually the median age of the entire ICU population is already above 65 years, as shown in large, recent epidemiological studies.2–4 Nonetheless, ICU beds are limited and will probably decrease even more.5 These limitations pose great challenges to the ICU triage decision-making process. Indeed, older patients are usually subjected to strict triage before admittance to the ICU and this selection is partly due to the fact that biological age is often not precisely estimated by chronological age. Therefore, intensivists are shifting their attention from the chronological age itself and the traditional co-morbidity measures to the more comprehensive concept of frailty, as a predictor of poor prognosis. Frailty is a relatively new issue for the intensive care field and is defined as a status of increased susceptibility to stressors associated with a decline in reserve and function of a wide range of physiological systems.6,7 The Clinical Frailty Scale (CFS), in particular, is a simple and visual scale that allows categorizing individuals in nine classes of increasing frailty. Such tool was first used on a large scale within intensive care in a Canadian study6 and has been associated with 6-month mortality.8,9 Moreover, studies evaluating CFS in respect to health-related outcomes suggested that the impact of frailty was more important than chronological age itself.10 This, again, emphasizes that age should not be used as an exclusive criteria to decide ICU admission.11,12
Although frailty has been largely investigated in geriatric epidemiological studies, the recent introduction of this concept in ICU settings has led to have only scarce available literature on this issue. Supporting the hypothesis that frailty may be a relevant predictor of poor prognosis of older patients admitted to an ICU, this study aimed to evaluate the association between frailty at ICU admission with 30-days, ICU and one-year mortality.
MethodsStudy populationThis unmatched case-control study involved patients ≥80 years old admitted to the intensive care of the Ospedale Sant’Antonio in the city of Padova (Italy). Between June 2013 and December 2017, data of consecutive patients of 80 years of age or older admitted to ICU for medical reasons and survived or died over a 30-days period, were retrospectively collected until the required sample size was reached. Surgical patients were excluded from the study in order to avoid confounding factors due to technical surgical reasons and to obtain a homogeneous patients’ population. Patients with inclusion criteria were divided in two groups according to their 30-days survival.
For the sample size computation, we considered the 30-days mortality as primary outcome, while secondary outcomes were ICU mortality and one-year mortality. A recent prospective multicentre study in ICU patients reported that frailty at ICU admission was present in 54% of patients who died during a 30-days follow-up vs. 38% of those who survived.11 In our case-control study, considering an alpha of 5% and a power of 80% in a two-sided test, we estimated that a sample size of 302 patients (151 patients for group) was needed to detect the above mentioned difference in frailty proportion between older ICU patients survived vs. died at 30 days.
The study was conducted according to the declaration of Helsinki and its later amendments and all participants, or their next of kin for those with cognitive impairment, gave their informed consent for data treatment. Patients’ data were collected retrospectively from hospital records and analyzed anonymously.
Data collectionThe following variables were collected: socio-demographics characteristics; body mass index (BMI); reason for ICU admission; presence of acute and/or chronic diseases, including ischemic heart disease, congestive heart failure, peripheral vascular disease, cerebrovascular events, cognitive impairment, chronic obstructive pulmonary disease, (COPD), connective tissue disease, peptic ulcer disease, liver disease, diabetes mellitus, hemiplegia, moderate to severe chronic kidney disease (CKD), solid cancer, leukemia, and lymphoma; mean arterial pressure (MAP) at ICU admission; use of ICU advanced procedures [respiratory support (not needed vs. need for non-invasive ventilation, mechanical ventilation, or tracheostomy), administration of vasoactive drugs, initiation of renal replacement therapy]; ICU length of stay (days); hospital stay (days). Comorbidity was assessed via the Charlson Comorbidity Index (CCI).12 Frailty was assessed with the CFS, a simple tool that ranges from 1 to 9. In line with previous studies, frailty was defined as a CFS ≥5 points.10,13 CFS was derived from written information on the visual description of patients, recorded in the local hospital patients’ register by ICU physicians. Data on ICU, 30-days and one-year mortality were obtained from the local hospital records.
Statistical analysisNormally distributed continuous data were described as means and standard deviation (SD) and non-normal distributed data were described as median and interquartile range (IQR). Categorical variables were reported as numbers and percentages. Baseline characteristics, treatment and outcomes were compared between participants who died vs. survived over a 30-days follow-up. Comparisons between groups were performed by using the Student t-test or the Mann–Whitney U test, for the continuous variables normally or non-normally distributed, respectively, and through the Chi-square (or Fisher) test for the categorical ones.
The association between frailty and mortality was evaluated using logistic regression analysis. In particular, in Model 1, we tested in univariate analysis the risk of mortality as a function of patients’ characteristics, frailty, comorbidities, clinical complexity, cause of ICU admission and treatment administered. Variables that showed to be associated with the studied outcomes demonstrating a p-value <0.10, were entered into a multivariable model (Model 2). Moreover, a further multivariable model adjusted for variables indicating the clinical status of patients at ICU admission (frailty, GCS, CCI and cause of admission), was performed (Model 3). The strength of such associations was expressed as odds ratio (OR) and 95% confidence interval (95%CI). p-Values <0.05 were considered statistically significant. All analyses were performed with R software, version 3.5.2.
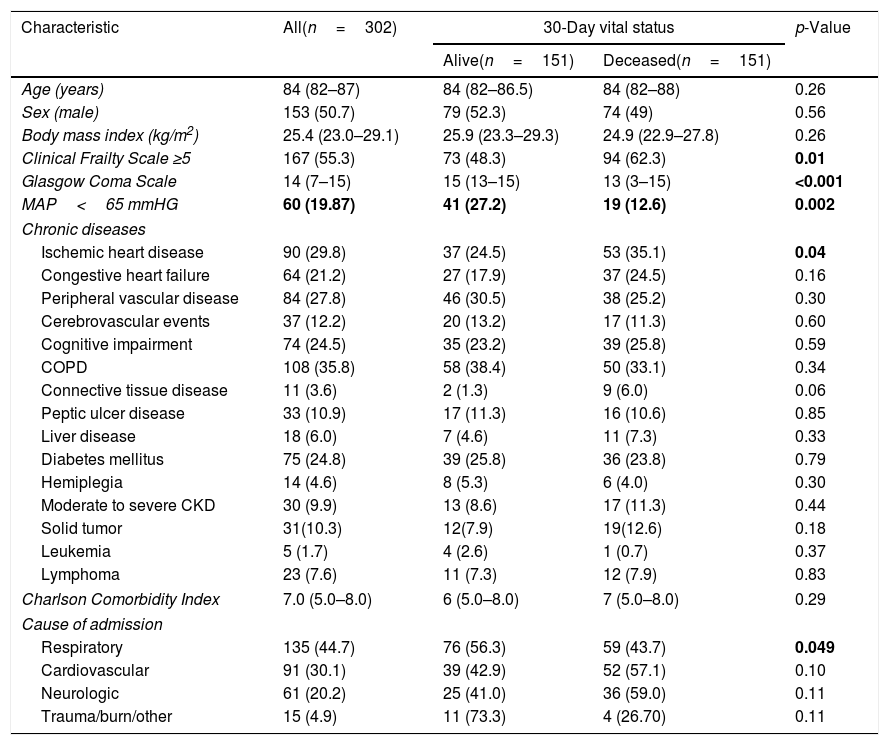
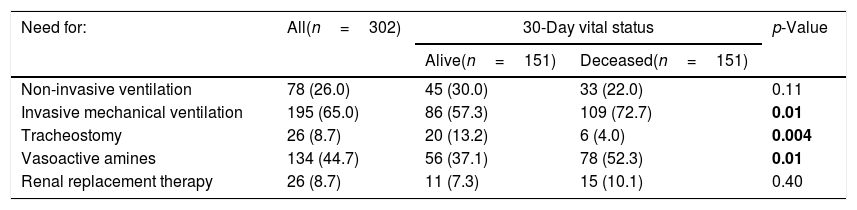
ResultsThe baseline characteristics of patients included in the study are reported in Table 1. As shown, the median age of the sample was 84 (82–87) years and 153 (50.7%) patients were men. The most frequent diagnosis at ICU admission was respiratory failure, followed by cardiovascular insufficiency. In the sample as a whole, the most common chronic conditions were COPD, diabetes and cardiovascular diseases, and around a quarter of patients reported a physician-based diagnosis of cognitive impairment. Compared with patients alive at the 30-days follow-up, those who deceased were more likely to have had lower GCS at ICU admission (median GCS 13 vs. 15, p<0.001) and to suffer from ischemic heart diseases. More than half of patients were classified as frail at ICU admission (55.3%), and the prevalence was higher among those who died than those who were alive after a 30-days period (62.3% vs. 48.3%, p=0.01). As regards the medical interventions performed during the ICU stay (Table 2), most of patients required intubation, mechanical ventilation and administration of vasoactive drugs, especially those who had worse prognosis at the follow-up. The median length of ICU stay was 5 (3–10) days in the overall population and was significantly longer in 30-days survivors compared with those who deceased [6 (4–14) vs. 4 (2–9) days, p<0.001].
Differences in the characteristics of patients at ICU admission by vital status after a 30-day follow-up.
| Characteristic | All(n=302) | 30-Day vital status | p-Value | |
|---|---|---|---|---|
| Alive(n=151) | Deceased(n=151) | |||
| Age (years) | 84 (82–87) | 84 (82–86.5) | 84 (82–88) | 0.26 |
| Sex (male) | 153 (50.7) | 79 (52.3) | 74 (49) | 0.56 |
| Body mass index (kg/m2) | 25.4 (23.0–29.1) | 25.9 (23.3–29.3) | 24.9 (22.9–27.8) | 0.26 |
| Clinical Frailty Scale ≥5 | 167 (55.3) | 73 (48.3) | 94 (62.3) | 0.01 |
| Glasgow Coma Scale | 14 (7–15) | 15 (13–15) | 13 (3–15) | <0.001 |
| MAP<65 mmHG | 60 (19.87) | 41 (27.2) | 19 (12.6) | 0.002 |
| Chronic diseases | ||||
| Ischemic heart disease | 90 (29.8) | 37 (24.5) | 53 (35.1) | 0.04 |
| Congestive heart failure | 64 (21.2) | 27 (17.9) | 37 (24.5) | 0.16 |
| Peripheral vascular disease | 84 (27.8) | 46 (30.5) | 38 (25.2) | 0.30 |
| Cerebrovascular events | 37 (12.2) | 20 (13.2) | 17 (11.3) | 0.60 |
| Cognitive impairment | 74 (24.5) | 35 (23.2) | 39 (25.8) | 0.59 |
| COPD | 108 (35.8) | 58 (38.4) | 50 (33.1) | 0.34 |
| Connective tissue disease | 11 (3.6) | 2 (1.3) | 9 (6.0) | 0.06 |
| Peptic ulcer disease | 33 (10.9) | 17 (11.3) | 16 (10.6) | 0.85 |
| Liver disease | 18 (6.0) | 7 (4.6) | 11 (7.3) | 0.33 |
| Diabetes mellitus | 75 (24.8) | 39 (25.8) | 36 (23.8) | 0.79 |
| Hemiplegia | 14 (4.6) | 8 (5.3) | 6 (4.0) | 0.30 |
| Moderate to severe CKD | 30 (9.9) | 13 (8.6) | 17 (11.3) | 0.44 |
| Solid tumor | 31(10.3) | 12(7.9) | 19(12.6) | 0.18 |
| Leukemia | 5 (1.7) | 4 (2.6) | 1 (0.7) | 0.37 |
| Lymphoma | 23 (7.6) | 11 (7.3) | 12 (7.9) | 0.83 |
| Charlson Comorbidity Index | 7.0 (5.0–8.0) | 6 (5.0–8.0) | 7 (5.0–8.0) | 0.29 |
| Cause of admission | ||||
| Respiratory | 135 (44.7) | 76 (56.3) | 59 (43.7) | 0.049 |
| Cardiovascular | 91 (30.1) | 39 (42.9) | 52 (57.1) | 0.10 |
| Neurologic | 61 (20.2) | 25 (41.0) | 36 (59.0) | 0.11 |
| Trauma/burn/other | 15 (4.9) | 11 (73.3) | 4 (26.70) | 0.11 |
Notes. Numbers are frequency (%) or median (interquartile range), as appropriate. Abbreviations: MAP, Mean Arterial Pressure; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease; AIDS, Acquired Immunodeficiency Syndrome.
Differences in medical interventions in the sample as a whole and by vital status after a 30-day follow-up.
| Need for: | All(n=302) | 30-Day vital status | p-Value | |
|---|---|---|---|---|
| Alive(n=151) | Deceased(n=151) | |||
| Non-invasive ventilation | 78 (26.0) | 45 (30.0) | 33 (22.0) | 0.11 |
| Invasive mechanical ventilation | 195 (65.0) | 86 (57.3) | 109 (72.7) | 0.01 |
| Tracheostomy | 26 (8.7) | 20 (13.2) | 6 (4.0) | 0.004 |
| Vasoactive amines | 134 (44.7) | 56 (37.1) | 78 (52.3) | 0.01 |
| Renal replacement therapy | 26 (8.7) | 11 (7.3) | 15 (10.1) | 0.40 |
Notes. Numbers are frequency (%).
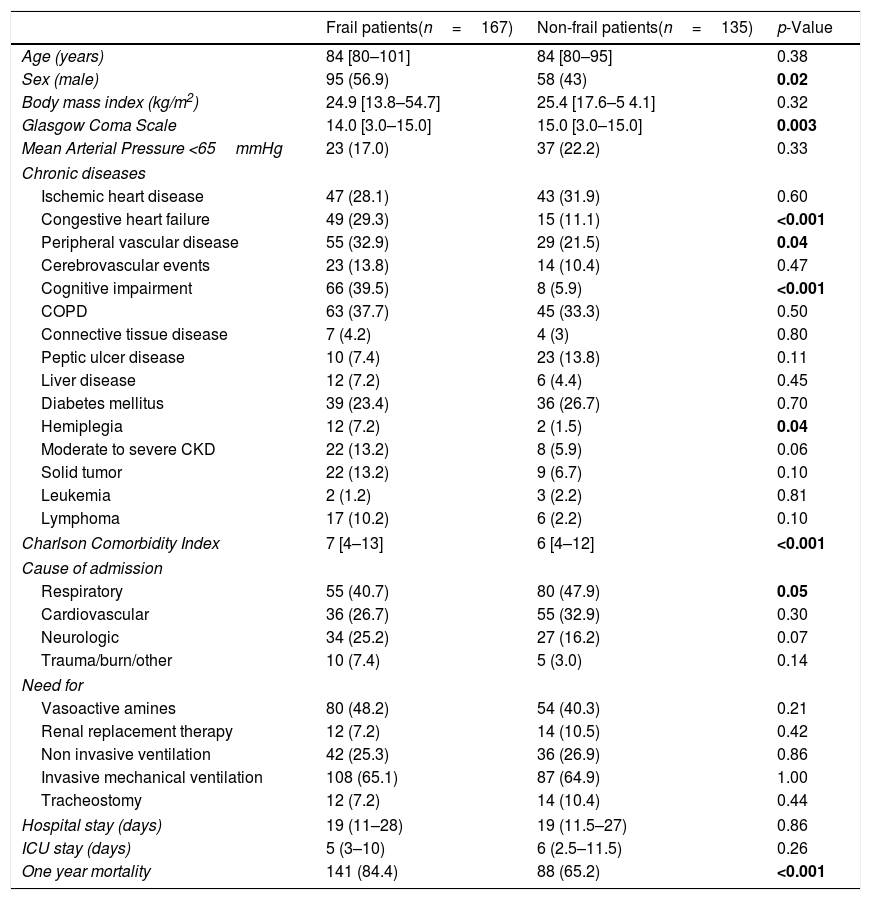
Differences in clinical outcomes between the 167 frail and 135 non-frail patients are reported in Table 3. As shown, frail individuals were more likely to be men, to have lower GCS, and a higher number of chronic diseases, especially congestive heart failure, peripheral vascular disease, hemiplegia and cognitive impairment. No significant differences between groups were found concerning the median length of hospital and ICU stay and the required medical interventions, while one-year mortality resulted to be higher in the frail (84.4%) than in the non-frail patients (65.2%, p<0.001).
Differences in clinical outcome between frail and non-frail patients.
| Frail patients(n=167) | Non-frail patients(n=135) | p-Value | |
|---|---|---|---|
| Age (years) | 84 [80–101] | 84 [80–95] | 0.38 |
| Sex (male) | 95 (56.9) | 58 (43) | 0.02 |
| Body mass index (kg/m2) | 24.9 [13.8–54.7] | 25.4 [17.6–5 4.1] | 0.32 |
| Glasgow Coma Scale | 14.0 [3.0–15.0] | 15.0 [3.0–15.0] | 0.003 |
| Mean Arterial Pressure <65mmHg | 23 (17.0) | 37 (22.2) | 0.33 |
| Chronic diseases | |||
| Ischemic heart disease | 47 (28.1) | 43 (31.9) | 0.60 |
| Congestive heart failure | 49 (29.3) | 15 (11.1) | <0.001 |
| Peripheral vascular disease | 55 (32.9) | 29 (21.5) | 0.04 |
| Cerebrovascular events | 23 (13.8) | 14 (10.4) | 0.47 |
| Cognitive impairment | 66 (39.5) | 8 (5.9) | <0.001 |
| COPD | 63 (37.7) | 45 (33.3) | 0.50 |
| Connective tissue disease | 7 (4.2) | 4 (3) | 0.80 |
| Peptic ulcer disease | 10 (7.4) | 23 (13.8) | 0.11 |
| Liver disease | 12 (7.2) | 6 (4.4) | 0.45 |
| Diabetes mellitus | 39 (23.4) | 36 (26.7) | 0.70 |
| Hemiplegia | 12 (7.2) | 2 (1.5) | 0.04 |
| Moderate to severe CKD | 22 (13.2) | 8 (5.9) | 0.06 |
| Solid tumor | 22 (13.2) | 9 (6.7) | 0.10 |
| Leukemia | 2 (1.2) | 3 (2.2) | 0.81 |
| Lymphoma | 17 (10.2) | 6 (2.2) | 0.10 |
| Charlson Comorbidity Index | 7 [4–13] | 6 [4–12] | <0.001 |
| Cause of admission | |||
| Respiratory | 55 (40.7) | 80 (47.9) | 0.05 |
| Cardiovascular | 36 (26.7) | 55 (32.9) | 0.30 |
| Neurologic | 34 (25.2) | 27 (16.2) | 0.07 |
| Trauma/burn/other | 10 (7.4) | 5 (3.0) | 0.14 |
| Need for | |||
| Vasoactive amines | 80 (48.2) | 54 (40.3) | 0.21 |
| Renal replacement therapy | 12 (7.2) | 14 (10.5) | 0.42 |
| Non invasive ventilation | 42 (25.3) | 36 (26.9) | 0.86 |
| Invasive mechanical ventilation | 108 (65.1) | 87 (64.9) | 1.00 |
| Tracheostomy | 12 (7.2) | 14 (10.4) | 0.44 |
| Hospital stay (days) | 19 (11–28) | 19 (11.5–27) | 0.86 |
| ICU stay (days) | 5 (3–10) | 6 (2.5–11.5) | 0.26 |
| One year mortality | 141 (84.4) | 88 (65.2) | <0.001 |
Notes. Numbers are frequency (%) or median (interquartile range), as appropriate. Abbreviations: COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease.
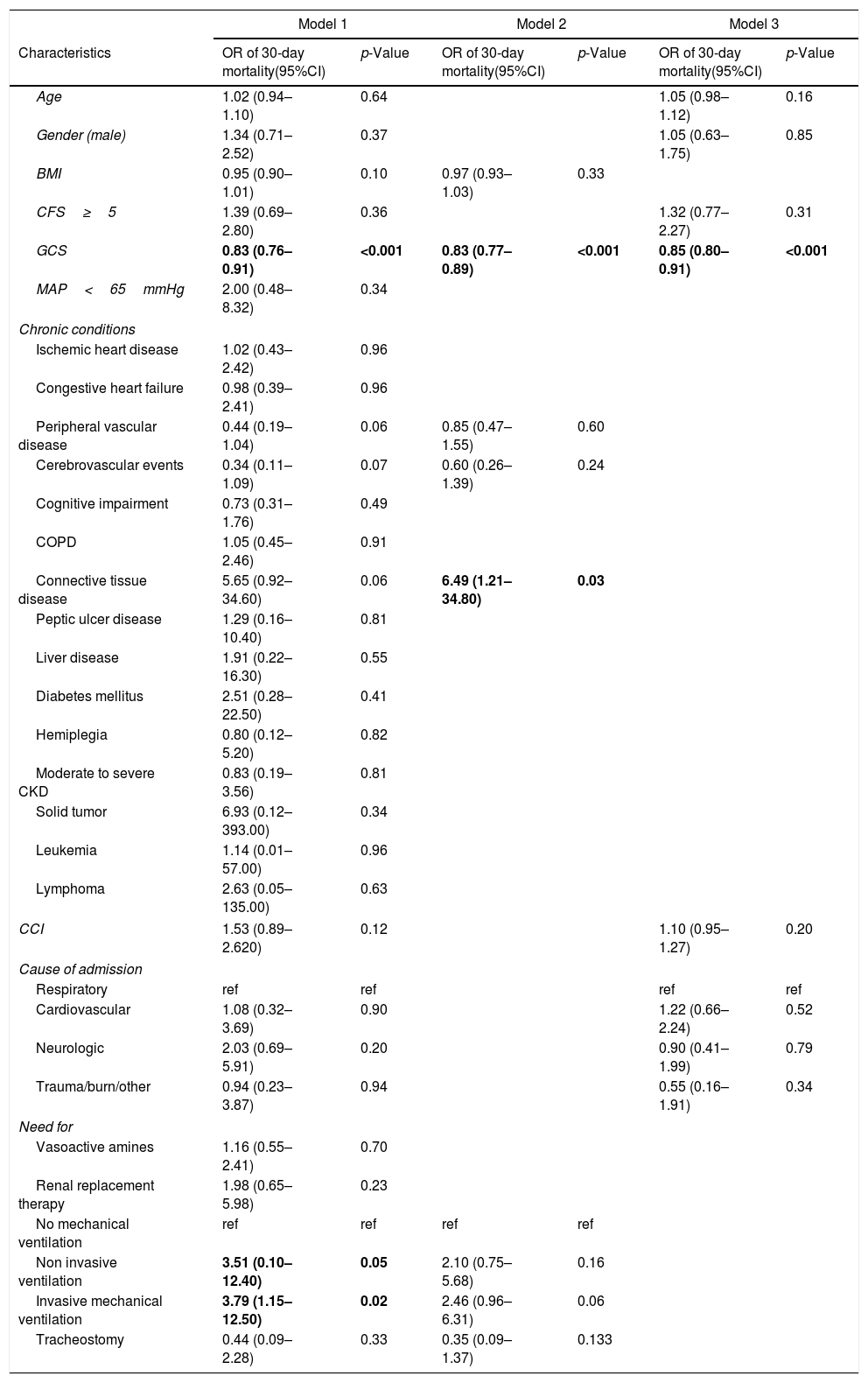
At univariate logistic regression analysis (Table 4), the factors that demonstrated to be significantly associated with 30-days mortality were GCS at ICU admission (OR=0.83, 95%CI: 0.76–0.91, per each 1-unit increase in GCS), and the need for non-invasive and invasive mechanical respiratory support (OR=3.51, 95%CI: 0.10–12.40; OR=3.79, 95%CI: 1.15–12.50, respectively). At the multivariable logistic regression models, only GCS at ICU admission and connective tissue disease but not frailty, were significantly associated with 30-day mortality (Table 4). Similar results were observed when considering ICU mortality (Supplementary Table S1). On the contrary, as reported in Supplementary Table S2, frailty resulted to be significantly associated with one-year mortality both at univariable (Model 1, OR=2.70, 95%CI: 1.22–5.99) and multivariable regression models (Model 2, OR=2.69, 95%CI: 1.48–4.89; and Model 3, OR=2.15, 95%CI: 1.15–4.03).
Logistic regression analysis on the association between frailty and, other characteristics of patients at ICU admission and use of ICU advanced procedures with 30-day mortality.
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| Characteristics | OR of 30-day mortality(95%CI) | p-Value | OR of 30-day mortality(95%CI) | p-Value | OR of 30-day mortality(95%CI) | p-Value |
| Age | 1.02 (0.94–1.10) | 0.64 | 1.05 (0.98–1.12) | 0.16 | ||
| Gender (male) | 1.34 (0.71–2.52) | 0.37 | 1.05 (0.63–1.75) | 0.85 | ||
| BMI | 0.95 (0.90–1.01) | 0.10 | 0.97 (0.93–1.03) | 0.33 | ||
| CFS≥5 | 1.39 (0.69–2.80) | 0.36 | 1.32 (0.77–2.27) | 0.31 | ||
| GCS | 0.83 (0.76–0.91) | <0.001 | 0.83 (0.77–0.89) | <0.001 | 0.85 (0.80–0.91) | <0.001 |
| MAP<65mmHg | 2.00 (0.48–8.32) | 0.34 | ||||
| Chronic conditions | ||||||
| Ischemic heart disease | 1.02 (0.43–2.42) | 0.96 | ||||
| Congestive heart failure | 0.98 (0.39–2.41) | 0.96 | ||||
| Peripheral vascular disease | 0.44 (0.19–1.04) | 0.06 | 0.85 (0.47–1.55) | 0.60 | ||
| Cerebrovascular events | 0.34 (0.11–1.09) | 0.07 | 0.60 (0.26–1.39) | 0.24 | ||
| Cognitive impairment | 0.73 (0.31–1.76) | 0.49 | ||||
| COPD | 1.05 (0.45–2.46) | 0.91 | ||||
| Connective tissue disease | 5.65 (0.92–34.60) | 0.06 | 6.49 (1.21–34.80) | 0.03 | ||
| Peptic ulcer disease | 1.29 (0.16–10.40) | 0.81 | ||||
| Liver disease | 1.91 (0.22–16.30) | 0.55 | ||||
| Diabetes mellitus | 2.51 (0.28–22.50) | 0.41 | ||||
| Hemiplegia | 0.80 (0.12–5.20) | 0.82 | ||||
| Moderate to severe CKD | 0.83 (0.19–3.56) | 0.81 | ||||
| Solid tumor | 6.93 (0.12–393.00) | 0.34 | ||||
| Leukemia | 1.14 (0.01–57.00) | 0.96 | ||||
| Lymphoma | 2.63 (0.05–135.00) | 0.63 | ||||
| CCI | 1.53 (0.89–2.620) | 0.12 | 1.10 (0.95–1.27) | 0.20 | ||
| Cause of admission | ||||||
| Respiratory | ref | ref | ref | ref | ||
| Cardiovascular | 1.08 (0.32–3.69) | 0.90 | 1.22 (0.66–2.24) | 0.52 | ||
| Neurologic | 2.03 (0.69–5.91) | 0.20 | 0.90 (0.41–1.99) | 0.79 | ||
| Trauma/burn/other | 0.94 (0.23–3.87) | 0.94 | 0.55 (0.16–1.91) | 0.34 | ||
| Need for | ||||||
| Vasoactive amines | 1.16 (0.55–2.41) | 0.70 | ||||
| Renal replacement therapy | 1.98 (0.65–5.98) | 0.23 | ||||
| No mechanical ventilation | ref | ref | ref | ref | ||
| Non invasive ventilation | 3.51 (0.10–12.40) | 0.05 | 2.10 (0.75–5.68) | 0.16 | ||
| Invasive mechanical ventilation | 3.79 (1.15–12.50) | 0.02 | 2.46 (0.96–6.31) | 0.06 | ||
| Tracheostomy | 0.44 (0.09–2.28) | 0.33 | 0.35 (0.09–1.37) | 0.133 | ||
Model 1 is unadjusted. Model 2 included all variables that were associated with 30-day mortality with a p-value <0.10 at the univariate Model 1. Model 3 is adjusted for clinical variables. Abbreviations: CCI, Charlson Comorbidity Index; CFS, Clinical Frailty Scale; GCS, Glasgow Coma Scale; MAP, mean arterial pressure; COPD, Chronic obstructive pulmonary disease; CKD, chronic kidney disease.
In our study, considering older patients admitted to an ICU, we found that frailty at ICU admission was not associated with 30-days mortality. On the contrary, frailty was significantly associated with one-year mortality, and such relationship seemed to mostly depend on the presence of pre-existing chronic diseases and on patients’ clinical complexity. Of note, one-year mortality was extremely high in this population and reached 84.4% among those classified as frail. In this regard, although no longitudinal data on health-related quality of life were collected, it should be pointed out that a not irrelevant number of patients who survived at the 30-day follow-up underwent tracheostomy, and were thus likely to experience a severe reduction of self-sufficiency and quality of life over their last years.
In this study, we decided to focus on a population of inpatients aged 80 years or older. These patients constitute one of the categories with the fastest growing representation in ICU. However, indications for ICU admission, triage criteria and the level of care for the oldest old are still matter of intense debate, making this issue of great relevance in the daily clinical practice. As well known, triage process should differ when dealing with younger vs. older patients.10 However, current evidence suggests that age per se is not necessarily associated with negative health-related outcomes, especially in critically ill patients.10 Therefore, there is increasing awareness among intensivists that the assessment of older patients in ICU should be performed with a comprehensive evaluation, including the concept of frailty.
In this regard, recent studies in older frail patients found an increased risk of mortality and a reduced quality of life.11,14,15 Conversely, other works reported contrasting results and strengthened the need for further investigations on this issue.16,17 Our work corroborates a recent retrospective study that considered a large sample of ICU patients aged 75 years or older.16 In that study, ICU survivors had significantly lower hospital frailty risk score than non-survivors and, as expected, frailty was significantly associated with negative health-related outcomes. However, hospital frailty risk score did not independently predict ICU mortality risk after adjustment for APACHE-II scores or SAPS-II scores. Other authors showed, instead, an independent association between frailty and adverse outcomes in ICU or Emergency Department settings.11,14,15 In a large prospective multicentric study, frailty, not-elective admission to the ICU and high SOFA score emerged as the three most important factors associated with short-term mortality in older patients.11
Overall, in line with previous findings, our study suggest that triage criteria for ICU admission should not depend only on chronological age, but that a comprehensive evaluation of older patients inclusive of frailty assessment, may play a fundamental role in predicting survival in such special population. Considering 30-day mortality as an outcome that could more likely capture the clinical vulnerability of older adults, our work supports the potential benefit of ICU treatments on short-term survival as long as clinical conditions at admission are not too much compromised, especially neurological functions. Even in that case, however, one-year mortality remains extremely high, and whether an extension of lifespan could preserve individuals’ self-sufficiency and quality of life is still a matter of debate. For this population of patients, indeed, so far possible alternatives are not admitting them to the ICU at all, privileging a hospitalization in a regular/acute care ward; or admitting them to the ICU and conducting all efforts to ensure a rapid ICU discharge, as suggested by previous studies.17–18
Our study has some limitations that need to be mentioned. First of all, biases linked to the monocentric and retrospective nature of our study are well-known and may impact on the generalizability of our results. A further related issue concerns the inclusion in the study only of patients ≥80 years, which could have influenced both the prevalence of frailty in our sample and the strength of its association with mortality. Second, the matching between cases and controls was not performed.19 However, the adjustment of our analyses for different sets of potential confounders should have reduced this possible bias and support our results. Third, severity of illness at ICU admission, evaluated with validated tools such as the Simplified Acute Physiology Score (SAPS) II score or the Sequential Organ Failure Assessment (SOFA) score, were not available for most patients, especially for the most critical ones, who died a few hours after ICU admission. Finally, we had no information about triage decision process before ICU admission. On the other hand, the high number of included patients in a relatively short period ensures uniformity in admission policy and clinical practice. Moreover, having collected data on a number of clinical parameters and procedures may represent a further strength of our work.
In conclusion, the admission of geriatric patients to ICUs is increasing over time and their clinical evaluation for triage need to be comprehensive considering parameters more likely associated with clinical outcomes, such as survival and quality of life. Frailty assessment may play an important role in this regard, but it should not be considered a priori as an exclusion criterion for admission to ICU. Further studies investigating this issue in specific subgroups of frail older patients in the ICU setting are warranted.
Authors’ contributionsLaura Pasin: study concept and design, acquisition of data, analysis and interpretation of data, preparation of manuscript.
Gianlorenzo Golino: study concept and design, acquisition of data, preparation of manuscript.
Sabrina Boraso: study concept and design, analysis and interpretation of data, preparation of manuscript.
Safaee Fakhr: study concept and design, acquisition of data, analysis and interpretation of data, preparation of manuscript.
Ivo Tiberio: study concept and design, analysis and interpretation of data, preparation of manuscript.
Caterina Trevisan: study concept and design, analysis and interpretation of data, preparation of manuscript.
FundingNone.
Conflicts of interestThe authors declare that they have no competing interests.