To evaluate the consequences of using nebulized drugs in patients subjected to noninvasive ventilation (NIV) with total face mask (TFM) and helmet.
DesignA descriptive analytical study of a prospective patient cohort was carried out.
AmbitPediatric intensive care unit (PICU) of a tertiary hospital.
PatientsConsecutive sampling was used to include all patients admitted to the PICU and requiring NIV with helmet or TFM over a period of 29 months. No patients were excluded.
InterventionsNebulized treatment was added according to medical criteria.
Variables of interestIndependent variables were age, sex, diagnosis, disease severity, ventilation parameters and nebulized drugs (if administered). Secondary outcomes were duration and failure of NIV, and length of PICU stay.
ResultsThe most frequent diagnoses were bronchiolitis (60.5%) and asthma (23%). Patients received NIV for a median of 43h. Nebulized drugs were administered in 40% of the cases during NIV, and no adverse effects were registered. Using Bayesian statistics, the calculated probability of suffering an adverse effect was 1.3% with helmet and 0.5% with TFM (high density 95% probability intervals). Patients with helmet and nebulized therapy were in more serious condition than those who did not receive nebulization; nevertheless, no differences were observed regarding the need to change to bilevel modality. With TFM, PICU stay was shorter for the same degree of severity (p=0.033), and the NIV failure rate was higher in patients who did not receive inhaled drugs (p=0.024).
ConclusionsThe probability of suffering an adverse effect related to nebulization is extremely low when using a helmet or TFM. Inhaled therapy with TFM may shorten PICU stay in some patients.
Evaluar las consecuencias de la medicación nebulizada en pacientes con ventilación no invasiva (VNI) con mascarilla facial total (MFT) y casco.
DiseñoEstudio analítico descriptivo sobre una cohorte prospectiva de pacientes.
ÁmbitoUCIP de hospital de tercer nivel.
PacientesTodos los pacientes ingresados en UCIP (muestreo consecutivo) con VNI con casco o MFT durante 29 meses. No se excluyeron pacientes.
IntervencionesSe añadió tratamiento nebulizado según criterio médico.
Variables de interésIndependientes: edad, sexo, diagnóstico, gravedad, parámetros ventilatorios y medicación nebulizada (si se utilizaba). Secundarias: duración, fallo de VNI y estancia en UCIP.
ResultadosLos diagnósticos más frecuentes fueron bronquiolitis (60,5%) y asma (23%). La mediana de conexión a VNI fue de 43 horas. Se administraron nebulizaciones durante la VNI en un 40% sin registrarse efectos adversos. La probabilidad calculada de tener un efecto adverso fue 1,3% con casco y 0,5% con MFT (estadística bayesiana, intervalo de probabilidad 95%). Los pacientes con casco y aerosolterapia tenían mayor gravedad que los que no recibieron nebulizaciones, sin encontrarse diferencias en la necesidad de cambiar a modalidad con doble nivel de presión. En los pacientes con MFT la estancia en UCIP fue menor (p=0,033) a pesar de no existir diferencias en el nivel de gravedad; la tasa de fallo de VNI fue mayor en los que no recibieron nebulizaciones (p=0,024).
ConclusionesLa probabilidad de tener un efecto adverso relacionado con la nebulización es baja utilizando casco o MFT. La terapia inhalada con MFT puede disminuir la estancia en UCIP en algunos pacientes.
In recent years, the use of non-invasive ventilation (NIV) in neonatal and pediatric intensive care units (NICU and PICU) settings has increased.1 The use of NIV reduces length of stay and hospitalization costs2 while improving patient comfort.
Helmet and total face mask (TFM) are two of the types of interfaces whose use in infants has been expanding in recent years. They offer several advantages in comparison with oronasal masks, such as fewer leaks, related to incorrect fitting of the interface or opening of the mouth, prevention of damage to the nasal mucosa and allowing modifications to fit the heads of younger children, as well as a high level of humidification.3
Several authors have reported good results with the use of Helmet and TFM in adult patients with acute respiratory failure (ARF).4–6 Better adaptation, less complications and similar efficacy to other interfaces have been described.5,7 In children, the helmet has been used in patients with hypoxemic ARF, leukemia and in preterm neonates, but there are no reports of the use of TFM in children.3,8,9
Many pediatric patients need nebulized drugs. In fact, asthma and bronchiolitis are two of the main three leading causes of NIV usage.8 Aerosol therapy for treatment of acute or acute-on-chronic respiratory failure in this setting may be delivered by pressurized metered-dose inhaler (pMDI) with a spacer and facemask, or nebulizer and facemask.
The use of nebulized drugs in patients treated with TFM and helmet is controversial because of the possibility of adverse effects, such as anisocoria due to nebulized ipratropium bromide.10,11
The effectiveness of the nebulization depends on several factors (drug characteristics, airways anatomy, patient's inhalation technique and nebulization system). There are three types of devices for nebulized drugs: ultrasonic, pneumatic (jet type) and membrane type. The particles with a mass from 1 to 5μm are those that are more likely to reach the most appropriate site of the bronchial Tree.12 Regarding the location of the nebulization system, results are very different; some authors obtain better results placing it between the interface and the leak port, while for others it is more effective to place it between the exhalation port and the ventilator for NIV.13,14
Therefore, the aim of our study was to evaluate the safety of aerosol therapy and his effect in infants using a helmet and TFM interfaces.
Patients and methodsEthical considerationsThe study was approved by the ethics committee of our hospital. On the admission to PICU an informed consent is given to all parents with the treatments they can receive according to medical criteria.
DesignDescriptive analytical study of a prospective cohort.
SamplingBy consecutive sampling, all patients admitted to the PICU requiring NIV with helmet or TFM in the PICU over a 29-month period (November 2013 to March 2016) were included. No patients were excluded.
MethodsInterfaces used were: helmet StarMed (Intersurgical®) and total full face mask PerforMax (Philips®).
Although in the StarMed technical information, the possibility of using nebulized medication is mentioned without having to disconnect the patient, in the case of the PerforMax this is not mentioned on its label. However, there is a specific elbow to the nebulizer that is compatible with this mask, so its use would not be contraindicated.
The decision to add nebulized treatment was made according to medical criteria (fundamentally depending on the diagnosis and the presence of bronchospasm at pulmonary auscultation), and clinical practice guidelines of the unit. Frequency of administration of medication was established according to clinical severity (usually in a range between every 2 and 6h) and was removed when it was not necessary (in PICU or outside) or no clinical response was observed.
Vibrating mesh (AeroNeb®) and jet nebulizers were used depending on availability in the unit and they were placed close to the patient (either between the leak port and the interface or 15–30cm proximal to the leak port).
Jet nebulizer was filled with a solution of 5ml and it was driven with oxygen at 6–8 liters per minute.
VariablesThe following independent variables were collected in every patient: age, sex, diagnosis, disease severity (using Wood Downes Ferrés – WDF – score, calculated at the moment of admission in PICU, in patients diagnosed with asthma and bronchiolitis), ventilation parameters and the use and type of nebulized drugs (if administered).
The main outcome of our analysis was the appearance of any side effects of nebulized drugs. A failure of NIV was considered when it was necessary to change from CPAP to bilevel mode or the need for intubation. Secondary outcomes were the failure of NIV, the evolution of disease severity (measured again using WD score), the duration of NIV, and length of stay in PICU.
Statistical analysisAll data were collected prospectively in a national data base (NIV-research) supported by the Spanish Respiratory Working Group of the Spanish Paediatric Intensive Care Society, and analyzed with R (with R-commander) and Epidat 4.1 software.
Descriptive statistics in continuous variables were done with percentiles (median and interquartile range, IQR) and in discrete ones with percentages. In both cases population 95% Confidence Intervals were used. When there were zero counts in the numerator of percentages, posterior high density 95% probability intervals using Bayesian descriptive analysis were calculated, based on conjugated Beta-Binomial model with Beta (½, ½) non-informative prior.
The statistical analysis was done in discrete variables with Fisher-Exact test and in continuous variables with Mann–Whitney U test, accepting p≤0.05 as the significance level.
For the multivariate analysis, the patients were divided in two different cohorts based on NIV interface used. In both, TFM and Helmet cohorts, multivariate Logistic or Cox regression models were fitted using Akaike Informative Criteria (AIC).
ResultsThe study sample consisted of 152 children treated with helmet or TFM. 61 patients received aerosol therapy during the NIV. The median age was 3.7months (IQR 1.6–17.1). The most frequent diagnoses were bronchiolitis (60.5%), asthma/bronchospasm (20.4%) and pneumonia (9.9%). Thirty percent were younger than 57 days.
If we study the diagnostics according to de type of interface, 63 patients (90%) with a helmet and 29 patients (35.4%) with TFM, have a bronchiolitis. The rest of patients who wear TFM have asthma or bronchospasm (36.5%) and pneumonia (17.1%). These differences in the type of pathology explain why the median age and weight are lower in patients with helmet (1.7 months, 4kg) compared to patients with TFM (15.6 months, 10kg).
The median length of stay in the PICU was 5 days (IQR 3–8). The median duration of ventilation was 43h (IQR 23.6–74.3). Characteristics of patients are shown in Tables 1 and 2.
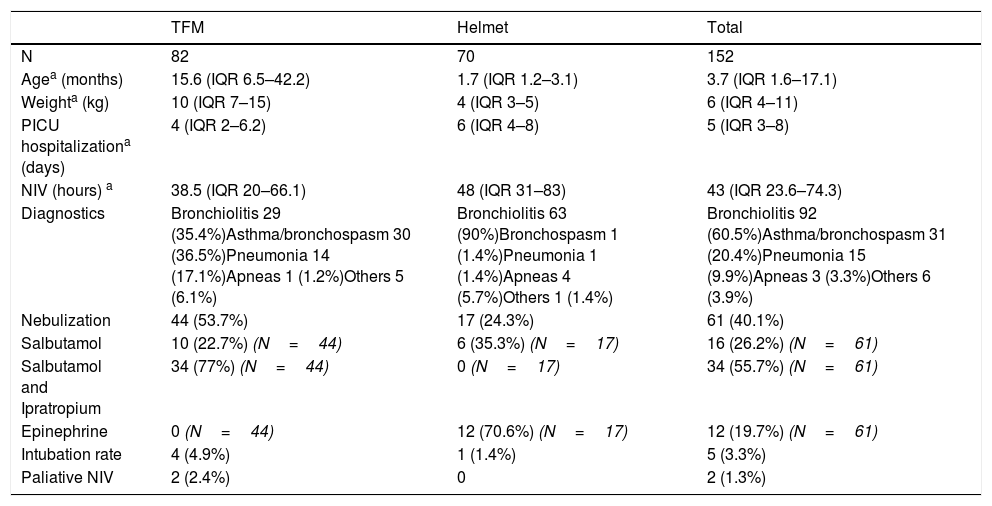
Characteristics of patients and interface used.
| TFM | Helmet | Total | |
|---|---|---|---|
| N | 82 | 70 | 152 |
| Agea (months) | 15.6 (IQR 6.5–42.2) | 1.7 (IQR 1.2–3.1) | 3.7 (IQR 1.6–17.1) |
| Weighta (kg) | 10 (IQR 7–15) | 4 (IQR 3–5) | 6 (IQR 4–11) |
| PICU hospitalizationa (days) | 4 (IQR 2–6.2) | 6 (IQR 4–8) | 5 (IQR 3–8) |
| NIV (hours) a | 38.5 (IQR 20–66.1) | 48 (IQR 31–83) | 43 (IQR 23.6–74.3) |
| Diagnostics | Bronchiolitis 29 (35.4%)Asthma/bronchospasm 30 (36.5%)Pneumonia 14 (17.1%)Apneas 1 (1.2%)Others 5 (6.1%) | Bronchiolitis 63 (90%)Bronchospasm 1 (1.4%)Pneumonia 1 (1.4%)Apneas 4 (5.7%)Others 1 (1.4%) | Bronchiolitis 92 (60.5%)Asthma/bronchospasm 31 (20.4%)Pneumonia 15 (9.9%)Apneas 3 (3.3%)Others 6 (3.9%) |
| Nebulization | 44 (53.7%) | 17 (24.3%) | 61 (40.1%) |
| Salbutamol | 10 (22.7%) (N=44) | 6 (35.3%) (N=17) | 16 (26.2%) (N=61) |
| Salbutamol and Ipratropium | 34 (77%) (N=44) | 0 (N=17) | 34 (55.7%) (N=61) |
| Epinephrine | 0 (N=44) | 12 (70.6%) (N=17) | 12 (19.7%) (N=61) |
| Intubation rate | 4 (4.9%) | 1 (1.4%) | 5 (3.3%) |
| Paliative NIV | 2 (2.4%) | 0 | 2 (1.3%) |
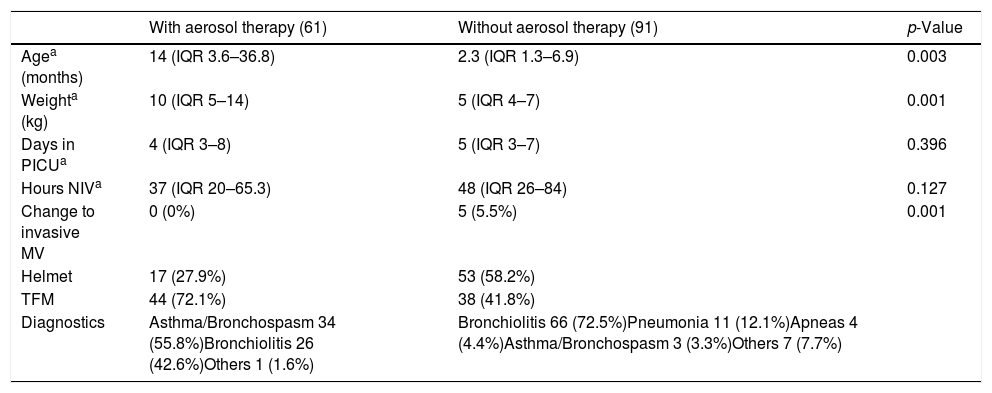
Characteristics of patients according to the use of nebulized drugs.
| With aerosol therapy (61) | Without aerosol therapy (91) | p-Value | |
|---|---|---|---|
| Agea (months) | 14 (IQR 3.6–36.8) | 2.3 (IQR 1.3–6.9) | 0.003 |
| Weighta (kg) | 10 (IQR 5–14) | 5 (IQR 4–7) | 0.001 |
| Days in PICUa | 4 (IQR 3–8) | 5 (IQR 3–7) | 0.396 |
| Hours NIVa | 37 (IQR 20–65.3) | 48 (IQR 26–84) | 0.127 |
| Change to invasive MV | 0 (0%) | 5 (5.5%) | 0.001 |
| Helmet | 17 (27.9%) | 53 (58.2%) | |
| TFM | 44 (72.1%) | 38 (41.8%) | |
| Diagnostics | Asthma/Bronchospasm 34 (55.8%)Bronchiolitis 26 (42.6%)Others 1 (1.6%) | Bronchiolitis 66 (72.5%)Pneumonia 11 (12.1%)Apneas 4 (4.4%)Asthma/Bronchospasm 3 (3.3%)Others 7 (7.7%) |
Analyzing separately the groups according to the interface, as can be seen in Table 3, in patients with helmet there are statistically significant differences in severity of WD score and length of stay in PICU between the group receiving aerosol therapy and the non-aerosol group (p=0.008 and p=0.046 respectively).
No side-effects due to drugs were observed. If Bayesian statistic is applied the probability of having an adverse effect is 1.3% with helmet (probability interval=0.000–0.135) and with TFM 0.5% (probability interval=0.000–0.055).
Using multivariate analysis and Cox regression (Tables 3 and 4), it can be observed that with helmet, nebulized therapy is used in the more severe cases and this use does not affect the failure rate nor the length of stay in PICU. Nevertheless, with TFM nebulized therapy is used in older patients and this use shortens the length of stay in PICU without affecting the failure rate.
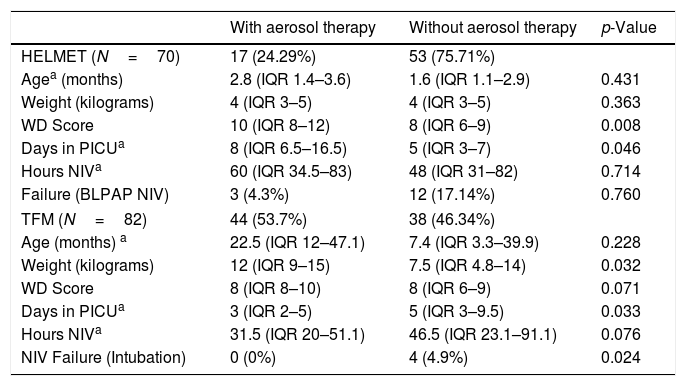
Comparison between patients with or without nebulized drugs according to the type of interface.
| With aerosol therapy | Without aerosol therapy | p-Value | |
|---|---|---|---|
| HELMET (N=70) | 17 (24.29%) | 53 (75.71%) | |
| Agea (months) | 2.8 (IQR 1.4–3.6) | 1.6 (IQR 1.1–2.9) | 0.431 |
| Weight (kilograms) | 4 (IQR 3–5) | 4 (IQR 3–5) | 0.363 |
| WD Score | 10 (IQR 8–12) | 8 (IQR 6–9) | 0.008 |
| Days in PICUa | 8 (IQR 6.5–16.5) | 5 (IQR 3–7) | 0.046 |
| Hours NIVa | 60 (IQR 34.5–83) | 48 (IQR 31–82) | 0.714 |
| Failure (BLPAP NIV) | 3 (4.3%) | 12 (17.14%) | 0.760 |
| TFM (N=82) | 44 (53.7%) | 38 (46.34%) | |
| Age (months) a | 22.5 (IQR 12–47.1) | 7.4 (IQR 3.3–39.9) | 0.228 |
| Weight (kilograms) | 12 (IQR 9–15) | 7.5 (IQR 4.8–14) | 0.032 |
| WD Score | 8 (IQR 8–10) | 8 (IQR 6–9) | 0.071 |
| Days in PICUa | 3 (IQR 2–5) | 5 (IQR 3–9.5) | 0.033 |
| Hours NIVa | 31.5 (IQR 20–51.1) | 46.5 (IQR 23.1–91.1) | 0.076 |
| NIV Failure (Intubation) | 0 (0%) | 4 (4.9%) | 0.024 |
Age, Weight, Wood Downes Score on admission in PICU, Days in PICU and Hours of NIV are expressed as median. IQR: interquartile range. Percentages are relative to the total number of patients with the same interface (helmet/TFM).
PICU: pediatric intensive care unit; NIV: non-invasive ventilation; WD: Wood Downes score; BLPAP: bilevel positive airway pressure; TFM: total face mask.
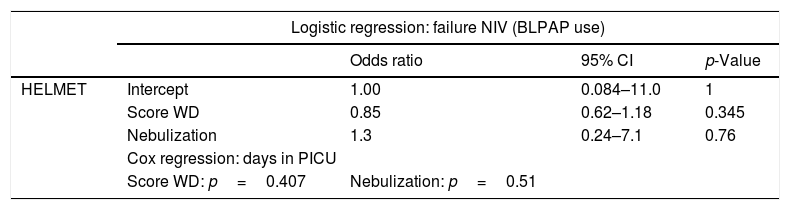
Logistic regression and Cox regression studies according to the type of interface.
| Logistic regression: failure NIV (BLPAP use) | ||||
|---|---|---|---|---|
| Odds ratio | 95% CI | p-Value | ||
| HELMET | Intercept | 1.00 | 0.084–11.0 | 1 |
| Score WD | 0.85 | 0.62–1.18 | 0.345 | |
| Nebulization | 1.3 | 0.24–7.1 | 0.76 | |
| Cox regression: days in PICU | ||||
| Score WD: p=0.407 | Nebulization: p=0.51 | |||
| Logistic regression: failure NIV (Intubation) | ||||
|---|---|---|---|---|
| Odds ratio | 95% CI | p-Value | ||
| TFM | Intercept | 0.105 | 0.03 to 0.38 | 0.00059 |
| Age (months) | 1.02 | 0.994 to 1.05 | 0.136 | |
| Nebulization | 5.55e−09 | 0.000 to Infinity | 0.994 | |
| Cox regression: days in PICU | ||||
| Age (months): p=0.457 | Nebulization: HR (95CI)=1.7 (1.07–2, p=0.019) | |||
NIV: non-invasive ventilation; CI: confidence interval; HR: hazard ratio; TFM: total face mask; WD: Wood Downes score on admission in PICU; PICU: pediatric intensive care unit.
However, if intubated or patients who died are excluded, no statistical differences are observed in the median of length of stay in patients with or without nebulized drugs (4 days with nebulized treatment, 5 days without inhaled drugs, p 0.99).
DiscussionThe increasing use of NIV in pediatric population, together with the need to associate inhaled drugs in a high percentage of cases (40.1%), makes this an important issue.
Although side effects with nebulized drugs in patients with face masks have been published,11 our cases appear to confirm that side effects are an extremely infrequent event.
The most important finding is that in our study the use of inhaled therapy with TFM (if required), reduces hospital stay without side-effects (61% of patients receiving these drugs have asthma). However, with helmet, it has no effect on failure or hospital stay (94% of the patients in which inhaled drugs were used presented with bronchiolitis).
Otherwise it is important to note that to our knowledge, this is the first report of use of TFM in children.
Nevertheless, our study has limitations. The main limitation is that it is a descriptive study and we do not have any control group to compare with.
The importance of nebulization as adjunctive therapy to ventilation (although not recommended in the latest guidelines of bronchiolitis – NICE guide –), the low frequency of adverse events related to its use and the minor clinical consequences, allows us to recommend the use of nebulized drugs, if necessary (for example in asthma and bronchospasm), when a patient uses a TFM or a helmet.
In conclusion, the probability of a pediatric patient having an adverse effect related to nebulization is extremely low using helmet or Total Face Mask. In our experience, aerosol therapy with helmet or TFM is safe and can reduce hospital stay in some patients.
Authors contributions- -
Lucía Rodríguez García: Study design, acquisition, analysis and interpretation of data. Preparation of the draft of the article. Final approval of the presented version.
- -
Alberto Medina Villanueva: Study design, acquisition, analysis and interpretation of data. Preparation of the draft of the article and critical review of it. Final approval of the presented version.
- -
Vicent Modesto i Alapont. Study design, analysis and interpretation of data. Article review. Final approval of the presented version.
- -
María Luisa Palacios Loro. Acquisition of data, critical review of the content of the article. Final approval of the presented version.
- -
Juan Mayordomo-Colunga. Analysis and interpretation of data. Article review. Final approval of the presented version.
- -
Ana Vivanco Allende. Analysis and interpretation of data. Article review. Final approval of the presented version.
- -
Corsino Rey Galán. Analysis and interpretation of data. Article review. Final approval of the presented version.
Authors have no conflict of interests to declare.
We thank all the PICU staff for their help.