The most common site of infection in immunocompromised oncological children is the lungs.1 The difficulties involved in achieving an early diagnosis are associated with its atypical presentation. Neutropenia reduces the inflammatory processes leading to lower clinical and radiological expressions.2 Therefore, some of these patients, like our case, can get worse while they are recovering from neutropenia.
The radiological presentation of pulmonary infiltrates can help achieve the differential diagnosis.1–3 The most common bacteria include Streptococcus pneumoniae, Staphylococcus, and Haemophlilus influenzae. However, in these patients, infections due to opportunistic bacteria should also be taken into consideration like Pseudomonas spp. and viral agents being the most common of all respiratory viruses1 and in the case of permanent neutropenias, fungal infections, being Candida the most common of all2 including Aspergillus.
This is the case of a 4-year-old kid of 15kg of weight diagnosed back on January 29th, 2020 of acute lymphoblastic leukemia type B without any other significant past medical history. He was administered prednisone (60mg/m2/day) followed by chemotherapy (the patient received his last dose on March 19).
Four days later he was admitted to the Day Hospital with signs of fever of 100.4°F and pancytopenia (420 leukocytes/μL and 40 neutrophils/μL). At home he remained asthenic but still without a fever. He was admitted to the pediatric oncology unit and administered cefepime (150mg/kg/day) followed by prophylactic cotrimoxazole.
After 2 days and due to the presence of persistent fever the patient was administered teicoplanin (10mg/kg/day) plus amphotericin B (5mg/kg/day). The hemocultures, urine culture, and PCR run to identify the presence of influenza virus and respiratory syncytial virus all tested negative.
The patient progressed with respiratory failure and required a nasal cannula. The thoracic x-ray performed revealed the opacification of left hemithorax consistent with an inflammatory-infectious process.
Therefore, pneumonia in aplastic patient is suggested in the epidemiological context of infection due to new coronavirus infection in his grandfather (who remains hospitalized), mother, and grandmother as confirmed on the polymerase chain reaction (PCR) test performed through nasopharyngeal aspirate swab obtained using the Cobas 6800 system (Roche). The PCR performed to discard the presence of coronavirus at admission had tested negative. After the positive diagnosis of the patient’s mother and grandmother, he is tested again after the thoracic x-ray and the PCR tests negative one more time.
Nonetheless, due to the patient’s clinical worsening and epidemiological situation it is decided to administer azithromycin (10mg/kg/day), hydroxychloroquine (100mg every 12h within the first day followed by 50mg every 12h starting on day 2) followed by lopinavir/ritonavir 2.25mL every 12h.
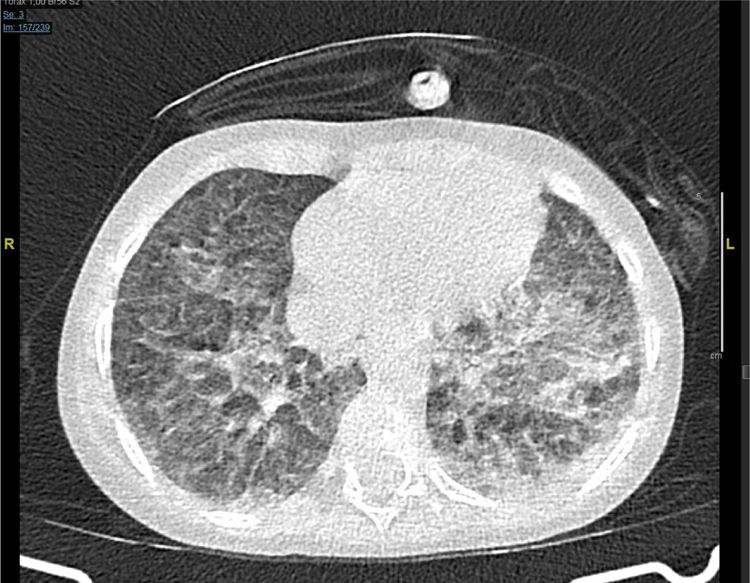
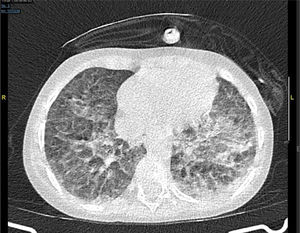
The next day the respiratory failure worsens despite the bone marrow aplasia recovery and a thoracic CT scan is performed that reveals the presence of both pseudonodular ground-glass opacities and bilateral peripheral opacities (Fig. 1).
High-flow oxygen therapy is started at 12 lmp and a Fi02 of 35%. The patient remains with persistent fever, tachypnea, subcostal retraction, and his admission to the pediatric ICU is authorized.
Initially, high-flow oxygen therapy is maintained while increasing parameters with good tolerance. However, the patient shows work of breathing with universal retraction and desaturation, and it is decided to proceed with orotracheal intubation and connection to invasive mechanical ventilation. A new PCR test to discard the presence of SARS-CoV-2 in bronchial aspirate is run again but it tests negative once again. Lopinavir/ritonavir, azithromycin, and cefepime are all withdrawn and meropenem (60mg/kg/day) is administered.
The respiratory pathogens panel test (IgM and IgG antibodies against Legionella pneumophila, Mycoplasma pneumoniae, and Chlamydophila pneumoniae), the galactomannan antigen blood test, and the viral load of cytomegalovirus all tested negative; no further serology tests of such control panel were run.
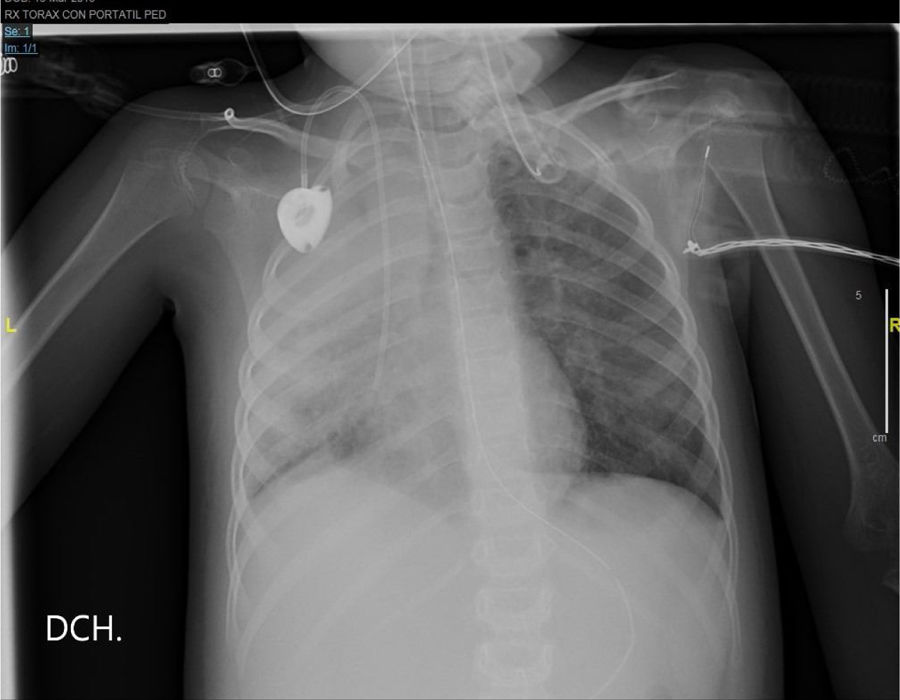
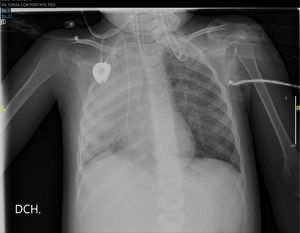
The thoracic x-ray performed the next day confirmed the complete opacification of the right lung with left paracardiac infiltration (Fig. 2).
The blood test performed at the ICU admission confirmed the following parameters: C-reactive protein (CRP), 11.9mg/dL; procalcitonin (PCT), 2.63ng/mL; triglycerides, 250mg/dL; lactate dehydrogenase, 780 U/L; ferritin, 1734μL/L; interleukin 6, 105pg/dL; lymphopenia, 750/μL; platelets, 70 000/μL, and D-dimer 2.72μg/mL. The CRP, PCT, triglyceride, and lactate dehydrogenase levels dropped gradually, ferritin levels went up to 2016μL/L on day 2, and IL-6 levels reached 141pg/dL on day 3 without further determinations. Lymphopenia and thrombocytopenia recovered on day 4 while D-dimer levels remained stable.
Due to these findings, the epidemiological situation, the situation of progressive respiratory failure, and the radiological images obtained, the case was discussed with the Unit of Infectious Diseases. It was decided to administer tocilizumab 8mg/kg in a single dose and run a total antibody test looking for the presence of IgM, IgG, and IgA antibodies against SARS-CoV-2 (two-step chemiluminescent immunoassay, Elecsys anti-SARS-CoV-2, Roche), and a new PCR in plasma (500mL of plasma mixed with the same amount of lysis solution) and in feces (fecal suspension in 500mL of physiological serum using the same procedure as in plasma processed using the Cobas 6800 system (Roche). Everything tested negative.
The next day, the PCR test to discard Pneumocystis jirovecii in bronchial aspirate tested positive and cotrimoxazole was titrated to therapeutic doses.
The patient’s respiratory condition started to improve, and he was extubated on day 8. The patient was discharged from the ICU and transferred to the hospital pediatric oncology unit. He showed eupneic breathing upon return to room air with oral tolerance to monotherapy with cotrimoxazole. The patient received no corticoids during his ICU stay.
A month later, the antibody test against the new coronavirus was run again but it tested negative one more time.
In light of the 2 diagnostic possibilities of our case, we were facing an epidemiological context with great exposure to SARS-CoV-2, compatible radiological and analytical findings, but negative test samples without ever running a PCR test in bronchoalveolar lavage (that performs better) or a rectal swab.4 We should not forget that inflammatory parameters are not specific of this infection. However, high levels of IL-6 and PCR are actually considered independent risk factors for its severity.5,6 High procalcitonin levels are suggestive of bacterial over-infection.7 In this case its discrete elevation was considered an acute-phase reactant. Pneumocystis is an environmental fungus that can cause false positive results.8 However, its diagnosis could not be excluded since we saw refractory infiltrates in the patient that did not respond to therapy within the first 24−72h. Still, the response to therapy was faster than expected.
Please cite this article as: González Moyano AB, Medina Ramos L, del Cañizo Moreira M, Merino de Lucas E, González Lorenzo M, Esteban García-Fontecha M. ¿SARS-CoV-2 o Pneumocystis jirovecii? A propósito de un caso. Med Intensiva. 2021;45:124–126.