To describe the factors associated to morbidity–mortality in pediatric patients with severe head injury (SHI).
Material and methodA review was made of the patients admitted to the Pediatric Intensive Care Unit (PICU) with SHI between July 1983 and December 2009.
ResultsOf the 389 patients with head injuries, 174 (45%) presented SHI. The mean age of these subjects was 67 (9) months, with a Glasgow Coma Score (GCS) of 5.5 (1.8) and a PRISM score of 10.6 (6.7). Thirty-nine percent of the patients showed diffuse encephalic injury (DEI) in the computed tomography (CT) study. Seventy-nine percent of the patients subjected to intracranial pressure monitoring (ICP) presented intracranial hypertension. These patients had a greater incidence of serious sequelae (66.7% vs 23.1%; p=0.01). Sequelae of clinical relevance were recorded in 59 patients (34%), and proved serious in 64% of the cases. The mortality rate among the patients with SHI was 24.7%, and mortality was significantly associated with a lower GCS score, hyperglycemia, intracranial hypertension and the presence of mydriasis or shock. The mortality rate associated to severe DEI was significantly higher than in the case of mild-moderate DEI (87.5% vs 7.2%; p<0.001).
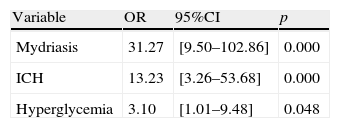
The independent mortality risk factors in the pediatric patients with SHI were found to be the presence of mydriasis (OR: 31.27), intracranial hypertension (OR: 13.23) and hyperglycemia (OR: 3.10).
Conclusions(a) SHI in pediatric patients was associated with high morbidity–mortality; (b) intracranial hypertension was associated to the development of serious sequelae; (c) independent mortality risk factors were the existence of mydriasis, intracranial hypertension and hyperglycemia.
Describir los factores asociados a la morbilidad y mortalidad de los pacientes pediátricos con traumatismo craneoencefálico grave (TCEG).
Material y métodoRevisión de los pacientes ingresados en una unidad de medicina intensiva pediátrica (UMIP) con TCEG en el periodo comprendido entre julio de 1983 y diciembre de 2009.
ResultadosDe los 389 pacientes con TCE ingresados en nuestra unidad durante el periodo de estudio, presentaron TCEG 174 (45%). La edad media de este grupo fue de 67±9 meses, con una puntuación media en la escala de Glasgow (GCS) de 5,5±1,8 y una puntuación PRISM media de 10,6±6,7. El 39% de los pacientes presentaron lesión encefálica difusa (LED) grave en la TAC. Un 79% de los pacientes en los que se monitorizó la presión intracraneal (PIC) presentaron hipertensión intracraneal (HIC). Estos pacientes tuvieron una mayor incidencia de secuelas graves que aquellos que no desarrollaron HIC (66,7 vs 23,1%; p=0,01).
Las secuelas de relevancia clínica se encontraron en 59 pacientes (34%), y fueron graves en el 64% de los mismos.
La mortalidad de los pacientes con TCEG fue de un 25% y se asoció de forma significativa a una menor puntuación del GCS, a la existencia de hiperglucemia o HIC, a la presencia de midriasis o shock y a la necesidad de ventilación mecánica. La mortalidad de la LED grave fue significativamente más elevada que la LED leve-moderada (87,5 vs 7,2%; p<0,001) y que la lesión focal (87,5 vs 36,1%; p<0,001). Los factores responsables de la mortalidad de forma independiente en los pacientes pediátricos con TCEG fueron la existencia de midriasis (OR: 31,27), HIC (OR: 13,23) e hiperglucemia (OR: 3,10).
Conclusionesa) Los TCEG en edad pediátrica asocian una alta morbilidad y mortalidad; b) la existencia de HIC se asoció al desarrollo de secuelas graves; c) los factores de riesgo de mortalidad de forma independiente fueron la existencia de midriasis, HIC e hiperglucemia.
In the pediatric population, head injuries (HIs) are the leading cause of trauma-related mortality and are responsible for sequelae as serious as mental retardation, childhood epilepsy and physical incapacitation.1–3
The adequate management of children with serious head injuries (SHIs) is crucial in order to minimize the associated complications.
Morbidity and mortality in patients with HIs are determined by the primary lesions inflicted at the time of trauma (related to the type of injuries and their location) and by the secondary lesions (hypoxia, ischemia, edema, intracranial hypertension [ICH]) – the effects of which manifest in later stages and are amenable to the adoption of preventive measures in the form of adequate resuscitation and stabilization.4–6
Advances in our knowledge of the physiopathology of the events following HIs have made it possible to develop new diagnostic and therapeutic methods. Nevertheless, morbidity and mortality remain high in these patients.7,9 Emphasis is therefore placed on the need to develop systems for improved monitorization of cerebral dynamics and for better prediction of patient outcome.6,10,11
The present study examines the factors influencing morbidity and mortality among pediatric patients with SHIs.
Material and methodsStudy designRetrospective (August 1983 to December 1998), prospective (January 1999 to December 2009).
Study setting- -
Reference population: children aged between 1 month and 14 years, with HIs, exhibiting a Glasgow Coma Scale (GCS) score of ≤8.
- -
Hospital center: third-level hospital serving as provincial reference center (The Canary Islands (Spain): Gran Canaria, Fuerteventura and Lanzarote), serving a pediatric population of 137,538 children.
- -
Quantitative variables: age, GCS score, pediatric risk of mortality score (PRISM), duration of mechanical ventilation, stay in ICU, Glasgow Outcome Scale (GOS).
- -
Qualitative variables: gender, origin, mortality, cause of HI, associated extracranial injuries, lesions on cranial CT scan, sequelae, presence of hyperglycemia, anemia, shock or pupil mydriasis, need for monitoring of intracranial pressure (ICP) and jugular venous oxygen saturation (SjO2), existence of ICH, and need for mechanical ventilation.
- -
GCS: following patient stabilization we used the first reflected value of the classical Glasgow scale12 or its modification for pediatric patients.13 The latter is distinguished from the classical scale only in terms of the verbal response section, which is scored as follows: babbling (5), irritable crying (4), crying in response to pain (3), complaint or sighing in response to pain (2), no response (1).
- -
SHI: GCS score ≤8.
- -
PRISM score: pediatric risk of mortality score.14
- -
Polytraumatized patient15,16: serious injuries affecting at least two body regions (skull/brain, thorax, abdomen, musculoskeletal system), or three major fractures. Serious injury is that affecting: (a) skull: unconsciousness or neurological focality, bleeding from nose, bleeding from ears, or facial fracture; (b) thorax: rib fractures, sternal fracture, pneumothorax, hemothorax, lung contusion, aortic rupture, cardiac tamponade or ruptured diaphragm; (c) abdomen: organ laceration or contusion, and (d) musculoskeletal system: vertebral body or arch fracture, fracture of the pelvis, femur, tibia, humerus, or amputation of extremities.
- -
Lesion evidenced by CT: we used the classification of the Trauma Coma Data Bank (TCDB).17,18 Severe diffuse brain injury (SDBI) was taken to represent diffuse brain injury (DBI) III and DBI IV.
- -
Shock: existence of systolic blood pressure <55mmHg in patients under 1 year of age and<65mmHg in those over 1 year, with organ repercussions and the need for fluid therapy (≥20ml/kg) and/or catecholamines for control.14
- -
Hyperglycemia: blood glucose>200mg/dl.
- -
Mechanical ventilation: patients requiring ventilation with intermittent positive pressure at the time of HI or in relation to the latter.
- -
Anemia: hemoglobin<8 g% or the need for transfusion of red cell concentrates ≥10ml/kg.
- -
Monitoring of ICP: performed with different pressure systems and in different locations according to the clinical case or technical availability.
- -
ICH: ICP>20mmHg on a sustained basis despite control of all the intracranial or extracranial factors capable of influencing its measurement.
- -
Mydriasis: non-reactive unilateral or bilateral dilatation of the pupil >4mm.
- -
SjO2: monitoring after retrograde internal jugular vein catheterization, with radiological verification of the location in the jugular bulbar zone. The determinations were performed on an intermittent basis.
- -
Arterio-jugular oxygen difference (Sa-jO2)19: difference between SaO2 and SjO2. Three possibilities were considered: (a) brain hyperemia: Sa-jO2<20%; (b) brain ischemia: Sa-jO2 >40%, and (c) normal: Sa-jO2 between 20 and 40%.
- -
Clinical outcome: at discharge from the PICU, the patients were classified into four categories according to the Glasgow Outcome Scale20: (a) death; (b) vegetative state: unable to reciprocally interact with the environment, or severe disability: able to follow instructions, unable to live independently; (c) moderate disability: able to live independently; and (d) good recovery: able to return to baseline situation before HI. Serious sequelae were considered to correspond to a GOS score of 2.
- -
Age groups: the population was classified into three groups: under 2 years, between 2 and 6 years, and over 6 years.
The data obtained were processed with the Statistical Package for Social Sciences (SPSS) version 17.0. The Student t-test was used for the comparison of means, while the chi-squared test or Fisher exact test was used for the comparison of percentages. The level of significance was established for alpha=0.05.
The multivariate analysis was based on the stepwise method, with input verification according to the significance of the scoring statistic and checking of elimination, based on the likelihood ratio test (partial maximum likelihood estimations) The accepted level of significance was 0.05.
Likewise, calibration analysis was carried out using the goodness of fit test and the Hosmer–Lemeshow χ2 statistic to evaluate the fit of the model.
ResultsA total of 389 pediatric patients were admitted with a diagnosis of HI. Forty-five percent, i.e., 174 children, suffered SHI, with a predominance of males (67%). The mean age was 67.9±41.6 months. Most patients were admitted to the ICU from the emergency area (61.5%), followed by transfer from other hospitals (38%). Traffic accidents were the cause of SHI in 56% of the cases, followed by falls (25%), other types of accidents (17%), and aggression as the least common cause (2%).
The mean stay in the ICU was 9.1±13 days, with a median of 5 days. The mean PRISM score was 10.77±6.7, while the mean duration of mechanical ventilation was 99.41±58h (median 48h).
Regarding the clinical course, 65 patients (37%) suffered hemodynamic instability and shock, while anemia and hyperglycemia were recorded in 112 (66%) and 66 patients (38%), respectively. Pure HIs were documented in 66% of the cases, while associated cranial fracture was diagnosed in 55% of the patients. In turn, 92.5% of the study series required mechanical ventilation.
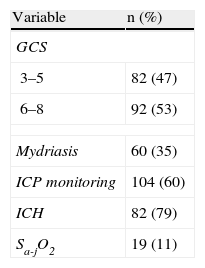
Table 1 reports the most important neurological monitorization parameters. In this context, the mean GCS score was 5.5±1.8, and 47% of the patients presented a GCS score of ≤5. Intracranial pressure was monitored in 104 patients (60%) with SHIs – ICH being diagnosed in 79% of the cases. In turn, SjO2 was monitored in 25% of the patients with ICH, revealing a predominance of low cerebral oxygen extraction (brain hyperemia) (in 70% of the cases) vs high cerebral oxygen extraction (brain ischemia) (recorded in 20% of the cases).
In relation to the CT findings, 71.7% of the patients presented diffuse cerebral lesions (severe in 55% of the cases). Among the focal lesions (28.3%), a predominance of evacuated focal lesions was recorded (75%).
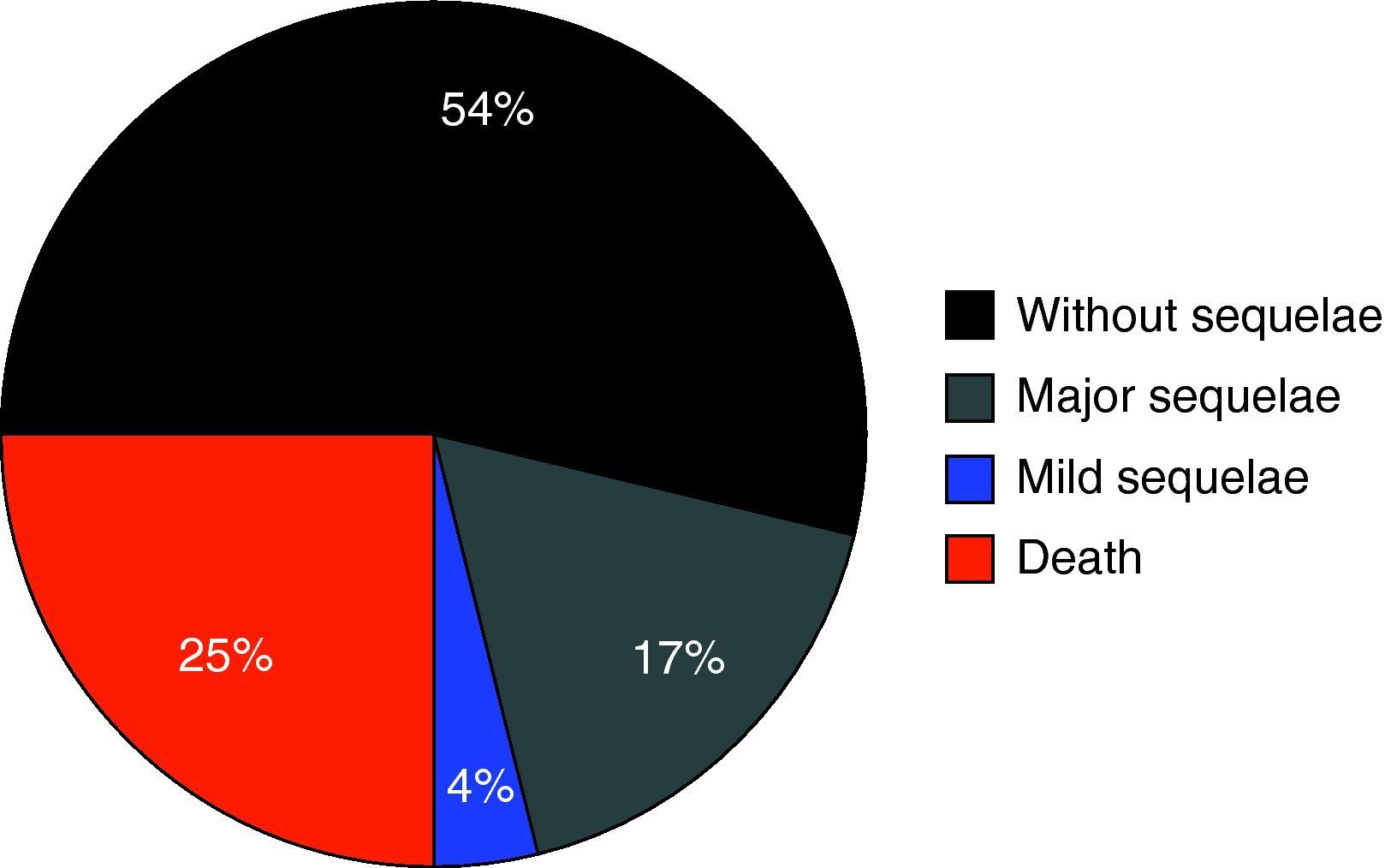
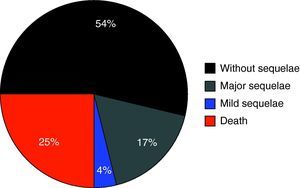
Fig. 1 shows the percentage distribution of the modified GOS corresponding to the patients at discharge from the PICU – revealing high morbidity (GOS2 and GOS3: 34.3%) and mortality (24.7%) in our population.
An analysis was made of a series of clinical variables classically related to morbidity and mortality in pediatric head injuries. The patients who developed ICH suffered a significantly higher incidence of serious sequelae (GOS=2) than those without ICH (66.7% vs 23.1%; p=0.01).
There were no significant differences in mortality according to patient age, considered either quantitatively (71.3±42.8 months vs 57.9±31.7 months; p=0.06) or qualitatively in the three different age groups or intervals considered (age<2 years: 26.7%; age 2–6 years: 31.4%; age >6 years: 31.7%; NS).
The patients with a lower GCS score globally suffered greater mortality, ranging from 5.6% among the patients with GCS 8 and from 56.4% for those with GCS 3.
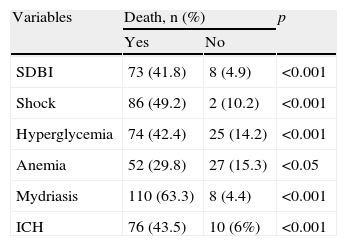
The patients who died showed comparatively higher PRISM scores (22.2±5.3 vs 8.9±4.9; p<0.001) and lower GCS scores (4.3±1.5 vs 5.9±1.7; p<0.001). Likewise, the patients with anemia, shock, hyperglycemia, ICH or mydriasis showed significantly greater mortality (Table 2). We recorded no differences in relation to mortality among the patients with associated polytraumatism vs the children with pure HIs (29% vs 23%; NS), or among those with cranial fracture vs those without (26% vs 23%; NS).
Factors associated to mortality in pediatric serious head injuries.
| Variables | Death, n (%) | p | |
| Yes | No | ||
| SDBI | 73 (41.8) | 8 (4.9) | <0.001 |
| Shock | 86 (49.2) | 2 (10.2) | <0.001 |
| Hyperglycemia | 74 (42.4) | 25 (14.2) | <0.001 |
| Anemia | 52 (29.8) | 27 (15.3) | <0.05 |
| Mydriasis | 110 (63.3) | 8 (4.4) | <0.001 |
| ICH | 76 (43.5) | 10 (6%) | <0.001 |
SDBI: severe diffuse brain injury; ICH: intracranial hypertension.
Regarding mortality associated to each of the lesions identified in the CT study, DBI IV was seen to predominate (58%), followed by DBI III (29%) and DBI V (28%). Severe DBI (III–IV) was associated to significantly greater mortality than DBI I–II (41.8 vs 4.9; p<0.001).
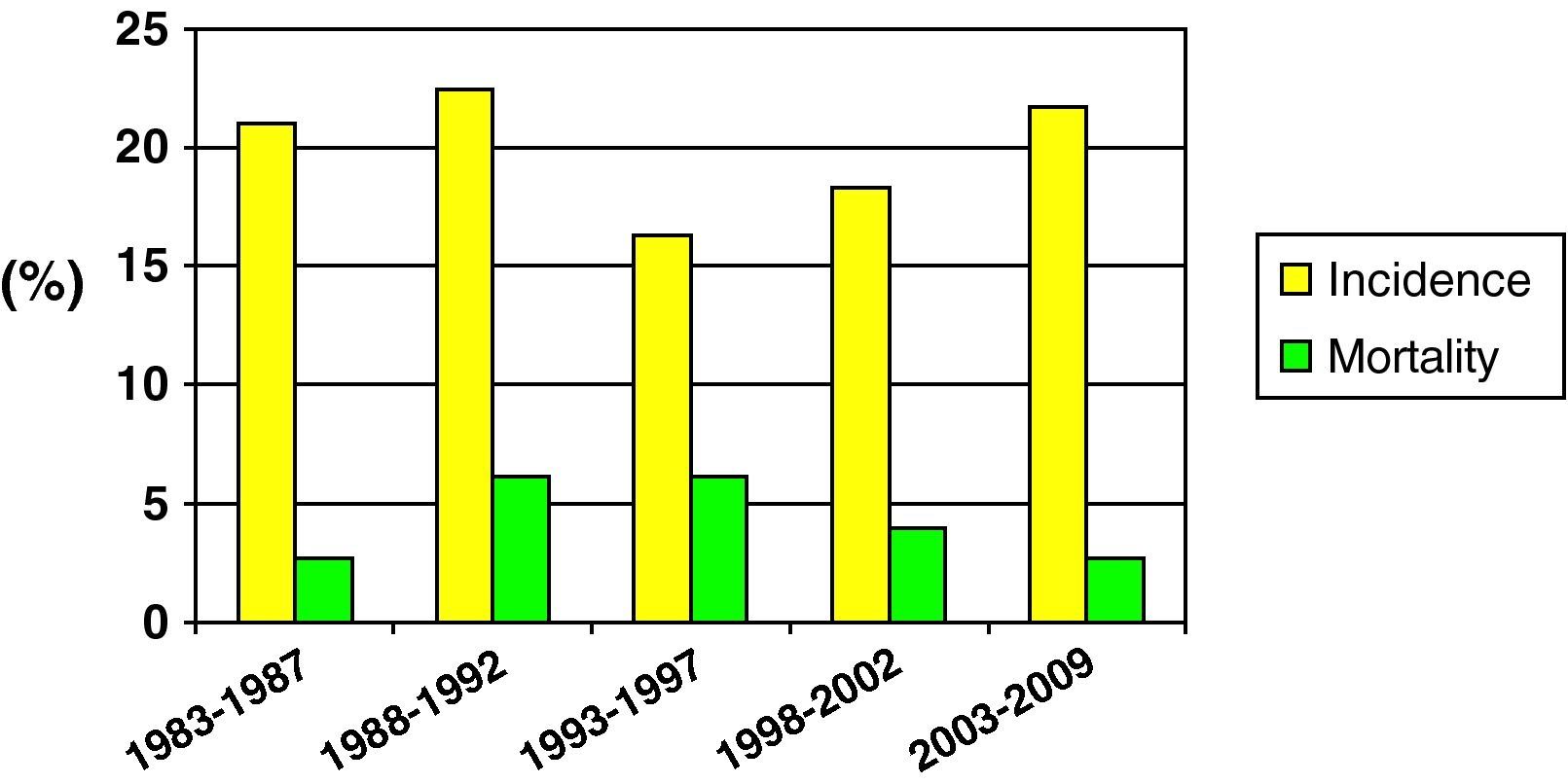
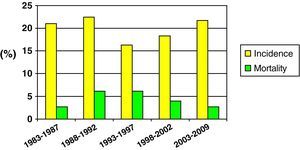
Fig. 2 shows the incidence and mortality findings related to SHIs in different time intervals during the study – a decrease in both parameters being observed over the global period of the study.
Mortality was compared in two different time periods (first 15 years of the study vs the last decade). Although mortality proved comparatively lower in the second period, the difference was not statistically significant (25.5% vs 15.1%; NS).
A multivariate analysis was performed to determine the factors independently responsible for mortality among the pediatric patients with SHIs. These were found to be the presence of mydriasis, ICH and hyperglycemia. Table 3 shows these factors together with the associated mortality risk corresponding to each of them.
Lastly, the good fit of the model was confirmed by calibration analysis (goodness of fit and Hosmer–Lemeshow χ2 statistic), yielding χ2=2.31 and p=0.80.
DiscussionThe present study describes 174 pediatric SHIs involving a total of 389 children with HIs (the last 10 years being subjected to prospective evaluation). A problem found on attempting to compare our data with those of other authors7,8,21–25 is that the published series do not tend to be homogeneous in aspects such as: (a) the age of the patients; (b) the severity of the HIs; or (c) the quality of pre- and in-hospital care (means of transport, promptness of resuscitation measures, diagnostic or therapeutic means, etc.).
A first aspect that should be underscored is the high morbidity recorded in the study population. In effect, one-third of all subjects suffered relevant sequelae – the latter being serious (vigil coma or disabling complications) in 22% of the children evaluated. Although assessment was based on the modified GOS (i.e., at discharge from the PICU, not 6 months after injury), this observation point to the need to maximize efforts to prevent secondary damage associated to HIs.
Of the factors classically associated to morbidity in pediatric HIs,9,18,26–29 we only recorded a significant association among the children that developed ICH. This is of crucial importance, since ICP was monitored in 60% of the patients admitted due to SHIs, and 79% of these cases presented ICH. Such data point to the need to monitor ICP in all children with SHIs.
The mortality rate in our series was 25%, which is similar to the figures reported in the literature.7,8,30 However, the incidence of SHIs in our study was 45%, and we moreover only considered patients between one month and 14 years of age. Ward31 studied 201 patients with SHIs, and the mortality rate after 12 months of follow-up was seen to be 24%, while Feickert et al.25 reported a rate of 22%.
Among the neurological variables, the GCS score was correlated to increased mortality (5.9±1.7 vs 4.2±1.5; p<0.001). In this context, 100% of the patients who died presented GCS ≤8. In our series, 50% of the patients showed GCS ≤5 – this group being associated to significantly greater mortality than the patients with GCS 6–8 (39% vs 12%; p<0.001).
Likewise, the existence of mydriasis or SDBI as evidenced by CT, indicative of ICH or at least diffuse brain edema, was correlated to increased mortality. Fernandez et al.23,34 reported this association in pediatric HIs, and although they analyzed both the first and the second CT scan in each patient, they only recorded a linear association between the initial CT and the clinical outcome.
Regarding the systemic variables,9,35–37,39,40 the presence of hyperglycemia, shock or anemia was associated to increased mortality. Mansfield38 recorded an increase in mortality of 150% in patients with SHIs and shock – this corresponding to a rise in mortality from 22% to 66% in pediatric patients with SHIs. In this same sense, White et al.9 recently concluded that elevated arterial pressure values are associated to greater survival.
The better prognosis of HIs in pediatric patients vs adults has been evidenced by many studies33,38,41 – mortality in turn being higher among the lower age ranges in the pediatric population.38,42,43 In our series, the highest mortality figures corresponded to patients under 6 years of age (30% vs 17.6%; p=0.06).
On the other hand, we observed no differences in mortality between patients with pure HIs or HIs associated to polytraumatism, in coincidence with the results published by Feickert et al.25 However, in the study of Tepas et al.,41 mortality associated to HIs with extracranial injuries almost doubled the mortality associated to pure HIs.
Another relevant observation was that mortality in pediatric SHIs was generally recorded in early phases, since the PICU stay in these cases was significantly shorter than in those patients who did not die (4.5 days vs 10.4 days; p<0.001). We therefore consider that the factors which should be analyzed in relation to mortality in pediatric patients are early-stage factors (upon admission, first 24h), since this is the period defining the prognosis of children with SHIs.
Lastly, in reference to mortality in pediatric SHIs, and despite the problem of sample homogenization, we recorded a clear tendency towards lessened mortality in the last decade (25.5% vs 15.1%). This may have been influenced by optimization in the diagnosis and treatment of patients with SHIs, as well as by improvements in traffic safety, the incorporation of more advanced safety systems in vehicles, etc.
In the multivariate analysis, the presence of ICH or of uni- or bilateral mydriasis as a reflection of ICH and an event prior to cerebral herniation represented determinant mortality factors, associated to a high odds ratio – in coincidence with the observations of other investigators.5,7–9,32 Although these two variables may raise doubts as possible confounding factors (association to each other and to mortality), the binary logistic regression analysis (stepwise method) and calibration analysis (goodness of fit and Hosmer–Lemeshow χ2 statistic) identified these two variables as independent mortality factors, with a good fit of the model. A third variable included in this analysis was hyperglycemia. Under anaerobic conditions, the brain shows an increased lactic acid output (anaerobic glycolysis) that favors an increase in osmolarity and brain edema.36,37 In our series, the presence of elevated blood glucose implied a three-fold increase in mortality risk among the children with SHIs.
In conclusion, and despite the inconvenience posed by the fact that part of this study was conducted on a retrospective basis, we can point to severe neurological damage (pupil mydriasis, ICH) and brain edema-favoring parameters such as hyperglycemia as factors of relevance in relation to mortality.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: López Álvarez JM, et al. Traumatismo craneoencefálico pediátrico grave (II): factores relacionados con la morbilidad y mortalidad. Med Intensiva. 2011;35:337–43.