To describe the epidemiology, clinical manifestations and evolutive characteristics of pediatric patients with severe head injury (SHI).
Material and methodA review was made of the patients admitted to the pediatric intensive care unit (PICU) with SHI between July 1983 and December 2009.
ResultsOf the 389 patients with head injuries admitted to the PICU during the study period, 174 (45%) presented SHI. The mean age in this group was of 67±9 months, with a Glasgow Coma Score (GCS) of 5.5±1.8 and a PRISM score of 10.7±6.7. The most frequent etiology of SHI was traffic accidents (56%), though these have decreased significantly in the last decade (58.5% vs 45.3%; p<0.001). Twenty-one percent of the patients required evacuation of the lesions detected by computed tomography (CT), and 39% presented severe diffuse encephalic injury (DEI). Seventy-nine percent of the patients in whom intracranial pressure (ICP) was monitored presented intracranial hypertension. Sequelae of clinical relevance were recorded in 59 patients (39%), and proved serious in 64% of the cases. The mortality rate in this patient series was 24.7%. Intracranial hypertension decreased significantly in the last decade (88% vs 54%; p<0.05), and clinical recovery has improved (23.3% vs 63.1%; p<0.001).
Conclusions(a) The incidence of traffic accidents has decreased in the last decade in the studied population; (b) patients with SHI in which ICP was monitored showed a high incidence of intracranial hypertension; (c) morbidity–mortality among pediatric patients with SHI has decreased over the course of the study period.
Describir las características epidemiológicas, clínicas y evolutivas de los pacientes pediátricos con traumatismo craneoencefálico grave (TCEG).
Material y métodoRevisión de los pacientes ingresados en una unidad de medicina intensiva pediátrica (UMIP) con TCEG en el periodo comprendido entre julio de 1983 y diciembre de 2009.
ResultadosDe los 389 pacientes con traumatismo craneoencefálico (TCE) ingresados en nuestra unidad durante el periodo de estudio, presentaron TCEG 174 (45%). La media de edad de este grupo fue 67±9meses, con una puntuación media en la escala de Glasgow (GCS) de 5,5±1,8 y una puntuación PRISM media de 10,7±6,7. La etiología más frecuente de los TCEG fueron los accidentes de tráfico (56%), aunque en la última década existe una disminución significativa de su incidencia (el 58,5 frente al 45,3%; p<0,001). Un 21% de los pacientes precisaron evacuación de la lesión objetivada en la TC, objetivándose en un 39% lesión encefálica difusa (LED) grave. Un 79% de los pacientes en los que se monitorizó la presión intracraneal (PIC) presentaron hipertensión intracraneal (HTC). Las secuelas de relevancia clínica se objetivaron en 59pacientes (39%), siendo graves en el 64% de ellos. La mortalidad de la población estudiada fue de un 24,7%. La incidencia de HTC fue significativamente menor en la última década estudiada (el 88 frente al 54%; p<0,05), con una mejor recuperación clínica (el 23,3 frente al 63,1%; p<0,001).
Conclusionesa) La incidencia de los accidentes de tráfico disminuyó en la última década en la población estudiada; b) los pacientes con TCEG en los que se monitorizó la PIC presentaron una alta incidencia de HTC, y c) la morbimortalidad de los TCEG pediátricos disminuyó a lo largo del periodo de estudio.
Head injuries (HIs) remain an important problem in the pediatric population, despite the efforts made to reduce their incidence,1–5 which in the developed countries has been estimated to be about 75–125 cases/100,000 children/year. Of these cases, approximately 7–10% are regarded as serious.1,6
In comparison with the general population,7 pediatric patients suffer a greater frequency of intracranial injuries, with a different response to injury and a better prognosis for one same degree of brain damage, as a result of anatomical and physiopathological factors.8
In pediatric patients, where brain maturation is in full process, it is essential to avoid secondary lesions which in association with the initial primary injury characterizing all HIs, can increase morbidity–mortality by up to 30–40%.1,6,9
Our more than 25 years of experience as a provincial reference center in the management of children with serious head injuries (SHIs) has allowed us to conduct the present study.
The objectives of the study have been: (a) to describe the main epidemiological, clinical and evolutive characteristics of pediatric patients with SHIs; and (b) to analyze their differences in different periods of the study and in different age groups.
Material and methodStudy: retrospective (August 1983–December 1998), prospective (January 1999–December 2009).
Setting: (a) reference population: children aged between 1 month and 14 years, with HIs, exhibiting a Glasgow Coma Scale (GCS) score of ≤8; (b) hospital center: third-level hospital serving as provincial reference center (The Canary Islands (Spain): Gran Canaria, Fuerteventura and Lanzarote), serving a pediatric population of 137,538 children.
Study variables- 1.
Quantitative variables: age, GCS score, pediatric risk of mortality score (PRISM), duration of mechanical ventilation, stay in ICU, Glasgow Outcome Scale (GOS).
- 2.
Qualitative variables: gender, origin, mortality, cause of HI, associated extracranial injuries, lesions on cranial CT scan, sequelae, presence of hyperglycemia, anemia, shock or pupil mydriasis, need for monitoring of intracranial pressure (ICP) and jugular venous oxygen saturation (SjO2), existence of intracranial hypertension (ICH), and need for mechanical ventilation.
- 1.
GCS: following patient stabilization we used the first reflected value of the classical Glasgow scale10 or its modification for pediatric patients.11 The latter is distinguished from the classical scale only in terms of the verbal response section, which is scored as follows: babbling (5), irritable crying (4), crying in response to pain (3), complaint or sighing in response to pain (2), no response (1).
- 2.
SHI: GCS score ≤8.
- 3.
PRISM score: pediatric risk of mortality score.12
- 4.
Polytraumatized patient13,14: serious injuries affecting at least two body regions (skull/brain, thorax, abdomen, musculoskeletal system), or three major fractures. Serious injury is that affecting: (a) skull: unconsciousness or neurological focality, bleeding from nose, bleeding from ears, or facial fracture; (b) thorax: rib fractures, sternal fracture, pneumothorax, hemothorax, lung contusion, aortic rupture, cardiac tamponade or ruptured diaphragm; (c) abdomen: organ laceration or contusion; and (d) musculoskeletal system: vertebral body or arch fracture, fracture of the pelvis, femur, tibia, humerus, or amputation of extremities.
- 5.
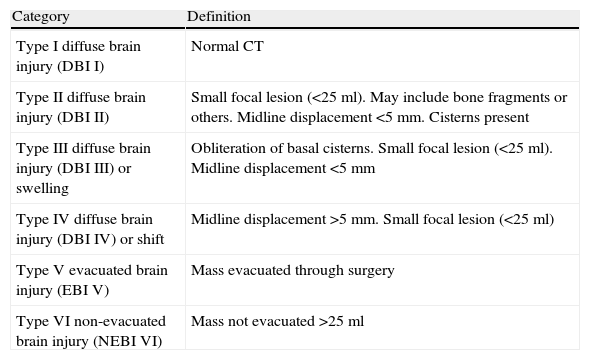
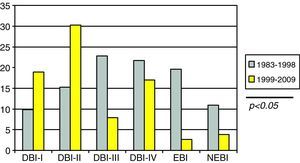
Lesion evidenced by CT: we used the classification of the Trauma Coma Data Bank (TCDB),15 which is shown in Table 1. Severe diffuse brain injury (SDBI) was taken to represent diffuse brain injury (DBI) III and DBI IV.
Table 1.Classification of the brain injuries according to the CT findings (TCDB criteria15).
Category Definition Type I diffuse brain injury (DBI I) Normal CT Type II diffuse brain injury (DBI II) Small focal lesion (<25ml). May include bone fragments or others. Midline displacement <5mm. Cisterns present Type III diffuse brain injury (DBI III) or swelling Obliteration of basal cisterns. Small focal lesion (<25ml). Midline displacement <5mm Type IV diffuse brain injury (DBI IV) or shift Midline displacement >5mm. Small focal lesion (<25ml) Type V evacuated brain injury (EBI V) Mass evacuated through surgery Type VI non-evacuated brain injury (NEBI VI) Mass not evacuated >25ml TCDB, Trauma Coma Data Bank; Cranial volume, under 2 years, 85% of the adult volume; over 8 years, same as in the adult.
- 6.
Shock: existence of systolic blood pressure <55mmHg in patients under 1 year of age and <65mmHg in those over 1 year, with organ repercussions and the need for fluid therapy (≥20ml/kg) and/or catecholamines for control.12
- 7.
Hyperglycemia: blood glucose >200mg/dl.
- 8.
Mechanical ventilation: patients requiring ventilation with intermittent positive pressure at the time of HI or in relation to the latter.
- 9.
Anemia: hemoglobin <8g% or the need for transfusion of red cell concentrates ≥10ml/kg.
- 10.
Monitoring of ICP: performed with different pressure systems and in different locations according to the clinical case or technical availability.
- 11.
ICH: ICP >20mmHg on a sustained basis despite control of all the intracranial or extracranial factors capable of influencing its measurement.
- 12.
Mydriasis: non-reactive unilateral or bilateral dilatation of the pupil >4mm.
- 13.
SjO2: monitoring after retrograde internal jugular vein catheterization, with radiological verification of the location in the jugular bulbar zone. The determinations were performed on an intermittent basis.
- 14.
Arterio-jugular oxygen difference (Sa-jO2)16: difference between SaO2 and SjO2. Three possibilities were considered: (a) brain hyperemia: Sa-jO2<20%; (b) brain ischemia: Sa-jO2>40%; and (c) normal: Sa-jO2 between 20 and 40%.
- 15.
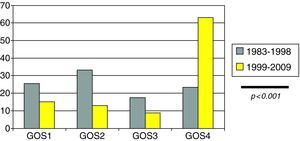
Clinical outcome: at discharge from the PICU, the patients were classified into four categories according to the Glasgow Outcome Scale (GOS)17: (a) death; (b) vegetative state: unable to reciprocally interact with the environment, or severe disability: able to follow instructions, unable to live independently; (c) moderate disability: able to live independently; and (d) good recovery: able to return to baseline situation before HI.
- 16.
Age groups: the population was classified into three groups: under 2 years, between 2 and 6 years, and over 6 years.
- 17.
Periods of study: (a) first 15 years (1983–1998); and (b) last decade (1999–2009).
The data obtained were processed with the Statistical Package for Social Sciences (SPSS) version 17.0. The Student's t-test was used for the comparison of means, while the chi-squared test or Fisher exact test was used for the comparison of percentages. The level of significance was established for alpha=0.05.
ResultsDuring the period of the study, 389 children were admitted to the PICU due to HI, this representing an incidence of 11 cases/100,000 children/year. Of these cases, 174 (45%) presented GCS≤8, and these were the patients included in the study, as they were taken to represent cases of SHI. Thus, the incidence of pediatric SHI was 5 cases/100,000 children/year. Males predominated (67%), and the mean patient age was 67.96±41.6 months, with a range of 1–166 months (Table 2). A total of 106 patients (61%) were admitted to the PICU from the emergency area, while 66 patients (38%) had been transferred from other hospitals.
Demographic and clinical variables of the study population.
| Age (months) | 67.9±41.6 |
| Males | 117 (67.2) |
| Females | 57 (32.8) |
| PRISM | 10.77±6.7 |
| GCS | 5.5±1.8 |
| Stay in PICU (days) | 9.1±13 [5] |
| Duration of mechanical ventilation (h) | 99.4±58 [48] |
| Mortality | 43 (24.7) |
Data expressed as mean±standard deviation [median] or n (%).
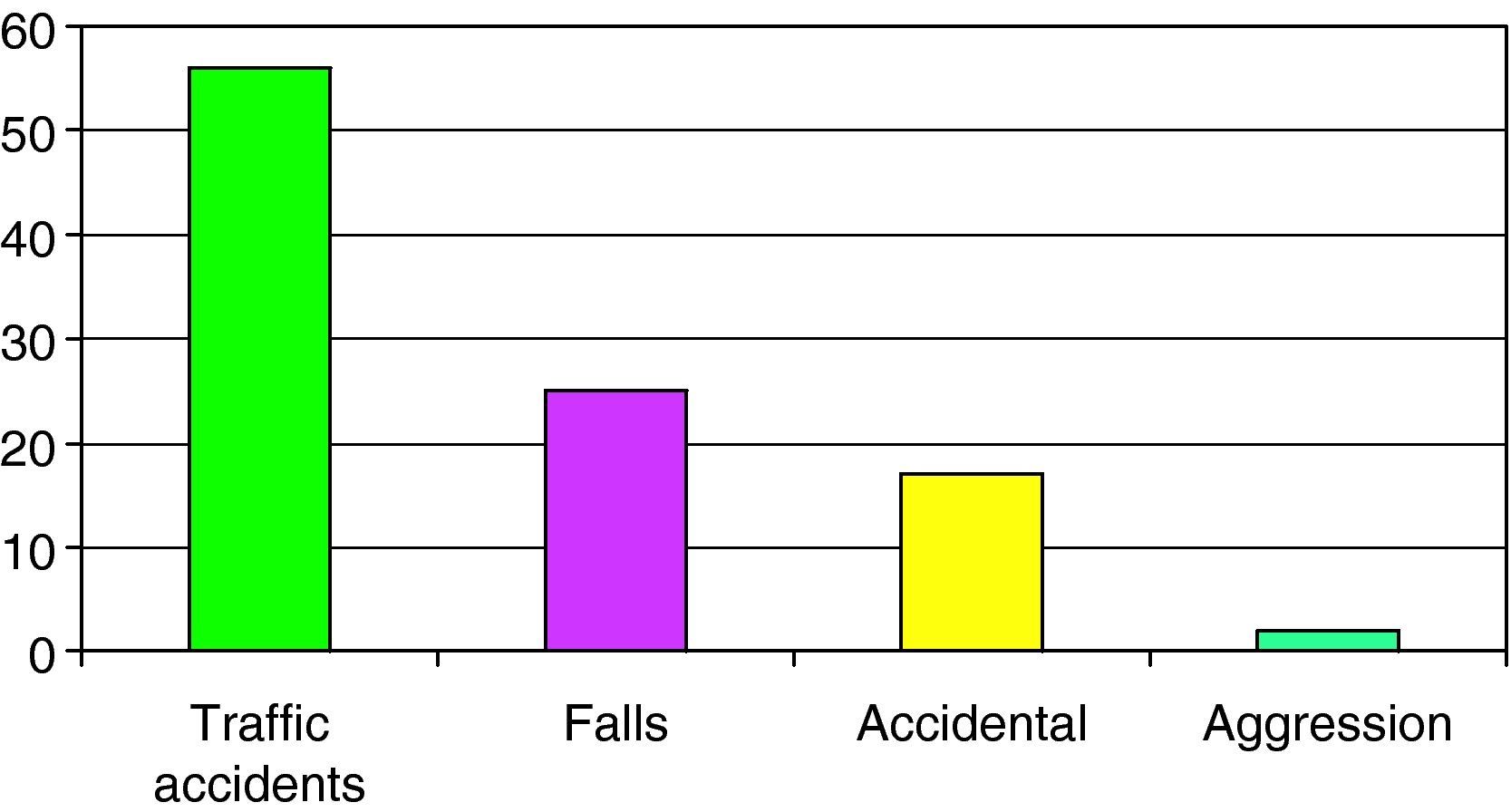
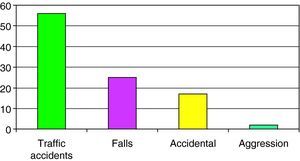
The causes of SHI are reported in Fig. 1, with a predominance of traffic accidents (56%), followed by falls from a height (24%).
Fifty-five percent of the study population suffered cranial fracture, and 58 patients (33%) presented other associated extracranial traumatisms. In turn, 66% of the children presented anemia, with an association of hyperglycemia or shock in 38% and 37% of the cases, respectively. Mechanical ventilation was provided in 92.5% of the patients.
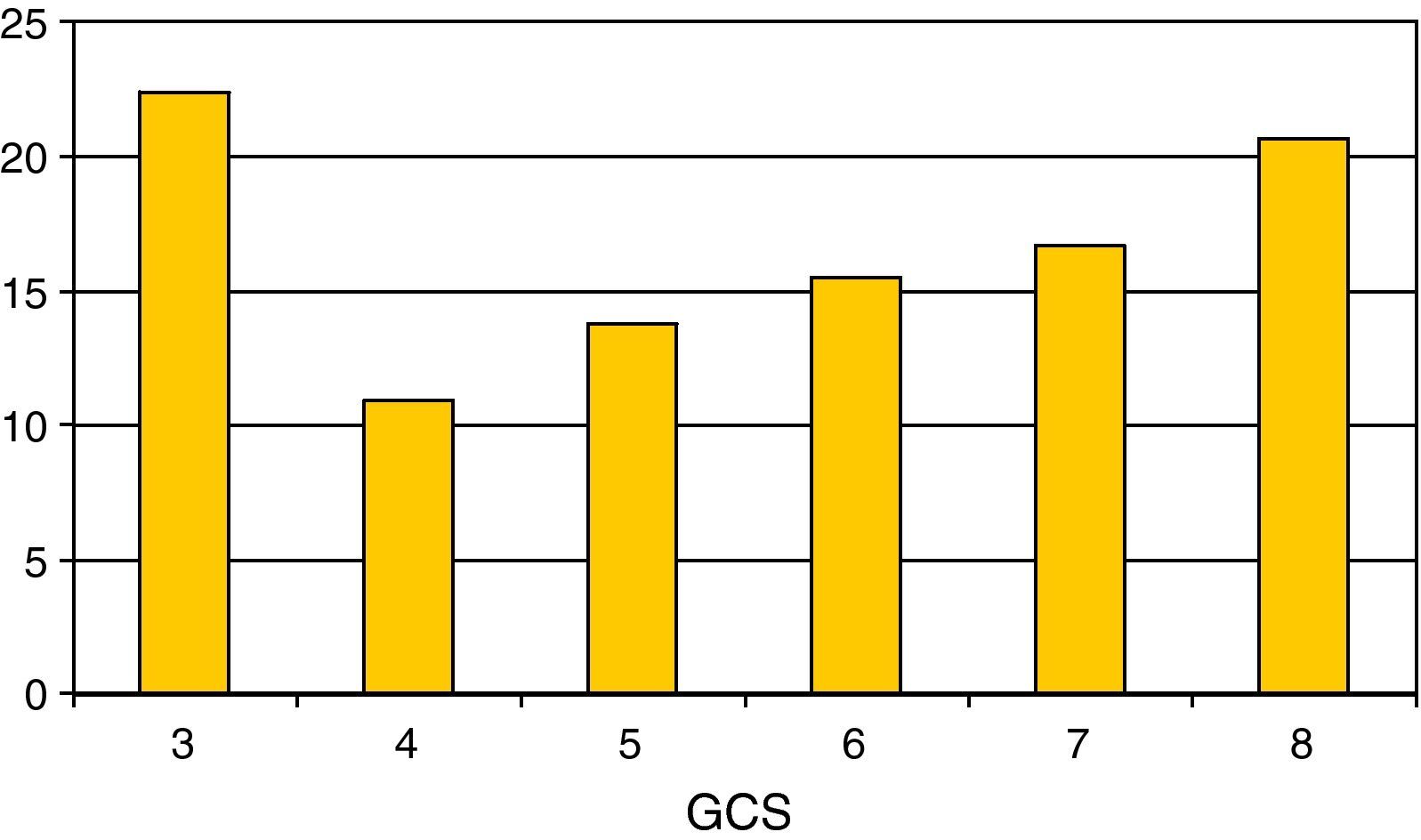
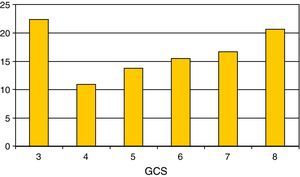
Regarding the most relevant neurological variables, the mean GCS was 5.55±1.8; Fig. 2 shows the percentage distribution. Thirty-five percent of the patients presented pupil mydriasis. ICP was monitored in 104 children (60%); 79% presented ICH. The jugular bulb was catheterized in 25% of the children with ICH; the determinations made revealed Sa-jO2 values compatible with brain hyperemia in 70% of the cases, with brain ischemia in 20%. The values proved normal in 10% of the cases.
Regarding the lesions evidenced by CT, it should be noted that in 55% of the cases of diffuse brain injury, the latter proved severe (SDBI).
The mean stay in the ICU was 9.1±13 days, with a median of 5 days, whereas the mean duration of mechanical ventilation was 99.41±58h, with a median of 48h. The mean PRISM score was 10.77±6.7.
The modified Glasgow Outcome Scale (GOS) score at discharge from the PICU was: (a) 43 patients (24.7%) died; (b) 38 patients (22%) were in a vegetative condition or presented severe disability; (c) 21 patients (12.3%) suffered moderate disability; and (d) 71 patients (41%) showed good recovery.
We analyzed some of the above commented aspects in relation to the three age groups into which the study population was divided. In children under 2 years of age, HIs were mainly caused by accidental falls (33%) and falls from heights (33%). Of note is the observation that aggressions or abuse were the cause of injury in 13% of the cases. After 2 years of age, in both the 2–6 years and over 6 years age groups, traffic accidents were the main cause of HIs (57.1% and 68.9%, respectively).
Cranial fractures were uniformly distributed in all three groups, in the same way as shock, anemia, hyperglycemia and ICH.
SDBI as evidenced by CT was seen to increase in frequency with age: 26.7% in those under 2 years of age, 34.7% in those aged 2–6 years, and 48.6% in those over 6 years of age. These differences came close to statistical significance (p=0.06).
The mortality rates in the three groups showed no significant differences (26.7%, 31.4% and 17.6%, respectively), though on comparing the patients under 6 years of age with those over 6 years, greater mortality was observed in the former (30%) versus the latter (17.6%) – the difference being almost significant (p=0.06).
A comparison was made of the two study periods (first 15 years versus the last decade), revealing the following differences:
- 1.
Traffic accidents as a cause of SHI in pediatric patients decreased significantly (58.5% vs 45.3%; p<0.001).
- 2.
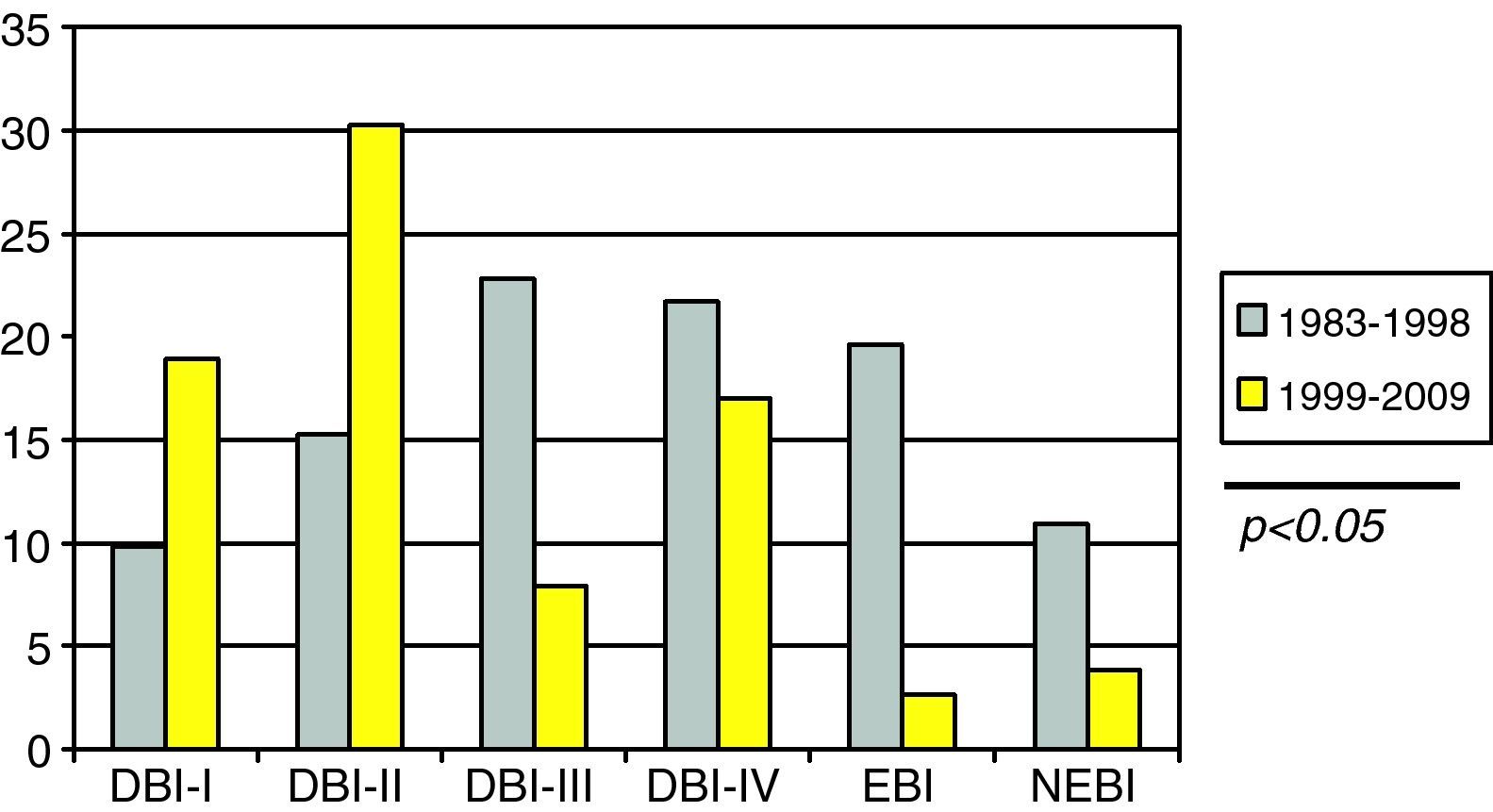
SDBI as evidenced by CT (Fig. 3) presented a significantly higher incidence in the first period versus the second (44.5% vs 24.5%; p<0.05).
- 3.
There were no significant differences between the two groups in terms of the monitoring of ICP (58.6% vs 63.8%; NS).
- 4.
There was a higher incidence of ICH in the children with HI in the first 15 years of the study versus the last 10 years (88% vs 54%; p<0.05).
- 5.
Mortality decreased from 25.5% to 15.1% in the last decade—the difference between the two periods being nonsignificant.
- 6.
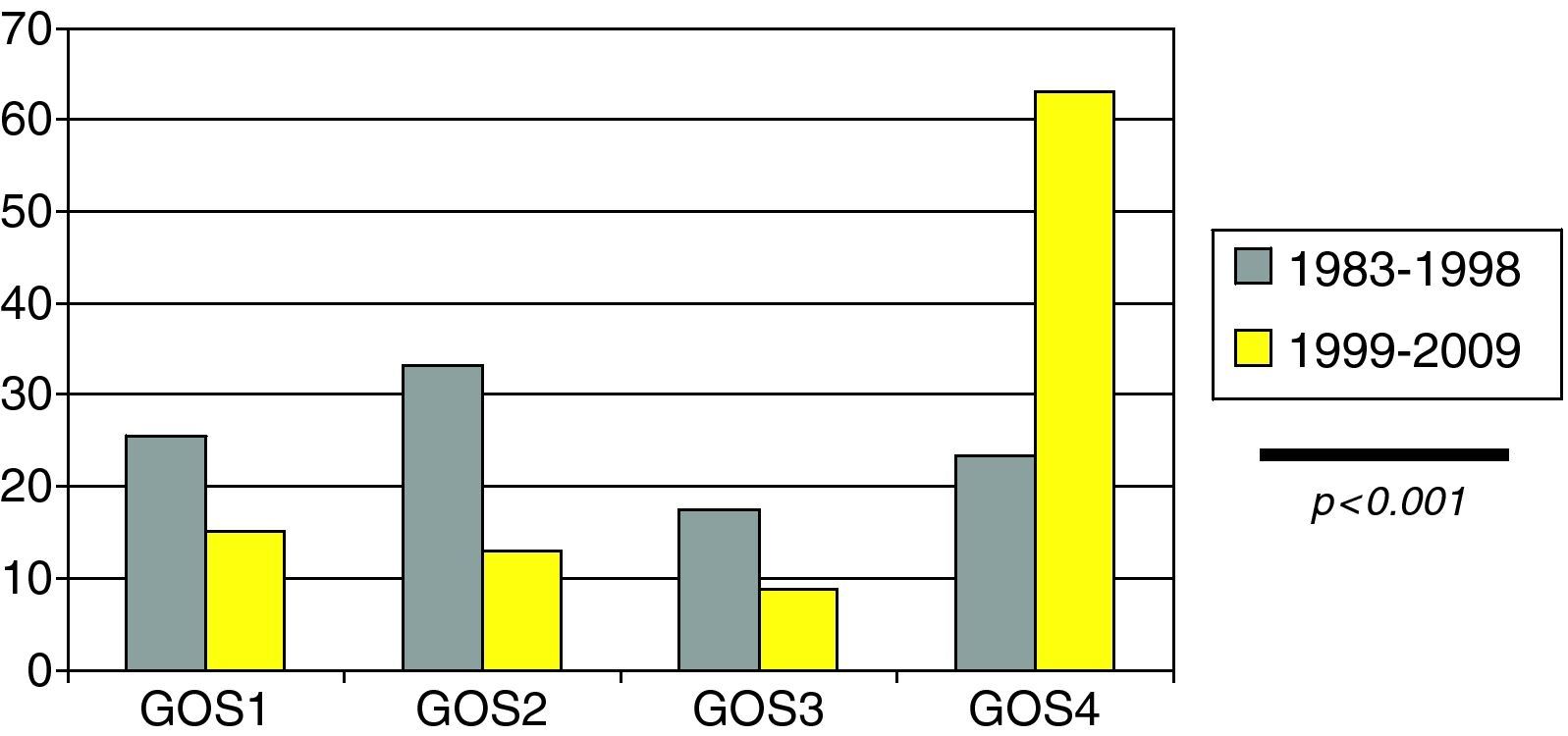
Clinical outcome in the form of adequate recovery as established from the modified GOS (Fig. 4) was significantly more common in the last decade (23.3% vs 63.1%; p<0.001).
In our study population, SHIs represented almost one-half of all HIs treated in the PICU, the incidence of pediatric SHIs being 5 cases/100,000 children/year—this figure being slightly lower than reported in other series, with incidences of between 7 and 12 cases/100,000 children/year.1,6 However, in the group of patients with SHI, 47% presented a GCS score of under 6.
The mean age in our series (5.6 years) was slightly lower than in current series which report ages of between 7 and 8 years.18–21 We found no differences in mortality between the three age groups studied, in coincidence with the observations of White et al.22 However, in most studies, mortality is greater in younger children, particularly among those under 6 years of age4,23–25; in our population the differences came close to statistical significance (30% for those under 6 years versus 17.6% among those over 6 years; p=0.06).
The most common cause of SHI in pediatric patients was traffic accidents, in coincidence with the reports of other authors.21,25 Of note was the observation that all recorded cases of aggression or abuse (2.3%) corresponded to the group of infants under two years of age; this fact should be taken into account in situations of neurological damage of indeterminate cause in this group of patients.
As commented above, practically one-half of all the pediatric patients with HIs in our series presented GCS<6, i.e., these corresponded to cases of SHI. Another finding was the existence of pupil mydriasis in one-third of the population, which coincides with the incidence recently reported by Bahlout et al.,1 and which together with the GCS score reflects the severity of the cases analyzed. In this same line, practically 80% of our patients subjected to ICP monitorization presented ICH. We therefore consider ICP monitoring to be a priority in children with SHIs.26
A significant observation was that 25% of the patients with ICH were subjected to SjO2 monitorization; this is relevant in view of the limited data available on this point in the literature related to pediatric patients. Although starting in the year 1993 a progressive increase in SjO2 monitoring was recorded among the patients with persistent ICH, this technique has not been standardized—possibly because of problems of interpretation, since it constitutes a local measure of cerebral blood flow and oxygen consumption (VOc) and of their adequate correlation in concrete clinical situations (brain death, barbiturate coma, etc.). Likewise, continuous monitorization systems tend to present the inconvenience of requiring frequent calibrations. Nevertheless, in our series the predominant pattern corresponded to brain hyperemia, coinciding with the clinical and radiological findings in pediatric SHI, characterized by an increase in cerebral blood flow and of the risk of brain edema.27–29
In our study, the incidence of brain edema regarded as SDBI (grades III and IV according to the criteria of the TCDB15) was approximately 40%, and very similar to the figures published by Esparza et al.30 and Bahlout et al.1 On analyzing the CT lesions in the different age groups, we noted a lesser incidence of SDBI among the younger patients. This could be explained by anatomical factors such as the persistence of open sutures, conferring greater skull and brain elasticity and plasticity in these early ages.
Other extracerebral factors such as anemia, the existence of shock or hyperglycemia, were found to be more frequent than in other studies.1,3
The mean PRISM score in our patients was 10.6, which is intermediate in comparison with the values recorded for other critical patients (sepsis, cardiogenic shock, severe respiratory failure, etc.). This could be explained by considering that in only 33% of the cases was SHI associated to polytraumatism, as a result of which the fundamental PRISM score is derived from the GCS score—since normally and apart from the neurological impairment, those patients with SHI who do not die present only minimal organ dysfunction (PRISM 8.9±5 vs 22.2±5.3; p<0.001).
As regards the clinical course and prognosis, we must underscore the important morbidity (34%) and mortality (25%) among the children with SHI as evidenced by the modified GOS at discharge from the PICU. In general, many children, once moved to the ward and subjected to standardization of the rehabilitation-physiotherapy measures, with an increased contact with close relatives, experience improvement of their initial sequelae.
The time course of the studied variables shows a tendency towards fewer traffic accidents as a cause of SHIs in pediatric patients, with a reduction in mortality (which although not statistically significant does seem evident, from 25.5% to 15.1%), and greater clinical recovery during the last decade.
Although the monitoring of ICP showed no significant differences, there was a greater incidence of ICH in the first period studied, and the severity of diffuse brain damage as evidenced by CT was also greater.
These differences observed in the children with SHI (fundamentally lesser severity and improved recovery) in the course of the 25 years of the study can be explained by improved care—though other contributing factors must also be taken into account: improvements in traffic safety, retention systems and devices in vehicles, prompt patient treatment and out-hospital resuscitation, etc.
This study thus focuses on the epidemiological, clinical and evolutive aspects that confirm the data reported by the few published series, and moreover also contributes new aspects that have been very little explored in the literature on serious head injuries.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: López Álvarez JM, et al. Traumatismo craneoencefálico pediátrico grave (I). Epidemiología, clínica y evolución. Med Intensiva. 2011;35:331–6.